Abstract
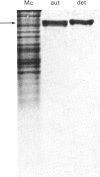
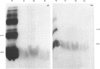
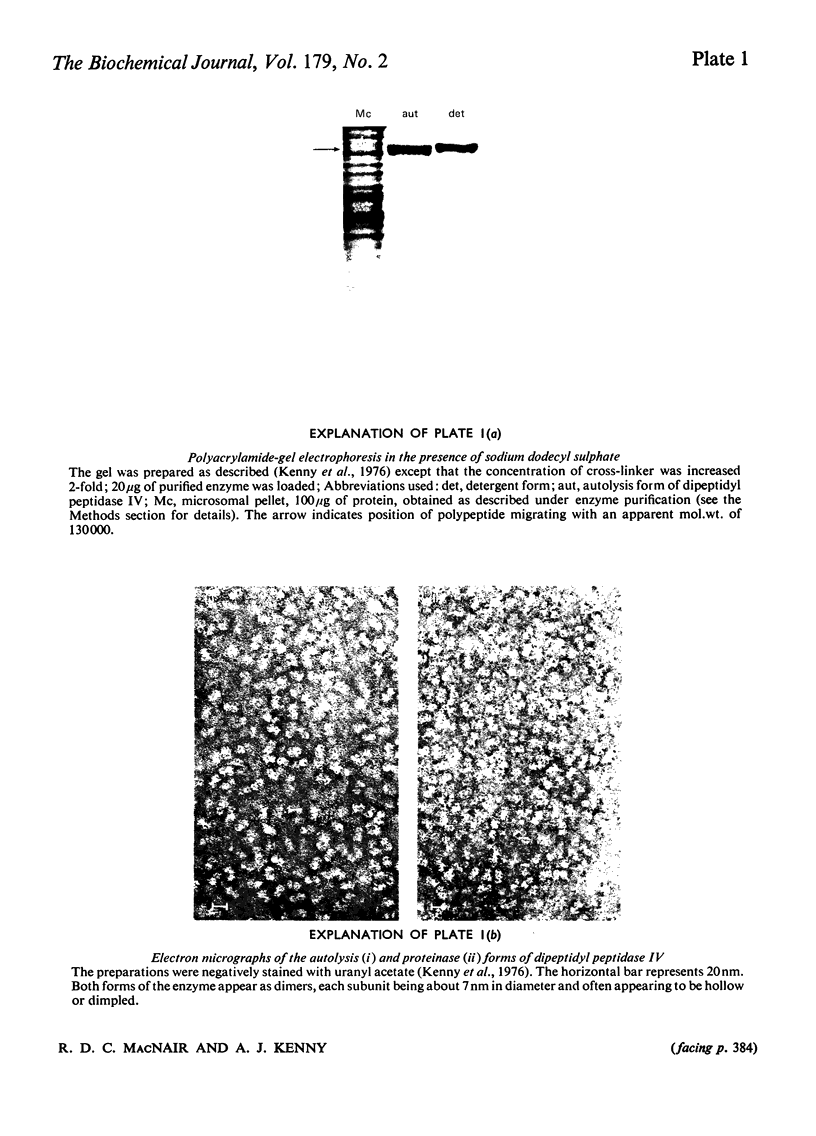
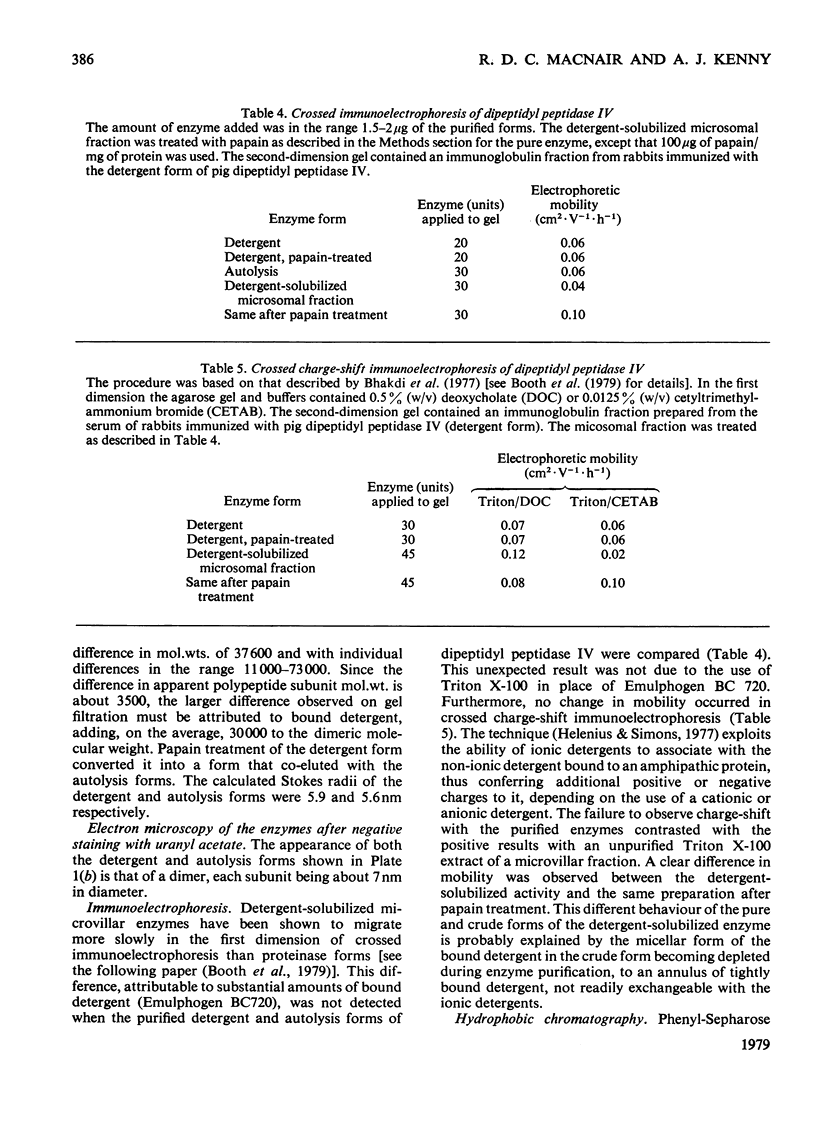
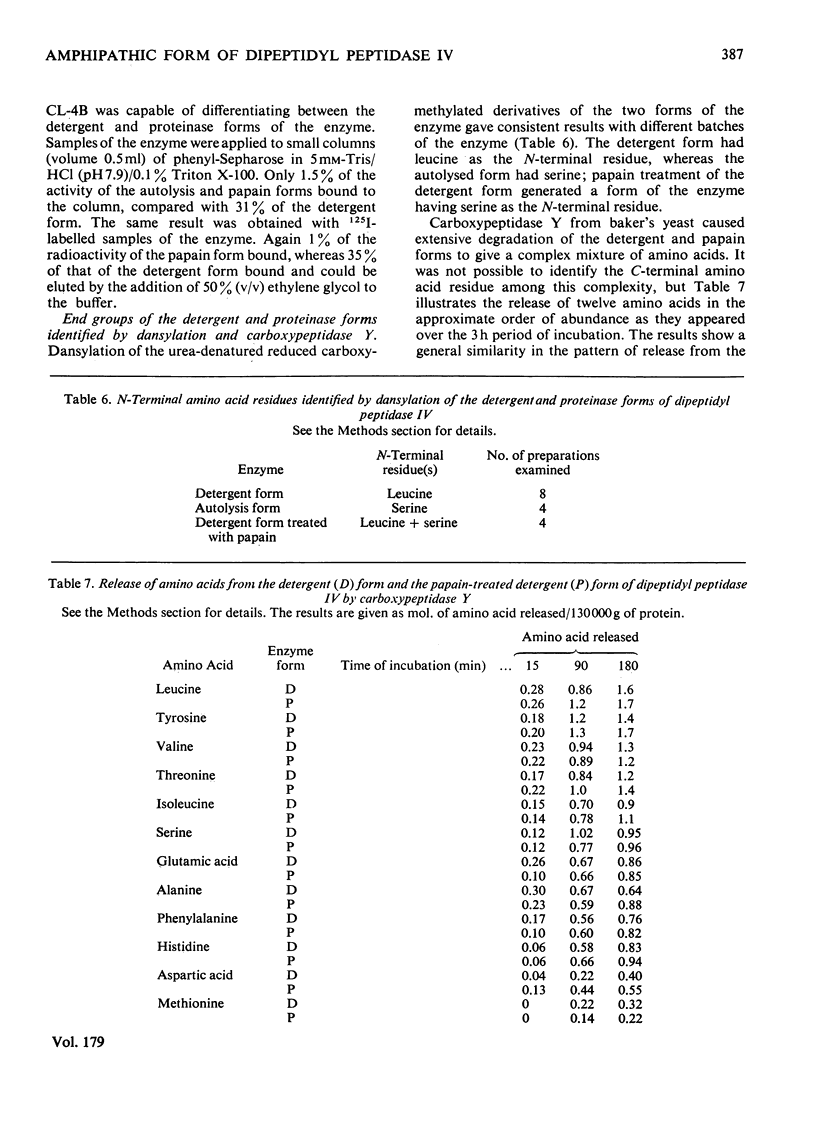
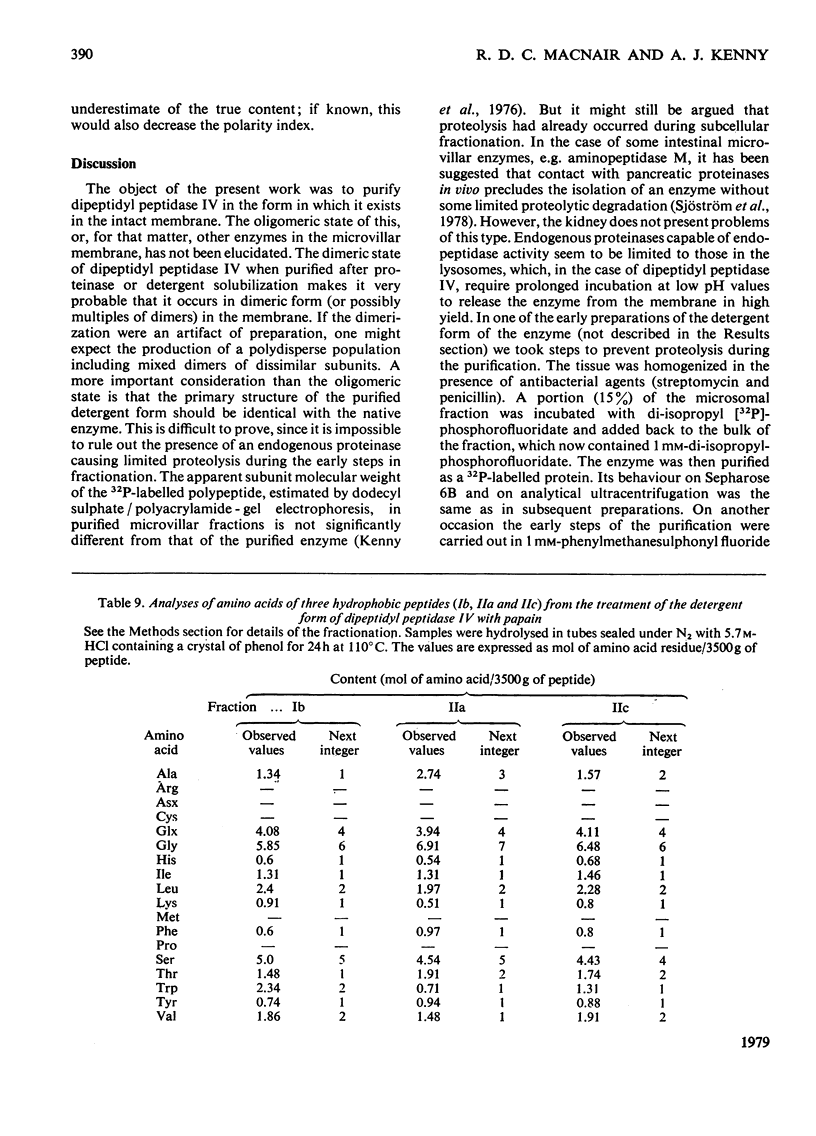
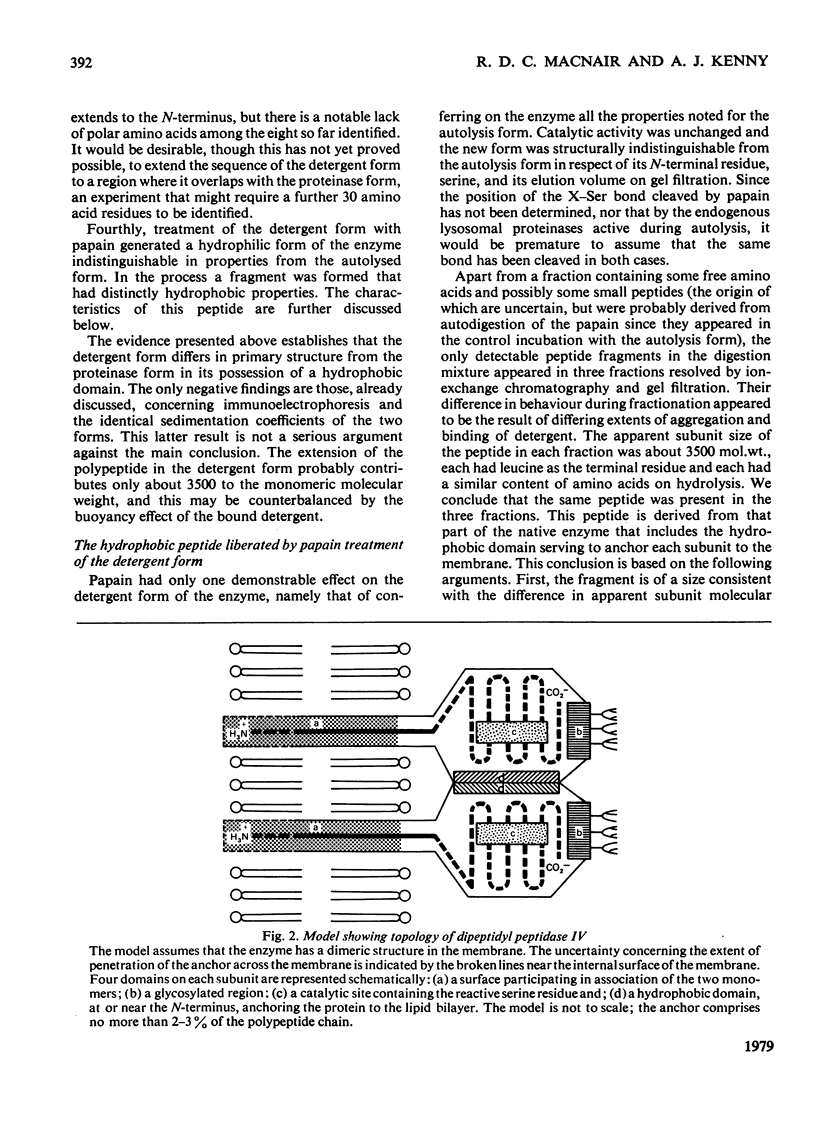
Dipeptidyl peptidase IV was solubilized from the microvillar membrane of pig kidney by Triton X-100. The purified enzyme was homogeneous on polyacrylamide-gel electrophoresis and ultracentrifugation, although immunoelectrophoresis indicated that amino-peptidase M was a minor contaminant. A comparison of the detergent-solubilized and proteinase (autolysis)-solubilized forms of the enzyme was undertaken to elucidate the structure and function of the hydrophobic domain that serves to anchor the protein to the membrane. No differences in catalytic properties, nor in sensitivity to inhibition by di-isopropyl phosphorofluoridate were found. On the other hand, several structural differences could be demonstrated. Both forms were about 130,000 subunit mol.wt., but the detergent form appeared to be larger by no more than about 4,000. Electron microscopy showed both forms to be dimers, and gel filtration revealed a difference in the dimeric mol.wt. of about 38 000, mainly attributable to detergent molecules bound to the hydrophobic domain. Papain converted the detergent form into a hydrophilic form that could not be distinguished in properties from the autolysis form. A hydrophobic peptide of about 3500 mol.wt. was identified as a product of papain treatment. The detergent and proteinase forms differed in primary structure. Partial N-terminal amino acid sequences were shown to be different, and the pattern of release of amino acids from the C-terminus by carboxypeptidase Y was essentially similar. The results are consistent with a model in which the protein is anchored to the microvillar membrane by a small hydrophobic domain located within the N-terminal amino acid sequence of the polypeptide chain. The significance of these results in relation to biosynthesis of the enzyme and assembly in the membrane is discussed.
Full text
PDF



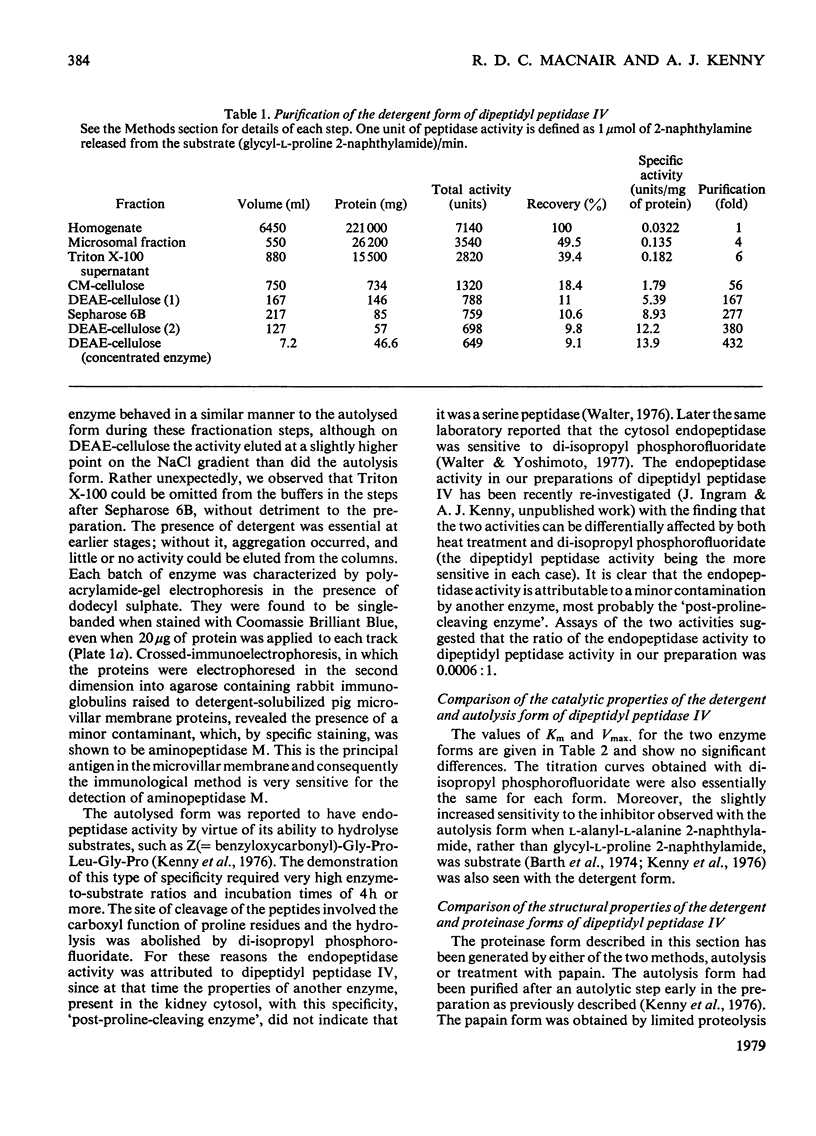






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Barth A., Schulz H., Neubert K. Untersuchungen zur Reinigung und Charakterisierung der Dipeptidylaminopeptidase IV. Acta Biol Med Ger. 1974;32(2-3):157–174. [PubMed] [Google Scholar]
- Bhakdi S., Bhakdi-Lehnen B., Bjerrum O. J. Detection of amphiphilic proteins and peptides in complex mixtures. Charge-shift crossed immunoelectrophoresis and two-dimensional charge-shift electrophoresis. Biochim Biophys Acta. 1977 Oct 3;470(1):35–44. doi: 10.1016/0005-2736(77)90059-1. [DOI] [PubMed] [Google Scholar]
- Bhatti T., Chambers R. E., Clamp J. R. The gas chromatographic properties of biologically important N-acetylglucosamine derivatives, monosaccharides, disaccharides, trisaccharides, tetrasaccharides and pentasaccharides. Biochim Biophys Acta. 1970 Nov 24;222(2):339–347. doi: 10.1016/0304-4165(70)90122-4. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A. G. A novel system for the two-dimensional electrophoresis of membrane proteins. Biochem J. 1977 Apr 1;163(1):165–168. doi: 10.1042/bj1630165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A. G., Hubbard L. M., Kenny A. J. Proteins of the kidney microvillar membrane. Immunoelectrophoretic analysis of the membrane hydrolases: identification and resolution of the detergent- and proteinase-solubilized forms. Biochem J. 1979 May 1;179(2):397–405. doi: 10.1042/bj1790397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. Proteins of the kidney microvillus membrane. Identification of subunits after sodium dodecylsullphate/polyacrylamide-gel electrophoresis. Biochem J. 1976 Nov;159(2):395–407. doi: 10.1042/bj1590395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P. N., Blobel G. The role of organelles in the chemical modification of the primary translation products of secretory proteins. FEBS Lett. 1976 Dec 31;72(2):215–226. doi: 10.1016/0014-5793(76)80973-8. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Vanderkooi G. The low polarity of many membrane proteins. Proc Natl Acad Sci U S A. 1972 Apr;69(4):930–932. doi: 10.1073/pnas.69.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clamp J. R., Bhatti T., Chambers R. E. The determination of carbohydrate in biological materials by gas-liquid chromatography. Methods Biochem Anal. 1971;19:229–344. doi: 10.1002/9780470110386.ch3. [DOI] [PubMed] [Google Scholar]
- Colbeau A., Maroux S. Integration of alkaline phosphatase in the intestinal brush border membrane. Biochim Biophys Acta. 1978 Jul 20;511(1):39–51. doi: 10.1016/0005-2736(78)90063-9. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigli I., von Zabern I., Porter R. R. The isolation and structure of C4, the fourth component of human complement. Biochem J. 1977 Sep 1;165(3):439–446. doi: 10.1042/bj1650439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros C., Labouesse B. Study of the dansylation reaction of amino acids, peptides and proteins. Eur J Biochem. 1969 Feb;7(4):463–470. doi: 10.1111/j.1432-1033.1969.tb19632.x. [DOI] [PubMed] [Google Scholar]
- Habener J. F., Rosenblatt M., Kemper B., Kronenberg H. M., Rich A., Potts J. T., Jr Pre-proparathyroid hormone; amino acid sequence, chemical synthesis, and some biological studies of the precursor region. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2616–2620. doi: 10.1073/pnas.75.6.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. Charge shift electrophoresis: simple method for distinguishing between amphiphilic and hydrophilic proteins in detergent solution. Proc Natl Acad Sci U S A. 1977 Feb;74(2):529–532. doi: 10.1073/pnas.74.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Henning R., Milner R. J., Reske K., Cunningham B. A., Edelman G. M. Subunit structure, cell surface orientation, and partial amino-acid sequences of murine histocompatibility antigens. Proc Natl Acad Sci U S A. 1976 Jan;73(1):118–122. doi: 10.1073/pnas.73.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopsu-Havu V. K., Rintola P., Glenner G. G. A hog kidney aminopeptidase liberating N-terminal dipeptides. Partial purification and characteristics. Acta Chem Scand. 1968;22(1):299–308. doi: 10.3891/acta.chem.scand.22-0299. [DOI] [PubMed] [Google Scholar]
- Katz F. N., Rothman J. E., Lingappa V. R., Blobel G., Lodish H. F. Membrane assembly in vitro: synthesis, glycosylation, and asymmetric insertion of a transmembrane protein. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3278–3282. doi: 10.1073/pnas.74.8.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny A. J., Booth A. G., George S. G., Ingram J., Kershaw D., Wood E. J., Young A. R. Dipeptidyl peptidase IV, a kidney brush-border serine peptidase. Biochem J. 1976 Jul 1;157(1):169–182. doi: 10.1042/bj1570169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny A. J., Booth A. G., Macnair R. D. Peptidases of the kidney microvillus membrane. Acta Biol Med Ger. 1977;36(11-12):1575–1585. [PubMed] [Google Scholar]
- Kenny A. J., Booth A. G. Microvilli: their ultrastructure, enzymology and molecular organization. Essays Biochem. 1978;14:1–44. [PubMed] [Google Scholar]
- Maroux S., Louvard D., Baratti J. The aminopeptidase from hog intestinal brush border. Biochim Biophys Acta. 1973 Sep 15;321(1):282–295. doi: 10.1016/0005-2744(73)90083-1. [DOI] [PubMed] [Google Scholar]
- Maroux S., Louvard D. On the hydrophobic part of aminopeptidase and maltases which bind the enzyme to the intestinal brush border membrane. Biochim Biophys Acta. 1976 Jan 21;419(2):189–195. doi: 10.1016/0005-2736(76)90345-x. [DOI] [PubMed] [Google Scholar]
- Morrison T. G. Site of synthesis of membrane and nonmembrane proteins of vesicular stomatitis virus. J Biol Chem. 1975 Sep 10;250(17):6955–6962. [PubMed] [Google Scholar]
- Robinson N. C., Tanford C. The binding of deoxycholate, Triton X-100, sodium dodecyl sulfate, and phosphatidylcholine vesicles to cytochrome b5. Biochemistry. 1975 Jan 28;14(2):369–378. doi: 10.1021/bi00673a025. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Lenard J. Membrane asymmetry. Science. 1977 Feb 25;195(4280):743–753. doi: 10.1126/science.402030. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Sjöström H., Norén O., Jeppesen L., Staun M., Svensson B., Christiansen L. Purification of different amphiphilic forms of a microvillus aminopeptidase from pig small intestine using immunoadsorbent chromatography. Eur J Biochem. 1978 Aug 1;88(2):503–511. doi: 10.1111/j.1432-1033.1978.tb12476.x. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Waterfield M. D. Studies on the primary structure of the influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):93–97. doi: 10.1073/pnas.72.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A., Strominger J. L. Detergent-soluble HLA antigens contain a hydrophilic region at the COOH-terminus and a penultimate hydrophobic region. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2481–2485. doi: 10.1073/pnas.73.7.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson B., Danielsen M., Staun M., Jeppesen L., Norén O., Sjöström H. An amphiphilic form of dipeptidyl peptidase IV from pig small-intestinal brush-border membrane. Purification by immunoadsorbent chromatography and some properties. Eur J Biochem. 1978 Oct 16;90(3):489–498. doi: 10.1111/j.1432-1033.1978.tb12628.x. [DOI] [PubMed] [Google Scholar]
- Tomita M., Marchesi V. T. Amino-acid sequence and oligosaccharide attachment sites of human erythrocyte glycophorin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2964–2968. doi: 10.1073/pnas.72.8.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Vannier C., Louvard D., Maroux S., Desnuelle P. Structural and topological homology between porcine intestinal and renal brush border aminopeptidase. Biochim Biophys Acta. 1976 Nov 11;455(1):185–199. doi: 10.1016/0005-2736(76)90163-2. [DOI] [PubMed] [Google Scholar]
- Wacker H., Lehky P., Vanderhaeghe F., Stein E. A. On the subunit structure of particulate aminopeptidase from pig kidney. Biochim Biophys Acta. 1976 Apr 8;429(2):546–554. doi: 10.1016/0005-2744(76)90302-8. [DOI] [PubMed] [Google Scholar]
- Walter R. Partial purification and characterization of post-proline cleaving enzyme: enzymatic inactivation of neurohypophyseal hormones by kidney preparations of various species. Biochim Biophys Acta. 1976 Jan 23;422(1):138–158. doi: 10.1016/0005-2744(76)90015-2. [DOI] [PubMed] [Google Scholar]
- Wirth D. F., Katz F., Small B., Lodish H. F. How a single Sindbis virus mRNA directs the synthesis of one soluble protein and two integral membrane glycoproteins. Cell. 1977 Feb;10(2):253–263. doi: 10.1016/0092-8674(77)90219-7. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Walter R. Post-proline dipeptidyl aminopeptidase (dipeptidyl aminopeptidase IV) from lamb kidney. Purification and some enzymatic properties. Biochim Biophys Acta. 1977 Dec 8;485(2):391–401. doi: 10.1016/0005-2744(77)90174-7. [DOI] [PubMed] [Google Scholar]