Abstract
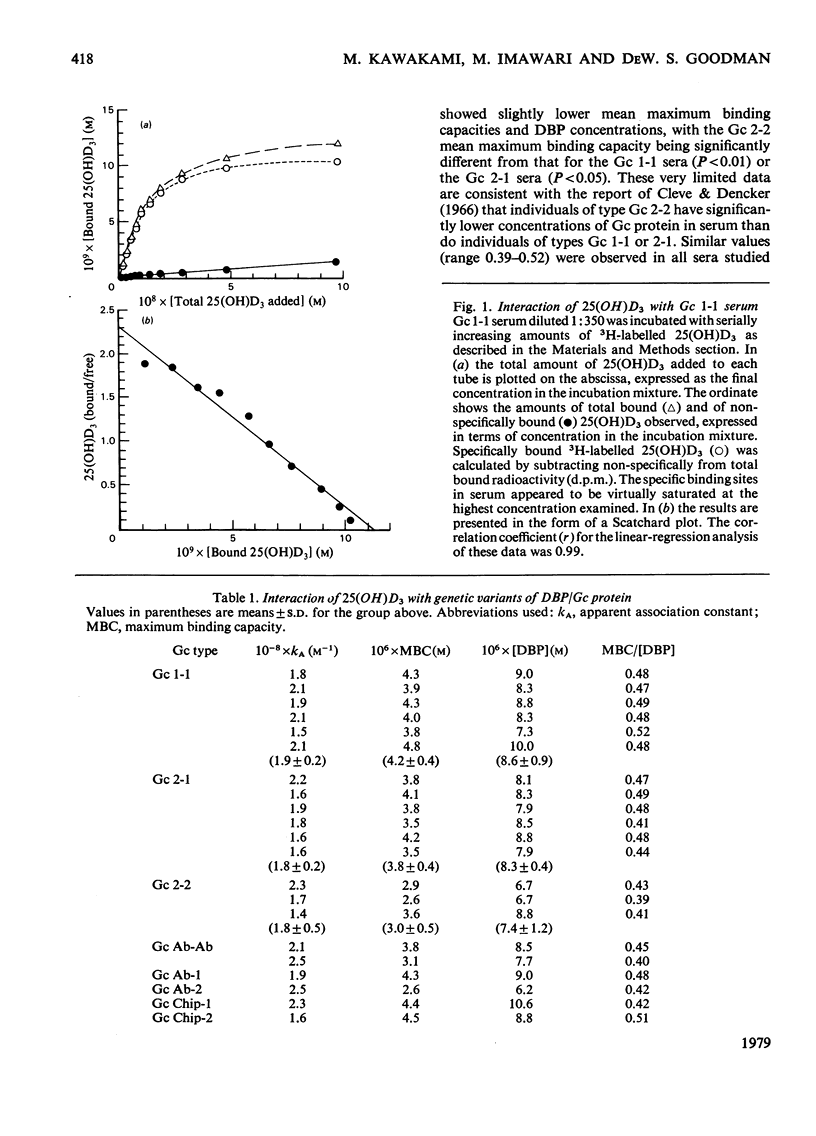
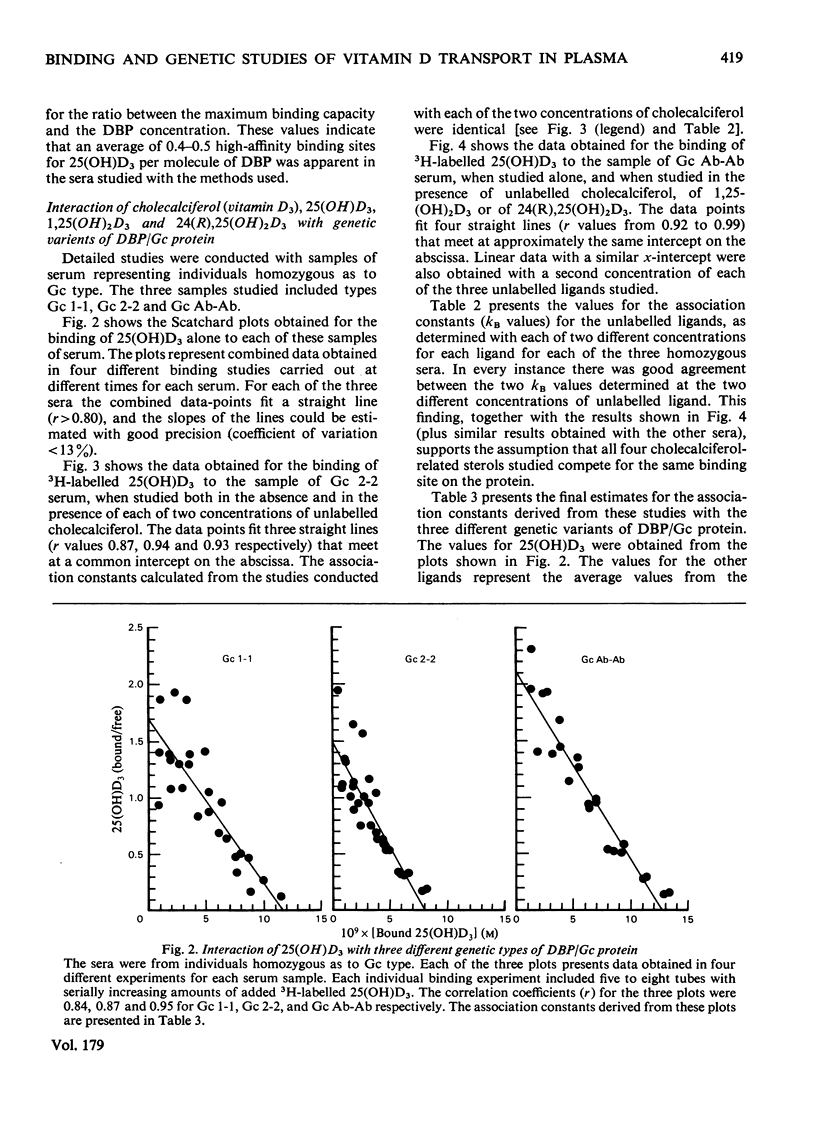
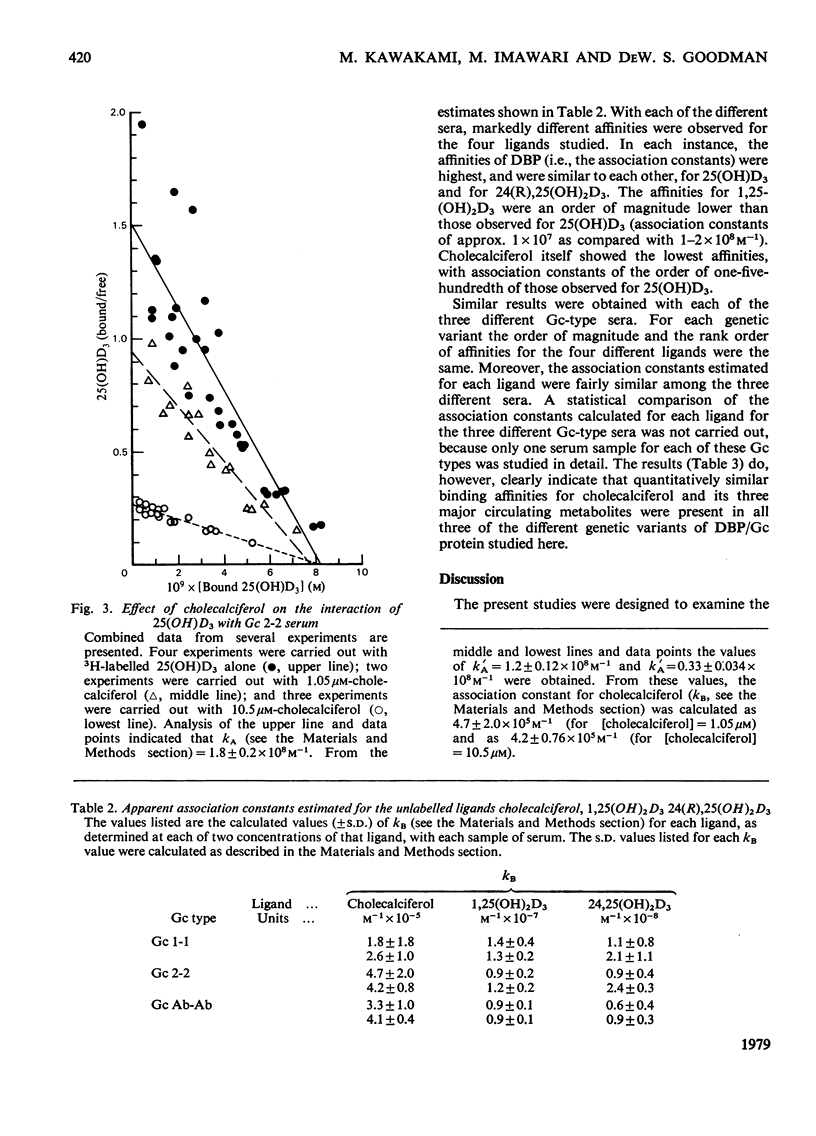
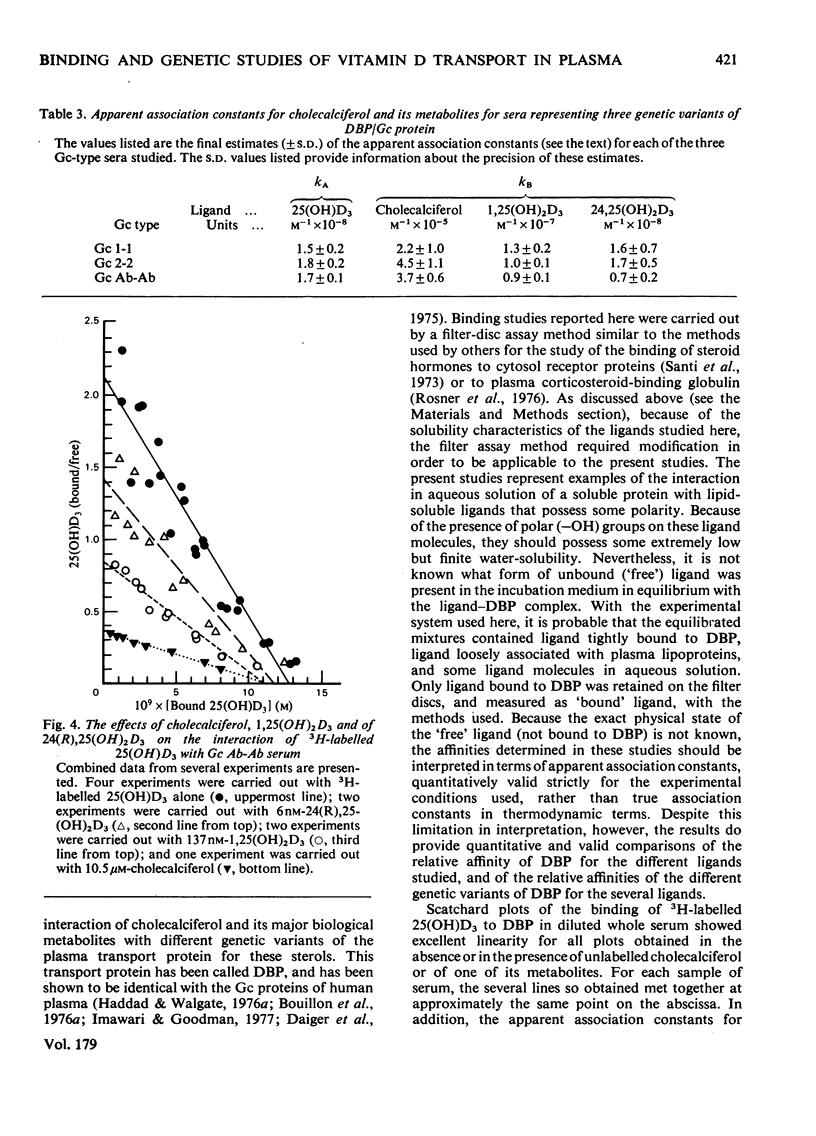
Cholecalciferol (vitamin D3) and its 25-hydroxy metabolite are transported in plasma bound to a specific protein, the binding protein for cholecalciferol and its metabolites (DBP). DBP is identical with the group-specific component (Gc) proteins, which are known to display genetic polymorphism. Studies were conducted to explore whether or not major differences in the transport of cholecalciferol and its biological metabolites might exist among persons with different Gc phenotypes. Detailed quantitative studies were first carried out on the interaction of 25(OH)D3 with DBP in 21 different samples of serum, representing eight different Gc phenotypes. The studies used a filter disc assay method that provided highly reproducible quantitative results with cholecalciferol-related sterols. The Gc phenotypes studied included the three common types (Gc 1-1, 2-1, and 2-2) and several uncommon genetic variants (Gc Ab-Ab, Ab-1, Ab-2, Chip-1, and Chip-2). The binding affinities for 25(OH)D3 observed with these different sera were all fairly similar to each other. More extensive studies were then conducted to compare the binding of four cholecalciferol-related sterols to each of three genetic variants of DBP, by using sera from homozygous persons with the Gc 1-1, Gc 2-2 and Gc Ab-Ab phenotypes. The ligands tested included cholecalciferol, 25(OH)D3, 1,25(OH)2D3, and 24(R) 25(OH)2D3. The affinities of the three genetic types of DBP/Gc protein were found to be similar for each of the four cholecalciferol-related sterols. The apparent association constants for 25(OH)D3 and 24,25(OH)2D3 were similar (approx. 1--2 x 10(8) M-1); lesser affinities were observed for 1,25(OH)2D3 (kA approx. 1 x 10(7) M-1) and for cholecalciferol (kA approx. 3--4 x 10(5) M-1). Thus the common genetic variants of DBP/Gc protein, and the uncommon genetic variants studied here, all appear to have similar binding properties for cholecalciferol and its several metabolites.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouillon R., Kerkhove P. V., De Moor P. Measurement of 25-hydroxyvitamin D3 in serum. Clin Chem. 1976 Mar;22(3):364–368. [PubMed] [Google Scholar]
- Bouillon R., Van Baelen H., Rombauts W., De Moor P. The purification and characterisation of the human-serum binding protein for the 25-hydroxycholecalciferol (transcalciferin). Identity with group-specific component. Eur J Biochem. 1976 Jul 1;66(2):285–291. doi: 10.1111/j.1432-1033.1976.tb10518.x. [DOI] [PubMed] [Google Scholar]
- Bouillon R., Van Kerkhove P., De Moor P. Characteristics of the vitamin D binding protein in different species. Calcif Tissue Res. 1976 Aug;21 (Suppl):172–176. [PubMed] [Google Scholar]
- Bouillon R., van Baelen H., de Moor P. The measurement of the vitamin D-binding protein in human serum. J Clin Endocrinol Metab. 1977 Aug;45(2):225–231. doi: 10.1210/jcem-45-2-225. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brumbaugh P. F., Haussler D. H., Bressler R., Haussler M. R. Radioreceptor assay for 1 alpha,25-dihydroxyvitamin D3. Science. 1974 Mar 15;183(4129):1089–1091. doi: 10.1126/science.183.4129.1089. [DOI] [PubMed] [Google Scholar]
- CLEVE H., KIRK R. L., PARKER W. C., BEARN A. G., SCHACHT L. E., KLEINMAN H., HORSFALL W. R. TWO GENETIC VARIANTS OF THE GROUP-SPECIFIC COMPONENT OF HUMAN SERUM: GC CHIPPEWA AND GC ABORIGINE. Am J Hum Genet. 1963 Dec;15:368–379. [PMC free article] [PubMed] [Google Scholar]
- Cleve H., Patutschnick W. The vitamin D binding of the common and rare variants of the group-specific component (Gc). An autoradiographic study. Hum Genet. 1977 Oct 14;38(3):289–296. doi: 10.1007/BF00402155. [DOI] [PubMed] [Google Scholar]
- Cleve H. The variants of the group-specific component. A review of their distribution in human populations. Isr J Med Sci. 1973 Sep-Oct;9(9):1133–1146. [PubMed] [Google Scholar]
- Daiger S. P., Cavalli-Sforza L. L. Detection of genetic variation with radioactive ligands. II. Genetic variants of vitamin D-labeled group-specific component (Gc) proteins. Am J Hum Genet. 1977 Nov;29(6):593–604. [PMC free article] [PubMed] [Google Scholar]
- Daiger S. P., Schanfield M. S., Cavalli-Sforza L. L. Group-specific component (Gc) proteins bind vitamin D and 25-hydroxyvitamin D. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2076–2080. doi: 10.1073/pnas.72.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisman J. A., Hamstra A. J., Kream B. E., DeLuca H. F. 1,25-Dihydroxyvitamin D in biological fluids: a simplified and sensitive assay. Science. 1976 Sep 10;193(4257):1021–1023. doi: 10.1126/science.1085035. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCHFELD J. The Gc-system. Immuno-electrophoretic studies of normal human sera with special reference to a new genetically determined system (Gc). Prog Allergy. 1962;6:155–186. [PubMed] [Google Scholar]
- Haddad J. G., Hillman L., Rojanasathit S. Human serum binding capacity and affinity for 25-hydroxyergocalciferol and 25-hydroxycholecalciferol. J Clin Endocrinol Metab. 1976 Jul;43(1):86–91. doi: 10.1210/jcem-43-1-86. [DOI] [PubMed] [Google Scholar]
- Haddad J. G., Jr, Walgate J. 25-Hydroxyvitamin D transport in human plasma. Isolation and partial characterization of calcifidiol-binding protein. J Biol Chem. 1976 Aug 25;251(16):4803–4809. [PubMed] [Google Scholar]
- Haddad J. G., Jr, Walgate J. Radioimmunoassay of the binding protein for vitamin D and its metabolites in human serum: concentrations in normal subjects and patients with disorders of mineral homeostasis. J Clin Invest. 1976 Nov;58(5):1217–1222. doi: 10.1172/JCI108575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad J. G., Stamp T. C. Circulating 25-hydroxyvitamin D in man. Am J Med. 1974 Jul;57(1):57–62. doi: 10.1016/0002-9343(74)90768-2. [DOI] [PubMed] [Google Scholar]
- Hillman L. S., Haddad J. G. Human perinatal vitamin D metabolism. I. 25-Hydroxyvitamin D in maternal and cord blood. J Pediatr. 1974 May;84(5):742–749. doi: 10.1016/s0022-3476(74)80024-7. [DOI] [PubMed] [Google Scholar]
- Holick M. F., DeLuca H. F. A new chromatographic system for vitamin D3 and its metabolites: resoluation of a new vitamin D3 metabolite. J Lipid Res. 1971 Jul;12(4):460–465. [PubMed] [Google Scholar]
- Imawari M., Goodman D. S. Immunological and immunoassay studies of the binding protein for vitamin D and its metabolites in human serum. J Clin Invest. 1977 Mar;59(3):432–442. doi: 10.1172/JCI108657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imawari M., Kida K., Goodman D. S. The transport of vitamin D and its 25-hydroxy metabolite in human plasma. Isolation and partial characterization of vitamin D and 25-hydroxyvitamin D binding protein. J Clin Invest. 1976 Aug;58(2):514–523. doi: 10.1172/JCI108495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. Assay of vitamins D2 and D3, and 25-hydroxyvitamins D2 and D3 in human plasma by high-performance liquid chromatography. Clin Chem. 1978 Feb;24(2):287–298. [PubMed] [Google Scholar]
- Lambert P. W., Syverson B. J., Arnaud C. D., Spelsberg T. C. Isolation and quantitation of endogenous vitamin D and its physiologically important metabolites in human plasma by high pressure liquid chromatography. J Steroid Biochem. 1977 Sep;8(9):929–937. doi: 10.1016/0022-4731(77)90189-3. [DOI] [PubMed] [Google Scholar]
- McLaughlin M., Raggatt P. R., Fairney A., Brown D. J., Lester E., Wills M. R. Seasonal variations in serum 25-hydroxycholecalciferol in healthy people. Lancet. 1974 Mar 30;1(7857):536–538. doi: 10.1016/s0140-6736(74)92717-2. [DOI] [PubMed] [Google Scholar]
- Mourant A. E., Tills D., Domaniewska-Sobczak K. Sunshine and the geographical distribution of the alleles of the Gc system of plasma proteins. Hum Genet. 1976 Aug 30;33(3):307–314. doi: 10.1007/BF00286857. [DOI] [PubMed] [Google Scholar]
- Rosner W., Beers P. C., Awan T., Khan M. S. Identification of corticosteroid-binding globulin in human milk: measurement with a filter disk assay. J Clin Endocrinol Metab. 1976 Jun;42(6):1064–1073. doi: 10.1210/jcem-42-6-1064. [DOI] [PubMed] [Google Scholar]
- Santi D. V., Sibley C. H., Perriard E. R., Tomkins G. M., Baxter J. D. A filter assay for steroid hormone receptors. Biochemistry. 1973 Jun 19;12(13):2412–2416. doi: 10.1021/bi00737a007. [DOI] [PubMed] [Google Scholar]
- Taylor C. M., Hughes S. E., de Silva P. Competitive protein binding assay for 24,25-dihydroxycholecalciferol. Biochem Biophys Res Commun. 1976 Jun 21;70(4):1243–1249. doi: 10.1016/0006-291x(76)91035-4. [DOI] [PubMed] [Google Scholar]


