Abstract
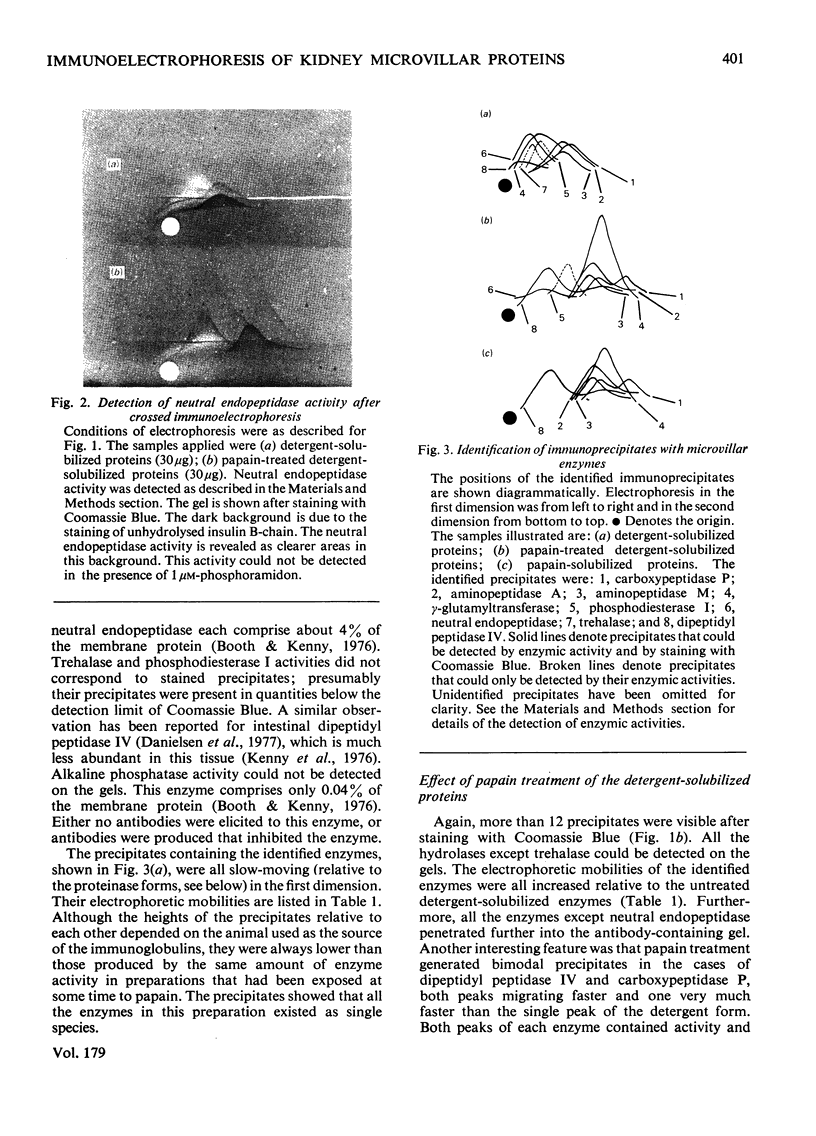
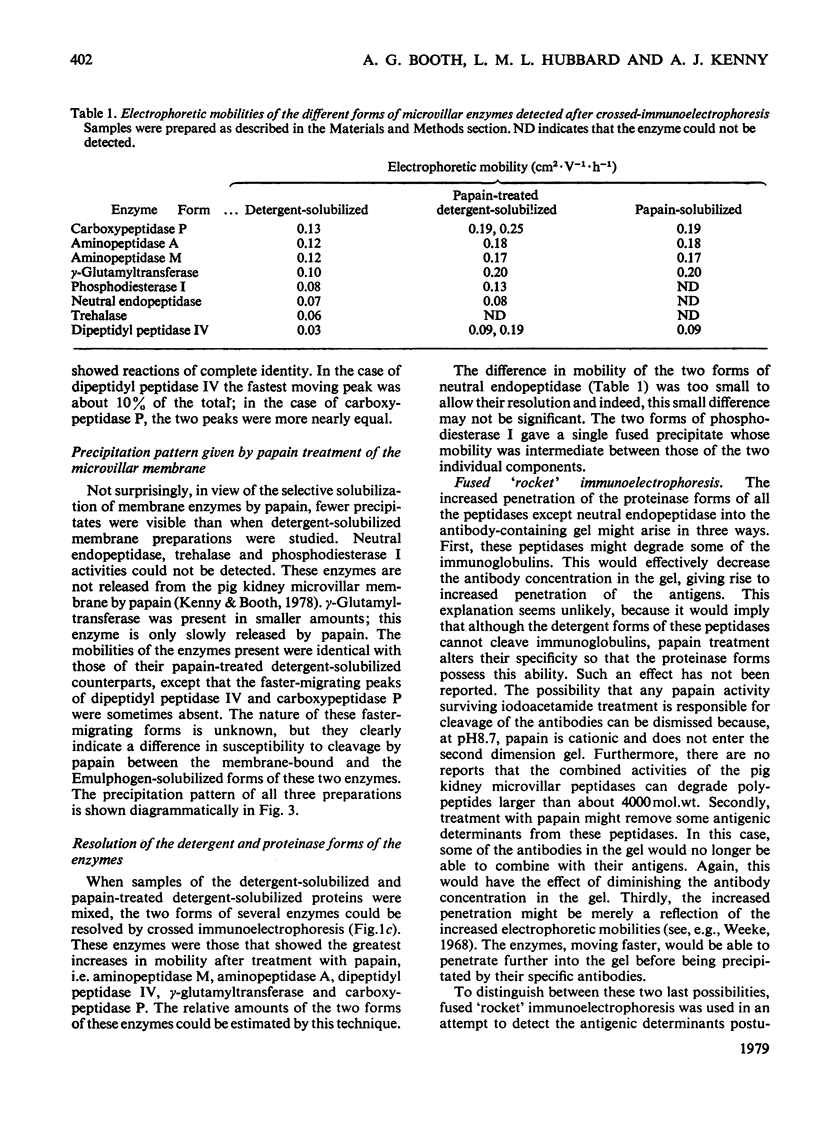
Antibodies raised in rabbits to detergent-solubilized pig kidney microvillar proteins have been used to investigate the membrane hydrolases by crossed immunoelectrophoresis. Eight enzymes were detected by specific staining methods: aminopeptidase M, dipeptidylpeptidase IV, neutral endopeptidase, aminopeptidase A, carboxypeptidase P, gamma-glutamyltransferase, trehalase and phosphodiesterase I. The mobility of all these enzymes, with the exception of trehalase and neutral endopeptidase, was increased by treatment of the detergent-solubilized preparation with papain. The difference between the detergent and proteinase forms of these enzymes is attributed to the removal of a small, non-antigenic peptide to which detergent is bound in significant quantities. This interpretation was further supported by experiments in which the microvillus fraction was labelled with an intramembrane photolabelling reagent, 1-azido-4-[125I]iodobenzene. After photolysis, the radioactivity in the membrane could be solubilized by detergent treatment but not by papain treatment. Radioautography after crossed charge-shift immunoelectrophoresis showed a good correlation between charge-shift (signifying the presence of detergent bound to a hydrophobic domain) and the presence of the label.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhakdi S., Bhakdi-Lehnen B., Bjerrum O. J. Detection of amphiphilic proteins and peptides in complex mixtures. Charge-shift crossed immunoelectrophoresis and two-dimensional charge-shift electrophoresis. Biochim Biophys Acta. 1977 Oct 3;470(1):35–44. doi: 10.1016/0005-2736(77)90059-1. [DOI] [PubMed] [Google Scholar]
- Bjerrum O. J. Immunochemical investigation of membrane proteins. A methodological survey with emphasis placed on immunoprecipitation in gels. Biochim Biophys Acta. 1977 Aug 9;472(2):135–195. doi: 10.1016/0304-4157(77)90016-8. [DOI] [PubMed] [Google Scholar]
- Booth A. G. A novel system for the two-dimensional electrophoresis of membrane proteins. Biochem J. 1977 Apr 1;163(1):165–168. doi: 10.1042/bj1630165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. Proteins of the kidney microvillus membrane. Identification of subunits after sodium dodecylsullphate/polyacrylamide-gel electrophoresis. Biochem J. 1976 Nov;159(2):395–407. doi: 10.1042/bj1590395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M., Sjöström H., Norén O., Dabelsteen E. Immunoelectrophoretic studies on pig intestinal brush border proteins. Biochim Biophys Acta. 1977 Oct 26;494(2):332–342. doi: 10.1016/0005-2795(77)90163-5. [DOI] [PubMed] [Google Scholar]
- George S. G., Kenny J. Studies on the enzymology of purified preparations of brush border from rabbit kidney. Biochem J. 1973 May;134(1):43–57. doi: 10.1042/bj1340043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. Charge shift electrophoresis: simple method for distinguishing between amphiphilic and hydrophilic proteins in detergent solution. Proc Natl Acad Sci U S A. 1977 Feb;74(2):529–532. doi: 10.1073/pnas.74.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmkamp R. W., Sears D. A. A label for the red cell membrane: diazotized diiodosulfanilic acid. Int J Appl Radiat Isot. 1970 Nov;21(11):683–685. doi: 10.1016/0020-708x(70)90127-4. [DOI] [PubMed] [Google Scholar]
- Kenny A. J., Booth A. G., George S. G., Ingram J., Kershaw D., Wood E. J., Young A. R. Dipeptidyl peptidase IV, a kidney brush-border serine peptidase. Biochem J. 1976 Jul 1;157(1):169–182. doi: 10.1042/bj1570169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny A. J., Booth A. G., Macnair R. D. Peptidases of the kidney microvillus membrane. Acta Biol Med Ger. 1977;36(11-12):1575–1585. [PubMed] [Google Scholar]
- Kenny A. J., Booth A. G. Microvilli: their ultrastructure, enzymology and molecular organization. Essays Biochem. 1978;14:1–44. [PubMed] [Google Scholar]
- Kenny A. J., Macnair R. D., Booth A. G. The topology of kidney microvillar enzymes. Biochem Soc Trans. 1978;6(5):825–829. doi: 10.1042/bst0060825a. [DOI] [PubMed] [Google Scholar]
- Louvard D., Maroux S., Vannier C., Desnuelle P. Topological studies on the hydrolases bound to the intestinal brush border membrane. I. Solubilization by papain and Triton X-100. Biochim Biophys Acta. 1975 Jan 28;375(2):235–248. [PubMed] [Google Scholar]
- Macnair D. C., Kenny A. J. Proteins of the kidney microvillar membrane. The amphipathic form of dipeptidyl peptidase IV. Biochem J. 1979 May 1;179(2):379–395. doi: 10.1042/bj1790379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda H., Aoyagi T., Takeuchi T., Umezawa H. Letter: A thermolysin inhibitor produced by Actinomycetes: phospholamidon. J Antibiot (Tokyo) 1973 Oct;26(10):621–623. doi: 10.7164/antibiotics.26.621. [DOI] [PubMed] [Google Scholar]
- Weeke B. A manual of quantitative immunoelectrophoresis. Methods and applications. 1. General remarks on principles, equipment, reagents and procedures. Scand J Immunol Suppl. 1973;1:15–35. doi: 10.1111/j.1365-3083.1973.tb03776.x. [DOI] [PubMed] [Google Scholar]
- Weeke B. Carbamylated human immunoglobulins tested by electrophoresis in agarose and antibody containing agarose. Scand J Clin Lab Invest. 1968;21(4):351–354. doi: 10.3109/00365516809077006. [DOI] [PubMed] [Google Scholar]