Abstract
Background
Anthracycline-induced cardiotoxicity (AIC) is characterized by a disruption in myocardial metabolism.
Objectives
The authors used a large animal model to test sodium-glucose cotransporter inhibitor therapy to prevent AIC.
Methods
Female large white pigs (n = 36) were used to identify the most translational AIC regimen: 6 triweekly intravenous doxorubicin injections (1.8 mg/kg each). Another group of 32 pigs were randomized (1:1:2) to doxorubicin plus empagliflozin 20 mg, doxorubicin plus empagliflozin 10 mg, or doxorubicin control. Pigs were serially examined using multiparametric cardiac magnetic resonance and magnetic resonance spectroscopy. At the end of the 21-week follow-up period, blood samples were obtained to measure myocardial metabolic substrate extraction, and the left ventricle was harvested and processed for analysis using metabolomics, transmission electron microscopy, mitochondrial respirometry, and histopathology.
Results
Final left ventricular ejection fraction (LVEF), the prespecified primary outcome, was significantly higher in pigs receiving 20 mg empagliflozin than in the doxorubicin control group (median 57.5% [Q1-Q3: 55.5%-60.3%] vs 47.0% [Q1-Q3: 40.8%-47.8%]; P = 0.027). Final LVEF in pigs receiving 10 mg empagliflozin was 51% (Q1-Q3: 46.5%-55.5%; P = 0.020 vs 20 mg empagliflozin). The incidence of AIC events was 0%, 50%, and 72% in the empagliflozin 20 mg, empagliflozin 10 mg, and doxorubicin control groups, respectively. Empagliflozin 20 mg treatment resulted in enhanced ketone body consumption by the myocardium, preserved magnetic resonance spectroscopy–measured cardiac energetics, and improved mitochondrial structure and function on transmission electron microscopy and respirometry. These changes were more modest with the 10-mg empagliflozin dose.
Conclusions
Sodium-glucose cotransporter-2 inhibitor therapy with empagliflozin exerts a dose-dependent cardioprotective effect against AIC. The improved LVEF was accompanied by enhanced ketone body consumption, improved cardiac energetics, and preserved mitochondrial structure and function.
Key Words: anthracycline, cardiomyopathy, cardio-oncology, cardioprotection, doxorubicin cardiotoxicity, heart failure, imaging, ketosis, metabolism, myocardial energetics, pigs, sodium glucose contransporter-2 inhibitors, treatment
Central Illustration
Long-term cancer survivors have a higher risk for death of cardiovascular complications than from cancer.1 One of the main contributors to the increased cardiovascular morbidity and mortality in this vulnerable population is cardiotoxicity caused by cancer treatment.1
Anthracyclines, administered alone or in combination, remain the first-line therapy for many cancer types. Anthracyclines can cause irreversible injury to the myocardium and concerns about anthracycline-induced cardiotoxicity (AIC) limit the use of these effective anticancer drugs.1 In 5% of patients treated with anthracyclines, severe AIC occurs, progressing to chronic heart failure (HF).2 Although AIC has been known for decades, there is a lack of effective preventive therapies.3
AIC is associated with myocardial metabolic alterations, characterized by a switch in the preferred substrate for adenosine triphosphate (ATP) production from mitochondrial oxidation to anaerobic glycolysis.4 This metabolic switch is proposed to be mechanistically involved in the development of AIC.4 Sodium-glucose cotransporter-2 inhibitor (SGLT2i) therapy has been shown to improve outcomes in a wide spectrum of patients with HF, irrespective of left ventricular ejection fraction (LVEF) or glycemic status. SGLT2i therapy is not only beneficial in overt HF but also prevents HF onset in patients at high risk.5 In post–myocardial infarction HF, SGLT2i therapy has been shown to modulate myocardial substrate use by favoring the use of fatty acids and ketone bodies as the main metabolic substrates,6 suggesting potential benefit as a preventive therapy against AIC. However, robust evidence for such a benefit has been lacking.
Methods
All studies conformed to European Union directive 2010/63EU and recommendation 2007/526/EC regarding the protection of animals used for experimental and other scientific purposes and were approved by the local institutional and regional animal research committees.
Study design
Figure 1 shows the various study designs.
Figure 1.
Study Design
See text for a detailed description of study designs. AIC = anthracycline-induced cardiotoxicity; Dox = doxorubicin; Empa = empagliflozin; SGLT2i = sodium-glucose cotransporter-2 inhibitor.
Study 1: refinement of an intravenous anthracycline cardiotoxicity model
To identify an anthracycline regimen closely matching the clinical scenario with minimal side effects, we tested different doxorubicin concentrations. Pigs received 6 injections at 3-week intervals. Thirty-six pigs were divided into 5 groups: no treatment and 4 doxorubicin doses (1, 1.8, 2.3, 3 mg/kg). Cardiac magnetic resonance (CMR) imaging was used to track cardiotoxicity progression.
Study 2: preclinical trial testing the ability of empagliflozin to protect against AIC
Using the selected dose in study 1 (1.8 mg/kg), we conducted a preclinical trial testing the protective effect of empagliflozin against AIC in 32 pigs, randomized 2:1:1 to no empagliflozin (doxorubicin control), 10 mg/d, or 20 mg/d. Treatment and follow-up lasted 21 weeks, with serial CMR scans per animal assessing anatomy, function, and tissue characterization. Myocardial energetics were evaluated using 31P magnetic resonance spectroscopy (MRS). At the study’s end, myocardial metabolic substrate use was quantified from blood samples obtained from the commencement and conclusion of the coronary circulation (the aortic root and coronary sinus).6 Pigs were sacrificed, and left ventricular (LV) samples were analyzed using histopathology, transmission electron microscopy, respirometry, and metabolomics to assess mitochondrial function and energy substrates.
The weights of the pigs across the span of the study are presented in Supplemental Figure 1.
In the Supplemental Appendix, protocols for intravenous doxorubicin infusion, animal care, and humane endpoint criteria can be found. In both studies, pigs were clinically monitored and sacrificed upon meeting humane endpoint criteria.
CMR imaging
Studies were performed using a 3-T Achieva Tx whole-body scanner (Philips Healthcare) equipped with a 32-element phased-array cardiac coil. The CMR protocol included a standard segmented cine steady-state free precession sequence to provide high-quality anatomical references, a T2 gradient spin echo mapping sequence, and native and postcontrast T1 mapping and late gadolinium enhancement sequences (see the Supplemental Appendix for additional information).
Phosphorus-31 magnetic resonance spectroscopic analysis of myocardial energetics
Cardiac electrocardiographically triggered localized 31P mitral regurgitation spectroscopic data were obtained for the noninvasive assessment of human myocardial high-energy phosphate metabolism (see the Supplemental Appendix for additional information).
Definition of AIC
The incidence of AIC in experimental groups was calculated according to the definition of cardiotoxicity in the European Society of Cardiology 2022 cardio-oncology guidelines.1 Moderate AIC was thus defined as a change in CMR-measured LVEF between baseline and follow-up examinations of 10 absolute percentage points to a value from 40% to 49%, and severe AIC was defined as a change in CMR-measured LVEF between baseline and any follow-up examination of 10 absolute percentage points to a value <40%.
Ex vivo analysis
Pigs were euthanized by intravenous injection of pentobarbital (50 mg/kg), and basal, mid, and apical LV samples were collected for histologic, mitochondrial function, transmission electron microscopic, and protein expression analyses (see the Supplemental Appendix).
Myocardial metabolic substrate extraction
Gradient across the myocardium of different metabolic substrates (glucose, fatty acids, and ketone bodies) was measured in plasma samples obtained from aortic root and coronary sinus using a cardiac catheterization procedure. This was analyzed using commercial kits. Metabolic substrate gradients were normalized for perfusion measured by magnetic resonance (see the Supplemental Appendix for additional information on the cardiac catheterization procedure and metabolite quantification).
Gas chromatographic–mass spectrometric metabolomics of cardiac samples
Samples from the LV were homogenized, and metabolites were extracted in preparation for gas chromatographic–mass spectrometric data processing (see the Supplemental Appendix for additional information).
Data acquisition and statistics
Study data were collected and managed using REDCap electronic data capture tools hosted at Centro Nacional de Investigaciones Cardiovasculares Carlos III.
For study 1, sample size was calculated in line with the Animal Research: Reporting of In Vivo Experiments guidelines, resulting in 7 animals per doxorubicin concentration. For study 2, sample size was calculated to detect a 10% between-group difference in LVEF in the final CMR study (primary endpoint) with an SD of 6.5%, a predicted casualty rate of about 30% to 40%, power of 0.8, and a 2-sided significance level of 0.05 among the treated groups. The required sampled size was 8 animals per group (6 at the end of the study) on the basis of a 2-sided Student’s t-test with equal variance. The Shapiro-Wilk test was performed to test data normality. Pairwise comparisons between treatment and control groups were made using Student’s t-test after 1-way analysis of variance with Tukey correction. Comparisons over time within each group for all the CMR data were made using repeated 1-way analysis of variance with the Bonferroni post hoc test. Kaplan-Meier curves were used to compare survival. Data are presented as median (Q1-Q3) or mean ± SEM and were analyzed using RStudio version 1.4.1717 (Posit).
Results
Identification of the doxorubicin dose inducing AIC with the least systemic effects (study 1)
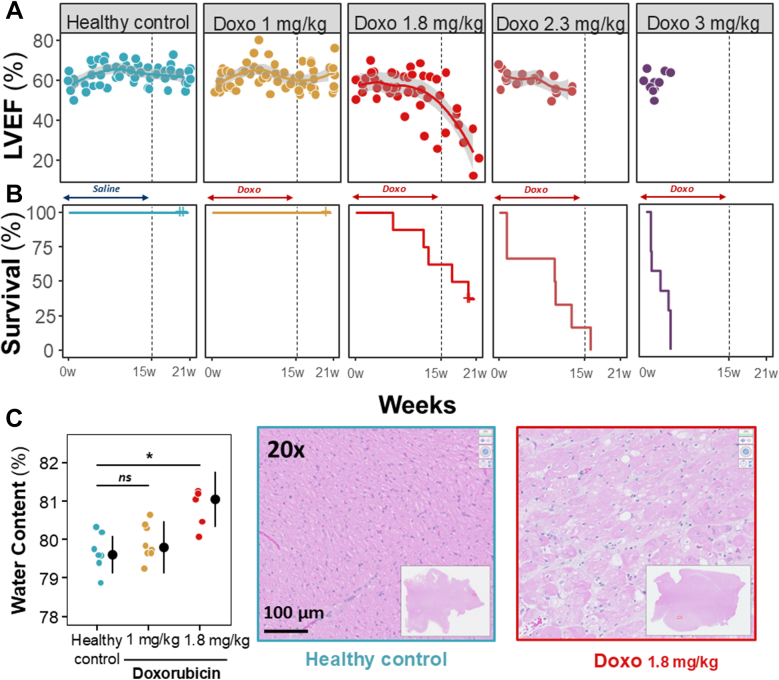
The lowest cumulative dose (1 mg/kg × 6 cycles) did not cause deaths or the humanitarian endpoint during the study period and produced no sign of AIC, with LVEF remaining unaltered throughout the follow-up (Figures 2A and 2B). A progressive increase in T2 relaxation times indicated mild myocardial injury, but this was insufficient to induce any deterioration in LV systolic function. The 2.3 and 3 mg/kg injection doses resulted in a 100% incidence of side effects meeting the prespecified humanitarian endpoint criteria, with no animals in these treatment groups completing all 6 doxorubicin cycles (Figures 2A and 2B).
Figure 2.
Results of Study 1
(A) In vivo trajectories for cardiac magnetic resonance–derived left ventricular ejection fraction (LVEF) in each doxorubicin (Dox) dose group, together with (B) Kaplan-Meier curves for humanitarian sacrifice. Discontinuous vertical lines indicate the end of Dox therapy at 15 weeks. (C) End of follow-up water content in the anterior myocardial wall. Colored dots represent individual animals. Black dots and lines indicate median (Q1-Q3). Representative hematoxylin and eosin histologic images are shown. Insets show low-magnification views. Scale bar, 100 μm. ∗P < 0.05. Data were compared using analysis of variance for parametric data, followed by Bonferroni correction (for paired comparisons) and Tukey correction (for unpaired comparisons) for multiple comparisons. For nonparametric data, the Wilcoxon rank sum test was used.
The 1.8 mg/kg injection dose induced a progressive decline in LVEF from the sixth cycle onward, resulting in a significantly lower median LVEF at the end of follow-up (59% [Q1-Q3: 55%-60%] at baseline vs 21% [Q1-Q3: 17%-29%] at the end of follow-up; P = 0.018) (Figures 2A and 2B). The 1.8 mg/kg doxorubicin regimen induced a significant and progressive prolongation in median T2 relaxation time, from 45 ms (Q1-Q3: 43-46 ms) at baseline (week 0), through 50 ms (Q1-Q3: 48-53 ms) after 3 treatment cycles (week 9) and 50 ms (Q1-Q3: 48-53 ms) after 5 cycles (week 15), to 55 ms (Q1-Q3: 53-56 ms) 6 weeks after the final doxorubicin injection (week 21) (P = 0.012 vs baseline). T2 data correlated with an increase in myocardial water content and intracardiomyocyte edema detected on histologic analysis (Figures 2C and 2D). Conversely, native T1 relaxation time and pre- and post-contrast T1-measured extracellular volume showed no significant change. Therefore, the 1.8 mg/kg doxorubicin regimen was selected as the closest mimic of clinical AIC.
Complete anatomical and functional CMR data from study 1 are shown in Supplemental Table 1.
Empagliflozin prevents AIC in a dose-dependent manner (study 2)
Treatment with empagliflozin resulted in an increase in median glycosuria, more marked with the 20-mg dose (393.5 mg/dL [Q-Q3: 81-1,536 mg/dL] vs 7 mg/dL [Q-Q3: 6.25-11 mg/dL] in the doxorubicin control group; P < 0.001). Empagliflozin 10 mg induced a milder increase in glycosuria (88 mg/dL [Q-Q3: 38.5-209.5 mg/dL]; P = 0.001 vs doxorubicin control).
Of note, empagliflozin 20 mg treatment was associated with a significant increase in median ketonemia (48.71 μmol/L [Q-Q3: 46.68-57.77μmol/L] vs 24.23 μmol/L [Q-Q3: 21.03-30.75 μmol/L] in the doxorubicin control group; P = 0.008). Although empagliflozin 10 mg increased median ketonemia (39.85 μmol/L [Q-Q3: 30.30-40.41 μmol/L]), it did so to a lesser extent than after the 20-mg dose (P = 0.004).
Humanitarian endpoint mortality was 37.5%, 25%, and 25% in the doxorubicin control, 10 mg empagliflozin, and 20 mg empagliflozin groups, respectively.
LVEF in doxorubicin control animals from study 2 was stable during the first 5 doxorubicin cycles and declined progressively thereafter (Figures 3A to 3C). Median LVEF in doxorubicin control pigs at baseline was 58% (Q1-Q3: 58%-63%), declining to 47% (Q1-Q3: 41%-48%) at study end (P = 0.009 vs baseline). Pigs allocated to 10 mg empagliflozin showed a similar but less pronounced decline in LVEF from the fifth doxorubicin cycle onward, declining from 62% (Q1-Q3: 58%-63%) at baseline to 51% (Q1-Q3: 47%-56%) at end of study (P = 0.022 vs baseline). Pigs allocated to 20 mg empagliflozin showed no significant deterioration in LVEF during the study: LVEF values at baseline and at the end of the study were 57% (Q1-Q3: 55%-62%) and 58% (Q1-Q3: 56%-60%) (P = 0.84).
Figure 3.
Cardiac Function and AIC Prevalence in the Randomized Preclinical Trial
(A) LVEF (%) from baseline to study end. Discontinuous vertical lines indicate the end of doxorubicin therapy. (B) LVEF (%) values at 21-week follow-up examination for each group. Colored dots represent individual animals, and colored shading represents the density. Box plots show median (Q1-Q3), and whiskers show 1.5 times the IQR. **P < 0.01. (C) Representative end of follow-up cine cardiac magnetic resonance (CMR) images of systole and diastole for each group. (D) End of follow-up AIC incidence in each experimental group according to the 2022 European Society of Cardiology cardio-oncology guideline definition.1 Data were compared using analysis of variance for parametric data, followed by Bonferroni correction (for paired comparisons) and Tukey correction (for unpaired comparisons) for multiple comparisons. For nonparametric data, the Wilcoxon rank sum test was used. Abbreviations as in Figures 1 and 2.
Median LVEF at the end of the 21-week follow-up period (Figure 3B), the primary study outcome, was significantly higher in pigs receiving empagliflozin 20 mg/d (58% [Q1-Q3: 56%-60%]) than in the doxorubicin control group (47% [Q1-Q3: 41%-48%]) (P = 0.003) and in pigs receiving empagliflozin 10 mg/d (51% [Q1-Q3: 47%-56%]) (P = 0.054 vs 20 mg empagliflozin). End-of-study LVEF did not differ significantly between pigs receiving 10 mg empagliflozin and the doxorubicin control group. Complete anatomical and functional CMR data for the 3 study groups are shown in Supplemental Tables 2 and 3.
The cumulative end-of-study prevalence of AIC events is presented in Figure 3D. Pigs receiving 20 mg empagliflozin had no AIC events, and pigs receiving 10 mg empagliflozin had a significantly lower cumulative and total incidence of AIC events than pigs in the doxorubicin control group. These data thus show that 20 mg empagliflozin prevented AIC and that 10 mg empagliflozin had some protective effect.
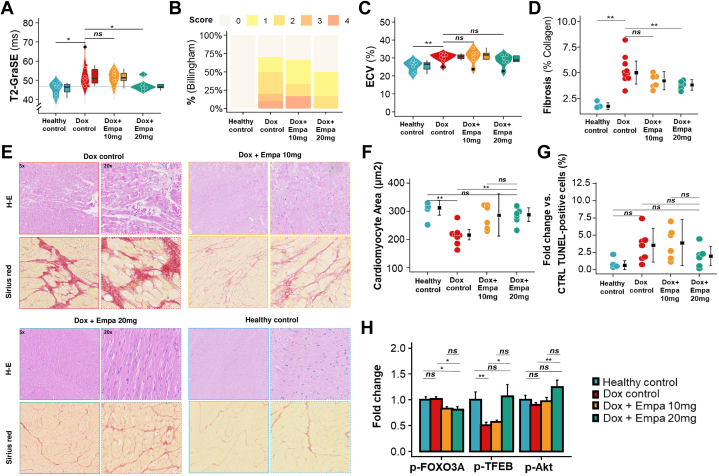
Empagliflozin attenuates AIC-induced tissue changes
Treatment with doxorubicin resulted in significantly atrophied cardiomyocytes compared with healthy controls (P = 0.008), as previously reported in mice.7 Treatment with 10 and 20 mg empagliflozin prevented atrophy observed in doxorubicin controls, with no significant differences compared with healthy controls (Figure 4F). This preventive effect against cardiac atrophy was also observed in LV mass measured by CMR, despite an overall increase in LV mass in all groups related to the pig’s increasing weight (Supplemental Tables 1-3).
Figure 4.
Myocardial Tissue Characterization
(A) Myocardium T2 values at end of follow-up. Colored dots represent individual animals, colored shading represents density, and black dots represent outliers. Box plots show median (Q1-Q3), and whiskers show 1.5 times the IQR. ∗P < 0.05. (B) Billingham score (calculated as percentage of cardiomyocyte vacuolization: 0, <0.5%; 1, 0.5%-1%; 2, 1%-3%; 3, 3%-5; and 4, >5%) at end of follow-up. (C) T1 mapping–based extracellular volume fraction (ECV). Box plots as in A. ∗∗P < 0.01. (D) Percentage collagen fractional area in the LV quantified from Sirius red–stained sections. (E) Representative histologic images of hematoxylin and eosin (H-E) staining to reveal cardiomyocyte vacuolization (top row) and Sirius red staining to detect fibrosis (bottom row). Paired images per condition show low-magnification (5×) and high-magnification (20×) views (scale bars, 100 and 50 μm). (F) Cardiomyocyte cross-sectional area (square micrometers). (G) Deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)–positive cells (%). Colored dots indicate individual animals. Black dots and lines indicate median (Q1-Q3). ∗∗P < 0.01. (H) Quantification of protein levels (fold increased) for p-FOXO3A, p-TFEB, and p-Akt. Data were compared using analysis of variance for parametric data, followed by Bonferroni correction (for paired comparisons) and Tukey correction (for unpaired comparisons) for multiple comparisons. For nonparametric data, the Wilcoxon rank sum test was used. CTRL = control; GraSE = gradient spin echo; other abbreviations as in Figure 1.
Compared with healthy controls, cardiac atrophy in doxorubicin controls was accompanied by an increase in DNA damage, indexed as the number of terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)–positive cardiac cells (P = 0.073). There was no difference in the density of TUNEL-positive cells between pigs treated with 20 mg empagliflozin and healthy controls. In contrast, pigs receiving 10 mg empagliflozin had a significant increase in DNA damage compared with healthy controls (P = 0.038) (Figure 4G). Representative images from wheat germ agglutinin and TUNEL analysis can be found in Supplemental Figure 3.
At the end of follow-up, doxorubicin control pigs displayed prolonged anteroseptal T2 values on CMR, typical of AIC and suggestive of intracellular edema in cardiomyocytes (Figure 4A). The T2 prolongation was prevented by long-term daily treatment with 20 mg empagliflozin (median: 46.6 ms [Q1-Q3: 46.1-47.8 ms] vs 51.0 ms [Q1-Q3: 48.5-55.7 ms] in doxorubicin control; P = 0.034); no effect was seen with the lower 10 mg/d dose (Figure 4A). Cardiomyocyte edema was confirmed by ex vivo histopathologic analysis of LV samples obtained after the sacrifice of animals at the end of the study, which revealed evident vacuolization and a corresponding increase in the Billingham cardiomyocyte vacuolization score in doxorubicin controls (Figure 4B). This effect was attenuated in the 20 mg empagliflozin group but not in the 10 mg empagliflozin group.
Western blot analysis revealed significant changes in the p-FOXO3A, AKT, and TFEB pathways in pigs receiving doxorubicin. Treatment with empagliflozin (10 and 20 mg) decreased FOXO3A phosphorylation. Empagliflozin 20 mg specifically enhanced AKT and TFEB phosphorylation compared with controls (Figure 4H, Supplemental Figure 5). In the final CMR examination (week 21), median T1-measured extracellular volume was increased in doxorubicin control pigs from 26% (Q1-Q3: 24%-27%) at baseline to 31% (Q1-Q3: 30%-32%) at week 21. Daily treatment with 20 mg empagliflozin ameliorated this CMR-measured extracellular expansion, but the effect was not statistically significant (Figure 4C). Conversely, ex vivo histologic analysis by Sirius red staining revealed a significantly lower extent of fibrosis in the 20 mg empagliflozin group (5.28% ± 1.77% in doxorubicin controls vs 3.79% ± 0.754% in the 20 mg empagliflozin group; P = 0.005), indicating a reduction in anthracycline-induced cardiac remodeling in pigs receiving the higher SGLT2i dose (Figures 4D and 4E).
Empagliflozin preserves myocardial energetics upon cumulative anthracycline exposure
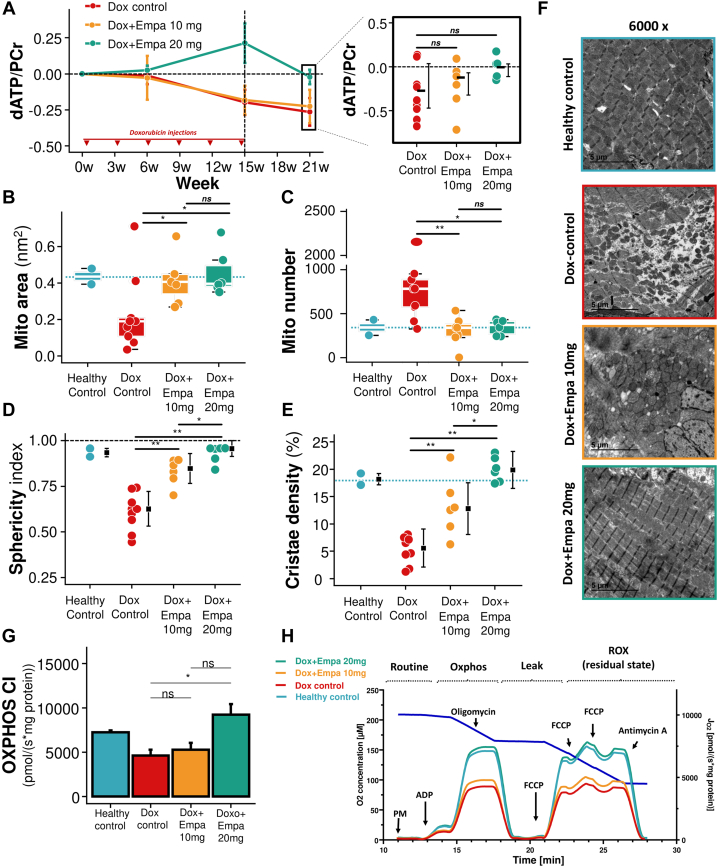
Myocardial energetics were measured in vivo using 31P MRS in each imaging session (Figure 5A). Doxorubicin control pigs showed a progressive deterioration in myocardial energetics with increasing cumulative doxorubicin exposure, with a median difference in the ATP/phosphocreatine (PCr) ratio between baseline and final follow-up (−0.306 [Q1-Q3: −0.469 to 0.035]). Pigs receiving 10 mg empagliflozin showed a milder deterioration in myocardial energetics, with the baseline and end of follow-up median ATP/PCr ratios differing by −0.157 (Q1-Q3: −0.322 to −0.068). Conversely, myocardial energetics in pigs receiving 20 mg empagliflozin did not deteriorate during the study period, with a median difference in ATP/PCr ratio between baseline and the end of follow-up of just −0.037 (Q1-Q3: −0.112 to 0.035).
Figure 5.
Mitochondrial and Metabolic Evaluation
(A) dATP/phosphocreatine (PCr) area over the study for the 3 groups and at the end of the study. Mitochondrial (Mito) area (B), number (C), sphericity index (D), and cristae density (E) in transmission electron microscopic (TEM) images. (F) Representative ultrastructural TEM images for each group. (G) Complex I oxidative phosphorylation (OXPHOS CI) respiration capacity. (H) Oroboros representative curves. Tukey box plot and individual colored plots indicated as previous figures. Black square and error bars represent median (Q1-Q3), colored bars represent mean and SE from (G). ∗P < 0.05 and ∗∗P < 0.01. Data were compared using analysis of variance for parametric data, followed by Bonferroni correction (for paired comparisons) and Tukey correction (for unpaired comparisons) for multiple comparisons. For nonparametric data, the Wilcoxon rank sum test was used. ADP = adenosine diphosphate; dATP = deoxyadenosine triphosphate; FCCP = carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone; PM = pyruvate, malate; ROX = respiratory rate oxygenation index; other abbreviations as in Figure 1.
Empagliflozin modified AIC-induced myocardial metabolic substrate use
The myocardial extraction rate for free fatty acids and glucose did not change between the doxorubicin control and empagliflozin groups (Figures 6A and 6B, top panels). However, the myocardial extraction rate of ketone bodies was significantly increased in pigs treated with empagliflozin 20 mg (P = 0.042 vs doxorubicin control) (Figure 6C, top panel). The myocardial extraction rate of ketone bodies in pigs treated with empagliflozin 10 mg did not differ from that in doxorubicin control animals. In healthy animals (not exposed to doxorubicin), 1-week treatment with empagliflozin 20 mg did not have any effect on myocardial extraction of metabolic substrates nor in mitochondrial respiration (Supplemental Figure 5).
Figure 6.
Metabolic Substrate Extraction of Myocardium and Tissue Metabolomics
Metabolic substrate gradients between plasma from aorta and coronary sinus corrected by CMR quantitative perfusion for (A) triglycerides, (B) glucose, and (C) ketone bodies. Tukey box plots represent median (Q1-Q3) (box and lines), the remainder of the data distribution (whiskers), and outliers (individual dots). Myocardial tissue metabolomic quantification for (D) fatty acids, (E) glucose and (F) ketone bodies. Bars represent means and SE, and colored dots represent individual data per group. Data were compared using Student’s t-test for parametric data and the Wilcoxon rank sum test for nonparametric data. GCMS = gas chromatography–mass spectrometry; other abbreviations as in Figure 1.
Metabolomic evaluation of LV tissue samples revealed no differences in free fatty acid or glucose levels between groups (Figures 6D and 6E, bottom panels). However, the myocardial tissue concentration of ketone bodies was significantly lower in pigs treated with empagliflozin 20 mg compared with doxorubicin controls (P = 0.016) and pigs treated with empagliflozin 10 mg (P = 0.18 vs empagliflozin 20 mg) (Figure 6F, bottom panel).
Altogether, these data suggest a heavy myocardial ketone body use for energy production in pigs treated with empagliflozin 20 mg.
Empagliflozin prevents mitochondrial structural and functional alterations associated with AIC
Mitochondrial structure
Representative transmission electron microscopic images obtained at the end of follow-up are shown in Figure 5F. Cardiomyocyte mitochondria in doxorubicin control LV samples had an overt fragmentation phenotype, with a median mitochondrial area of 0.172 mm2 (Q1-Q3: 0.066-0.345 mm2) vs 0.384 mm2 (Q1-Q3: 0.225-0.55 mm2) in healthy control pigs (P = 0.041) and a mean ± SEM mitochondria number of 830 ± 539 vs 342 ± 125 (P = 0.04) (Figures 5B and 5C). The mean ± SEM physiological spherical shape of cardiomyocyte mitochondria was significantly altered in doxorubicin control pigs (sphericity index 0.61 ± 0.1 vs 0.93 ± 0.03 in healthy controls; P = 0.000) (Figure 5D). In addition, the mean ± SEM density of mitochondrial cristae (a marker of respiratory efficiency) was significantly reduced the doxorubicin control pigs (5.19% ± 2.6% vs 18.2% ± 1.45% in healthy control pigs; P = 0.003) (Figure 5E).
The structural changes induced by doxorubicin were prevented by daily treatment with 20 mg empagliflozin, and the cardiomyocyte mitochondrial phenotype of pigs in this group closely resembled that of healthy control animals. Median mitochondrial area in the 20 mg empagliflozin group was significantly larger than that in doxorubicin control pigs (0.396 mm2 [Q1-Q3: 0.381-0.491 mm2] vs 0.191 mm2 [Q1-Q3: 0.108-0.209 mm2]; P = 0.050), and the median mitochondria number was correspondingly lower (372 [Q1-Q3: 240-432] vs 783 [Q1-Q3: 326-2,150]; P = 0.012). The mean ± SEM mitochondrial sphericity index in pigs receiving 20 mg empagliflozin was 0.93 ± 0.03, compared with 0.61 ± 0.1 in doxorubicin control pigs (P = 0.000), and mitochondrial cristae density was significantly higher (20% ± 2.26% vs 5.19% ± 2.6%; P = 0.000) (Figures 5B to 5E).
Mitochondrial function
Isolated mitochondria were studied using a high-resolution respirometer. Analysis of mitochondria isolated from doxorubicin control pigs revealed that the 6 cycles of doxorubicin caused a decrease in mitochondrial oxygen consumption in the complex I oxidative phosphorylation state compared with healthy controls (median 4,200 pmol · s−1 · mg−1 [Q1-Q3: 3,130-5,760 pmol · s−1 · mg−1] vs 7,370 pmol · s−1 · mg−1 [Q1-Q3: 6,920-7,540 pmol · s−1 · mg−1]; P = 0.060) (Figure 5G). Similarly, doxorubicin control pigs showed a decrease in mitochondrial oxygen consumption in the complex I electron transport chain state vs healthy controls (median 4,430 pmol · s−1 · mg−1 [Q1-Q3: 3,780-6,900 pmol · s−1 · mg−1] vs 7,810 pmol · s−1 · mg−1 [Q1-Q3: 7,270-8,450 pmol · s−1 · mg−1]; P = 0.060) (Figure 5H).
Median oxygen consumption was significantly better in pigs receiving 20 mg empagliflozin than in doxorubicin control pigs: complex I oxidative phosphorylation: 7,400 pmol · s−1 · mg−1 (Q1-Q3: 6,620-10,200 pmol · s−1 · mg−1) vs 4,200 pmol · s−1 · mg−1 (Q1-Q3: 3,130-5,760 pmol · s−1 · mg−1) (P = 0.042); complex I electron transport chain: 7,940 pmol · s−1 · mg−1 (Q1-Q3: 4,830, 11,000 pmol · s−1 · mg−1) vs 4,430 pmol · s−1 · mg−1 (Q1-Q3: 3,780, 6,900 pmol · s−1 · mg−1) (P = 0.11) (Figures 5G and 5H). All other mitochondrial function parameters are summarized in Supplemental Figure 2.
Overall, these data show that 20 mg empagliflozin preserves mitochondrial structure and function.
Discussion
In this study, we tested 2 empagliflozin doses as a preventive therapy in a large animal model of AIC. Exposure of 2-month-old sows to 6 cycles of intravenous 1.8 mg/kg doxorubicin at 3-week intervals resulted in a progressive decline in LVEF, a deterioration in cardiac energetics, altered myocardial metabolic substrate use, and severe structural and functional mitochondrial changes. These changes were mitigated by daily treatment with 20 mg empagliflozin during and after the doxorubicin treatment, and this effect was associated with a significant reduction in the incidence of AIC events. The 10 mg/d empagliflozin regimen resulted in milder cardioprotection. Improved myocardial energetics by empagliflozin 20 mg were observed, along with a significant increase in the cardiac use of ketone bodies as a fuel for ATP production. These findings establish high-dose empagliflozin as a promising cardioprotective intervention for testing in patients receiving anthracyclines and with a high risk for cardiotoxicity (Central Illustration).
Central Illustration.
SGLT2i Therapy Prevents Anthracycline-Induced Cardiotoxicity in a Dose-Dependent Manner in a Large Animal Model
In a pig model of anthracycline cardiotoxicity, empagliflozin exerted a dose-dependent cardioprotective effect. Empagliflozin 20 mg/d was associated with preserved systolic cardiac function, improved cardiac energetics, heavy ketone body consumption, and preserved mitochondrial structure and function. Empagliflozin 10 mg exerted a much milder cardioprotective effect. SGLT2i = sodium-glucose cotransporter-2 inhibitor.
Rodent models for AIC often fail to replicate human physiology, and drugs effective in these models may not succeed clinically.8 Large animals, such as pigs, offer a closer approximation to human cardiac responses, showing structural and functional changes similar to those observed in patients. These models also allow the use of clinical imaging tools such as CMR to detect early cardiac injury.8 The established intracoronary doxorubicin route in pigs is aggressive and does not fully mimic the clinical scenario.9,10 In this study, we developed a pig model of AIC, testing various doxorubicin doses to induce AIC while minimizing mortality and systemic side effects.
Intense efforts directed at identifying therapeutic strategies to prevent AIC have produced inconsistent results.11 Most interventions so far have tested nonspecific HF agents such as beta-blockers, angiotensin-converting enzyme inhibitors, mineralocorticoid receptor antagonists, and statins.12
Few studies have tested therapies targeting specific mechanisms involved in AIC. Here, focusing on the involvement of cardiac energetic imbalance in AIC,4 we tested the potential of the SGLT2i empagliflozin, a drug with known effects on cardiac energetics. Several large clinical trials have demonstrated positive cardiovascular effects of SGLT2i therapy in patients with HF, both with diabetes and without diabetes. Large animal models of myocardial infarction and post–myocardial infarction HF provide evidence that empagliflozin induces a shift in myocardial fuel use from glucose to ketone bodies and fatty acids, associated with low-grade ketonemia.6 In our study, we observed that treatment with empagliflozin 20 mg resulted in a significant increase in the myocardial extraction rate of ketone bodies. This was observed along with a significant reduction in the concentration of ketone bodies in the myocardium (as evaluated by metabolomics). Altogether, these data suggest a very high cardiac use of ketone bodies for energy production. In other experimental models of HF (not in the AIC context), SGLT2i therapy has not always been associated with enhanced ketone body consumption by the myocardium.13 As AIC, as opposed to other forms of HF, is mainly secondary to a cardiomyocyte metabolic disruption, SGLT2i therapy might be specifically suited for this specific cardiac condition.
Observational studies have shown that patients with cancer and diabetes receiving anthracycline plus SGLT2i therapy have lower rates of cardiac events and hospitalizations for HF.14, 15, 16 Additionally, a case-control study and retrospective cohort analysis showed that treatment with empagliflozin is associated with a reduced incidence of cancer therapy–related cardiac dysfunction in patients with diabetes.17,18 A rodent and cell culture study revealed that the cardioprotective effects of SGLT2i therapy depend on elevated serum ketone bodies.19
Given the mechanistic plausibility of AIC prevention with SGLT2i therapy, we developed the first randomized preclinical trial evaluating SGLT2i effectiveness in preventing AIC. The EMPACT (Empagliflozin in the Prevention of Cardiotoxicity in Cancer Patients Undergoing Chemotherapy Based on Anthracyclines; NCT05271162) trial will randomize 220 patients with cancer to 10 mg empagliflozin or placebo. The primary endpoint is LV systolic dysfunction, assessed using echocardiography and CMR. Our findings suggest that a higher empagliflozin dose might offer better cardioprotection if replicated in humans.
Echocardiography is the primary imaging tool recommended for the monitoring of cardiotoxicity,1 but the high spatial and temporal resolution of CMR make it the gold-standard method for the assessment of myocardial function.20 We used CMR to evaluate our primary endpoint, LVEF at the end of follow-up. Serial CMR assessments revealed that daily treatment with 20 mg empagliflozin preserves LV systolic function and prevents cardiotoxicity as defined by current European Society of Cardiology cardio-oncology guidelines (a decline of ≥10% or a decline of ≥5% to an LVEF of <50%).1 In a previous study from our group, we observed that T2 relaxation times was the first parameter altered in pigs undergoing AIC induction.9 In the present study, we also found that T2 relaxation times in pigs receiving the 25 mg/m2 dose regimen also displayed prolongation of T2 relaxation times after 3 doses of doxorubicin (from 45 to 50 ms in study 1) (Supplemental Table 1), a time when LVEF was unaffected. At the end of follow-up, in the former study, T2 relaxation times were remarkably elongated (73 ms),9 as opposed to the present study (55 ms). However, it should be noted that the AIC induction model was very different in each study: in the former, AIC was induced by 5 biweekly intracoronary doxorubicin injections (0.45 mg/kg each),9 and in the present study, doxorubicin was infused following a regimen more closely resembling the clinical scenario. Currently, T2 as an early marker of AIC in humans is being tested in a prospective clinical trial.21
Our study also provides evidence of cardioprotection with empagliflozin by mitigating cardiac atrophy, DNA damage, and cell death, which have been previously shown to precede systolic dysfunction in patients with AIC.7 Among other markers, we observed that empagliflozin 20 mg was associated with increased phosphorylation of AKT, which may be involved in the prevention of doxorubicin-induced cell death signaling, and phosphorylation of TFEB, a positive regulator of autophagy, by inactivating TFEB, which is known to exacerbate doxorubicin cardiotoxicity.22, 23, 24
To gain further insight into the underlying cardioprotective mechanism, we used 31P MRS and high-resolution respirometry to assess cardiac energetics and mitochondrial function, respectively. Phosphorus-31 MRS is the only noninvasive technique for quantifying cardiac metabolism. Our data suggest that 20 mg empagliflozin provides cardioprotection in AIC by improving cardiac energetics, likely through increased ketone body use, which enhances ATP generation and preserves mitochondrial function. In this regard, ketone bodies have been shown not only to be a more efficient fuel for generating ATP by the myocardium but also to preserve mitochondrial function and structure.25
Study limitations
The pig model of AIC used is highly translational; nevertheless, it is important to note that the adopted doxorubicin dose regimen in our model caused >50% mortality, which is not present in clinics. Another limitation is the lack of a tumor-bearing model, precluding us from studying the impact of empagliflozin on the antitumor activity of doxorubicin. There is some evidence, however, that suggests potential protective effects of SGLT2i therapy against cancer.26 Another way in which the model does not represent the cancer population is that the pigs were healthy juveniles. Although it has been shown that body mass index does not have a clinically meaningful effect on the pharmacokinetics of empagliflozin, we cannot completely rule out that the weight increase of pigs across the study influenced the drug effect.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Observational data suggest that SGLT2i therapy is associated with lower incidence of HF in patients with cancer and diabetes receiving anthracyclines; however, there is a lack of evidence from prospective randomized studies. Our preclinical trial results from a translatable large animal model provide important evidence that sodium-glucose cotransporter-2 inhibition with high-dose empagliflozin prevents AIC by preserving LV systolic function and mitochondrial structural integrity, function, and dynamics. The functional benefits are associated with an increased consumption of ketone bodies by the myocardium resulting in improved cardiac energetics.
TRANSLATIONAL OUTLOOK: The present study provides a strong basis for clinical trials testing SGLT2i therapy in patients at risk for AIC.
Funding Support and Author Disclosures
This work was supported by the European Commission (ERC-CoG 819775 to Dr Ibáñez, and ERC-CoG 725091 to Dr Sancho), Spanish Ministry of Science, Innovation and Universities (PID2022-140176OB-I00 to Dr Ibáñez, and PID2022-137712OB-I00 to Dr Sancho funded by MICIU/AEI/10.13039/501100011033 and by ERDF/EU), and the Red Madrileña de Nanomedicina en Imagen Molecular -Comunidad de Madrid (S2022/BMD-7403- RENIM-CM). Medina-Hernandez (ID LCF/BQ/DI22/11940004), and Dr Skoza (ID LCF/BQ/DI23/11990056) are Doctoral INPhINIT Fellows from la Caixa Foundation (ID 100010434). The CNIC is supported by the Instituto de Salud Carlos III (ISCIII), the Ministerio de Ciencia, Innovación y Universidades, and the Pro CNIC Foundation and is a Severo Ochoa Center of Excellence (grant CEX2020-001041-S funded by MICIN/AEI/10.13039/501100011033). Dr Sánchez-González is an employee of Philips. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
Simon Bartlett (Centro Nacional de Investigaciones Cardiovasculares Carlos III) provided English editing. The authors thank Lucía López-Palomar for her technical support and Santiago Rodriguez-Colilla and the comparative medicine staff for their help with animal care.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental methods, supplemental tables, supplemental figures, and supplemental references, please see the online version of this paper.
Contributor Information
Carlos Galán-Arriola, Email: carlos.galan@cnic.es.
Borja Ibáñez, Email: bibanez@cnic.es.
Appendix
References
- 1.Lyon A.R., Lopez-Fernandez T., Couch L.S., et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) Eur Heart J. 2022;43:4229–4361. doi: 10.1093/eurheartj/ehac244. [DOI] [PubMed] [Google Scholar]
- 2.Ibanez B., Moreno-Arciniegas A. The quest for an early marker of anthracycline-induced cardiotoxicity. JACC Basic Transl Sci. 2022;7:11–13. doi: 10.1016/j.jacbts.2021.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ibanez B., Gomes-Silva M. Remote ischemic conditioning for anthracycline cardiotoxicity: the need to protect the most vulnerable. JACC CardioOncol. 2023;5:356–359. doi: 10.1016/j.jaccao.2023.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tokarska-Schlattner M., Wallimann T., Schlattner U. Alterations in myocardial energy metabolism induced by the anti-cancer drug doxorubicin. C R Biol. 2006;329:657–668. doi: 10.1016/j.crvi.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Packer M. Six lessons learned from the use of SGLT2 inhibitors in patients with heart failure. Nat Rev Cardiol. 2022;19:499–500. doi: 10.1038/s41569-022-00736-3. [DOI] [PubMed] [Google Scholar]
- 6.Santos-Gallego C.G., Requena-Ibanez J.A., San Antonio R., et al. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol. 2019;73:1931–1944. doi: 10.1016/j.jacc.2019.01.056. [DOI] [PubMed] [Google Scholar]
- 7.Diaz-Guerra A., Villena-Gutierrez R., Clemente-Moragon A., et al. Anthracycline cardiotoxicity induces progressive changes in myocardial metabolism and mitochondrial quality control: novel therapeutic target. JACC CardioOncol. 2024;6:217–232. doi: 10.1016/j.jaccao.2024.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asnani A., Moslehi J.J., Adhikari B.B., et al. Preclinical models of cancer therapy-associated cardiovascular toxicity: a scientific statement from the American Heart Association. Circ Res. 2021;129:e21–e34. doi: 10.1161/RES.0000000000000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galan-Arriola C., Lobo M., Vilchez-Tschischke J.P., Lopez G.J., et al. Serial magnetic resonance imaging to identify early stages of anthracycline-induced cardiotoxicity. J Am Coll Cardiol. 2019;73:779–791. doi: 10.1016/j.jacc.2018.11.046. [DOI] [PubMed] [Google Scholar]
- 10.Galan-Arriola C., Villena-Gutierrez R., Higuero-Verdejo M.I., et al. Remote ischaemic preconditioning ameliorates anthracycline-induced cardiotoxicity and preserves mitochondrial integrity. Cardiovasc Res. 2021;117:1132–1143. doi: 10.1093/cvr/cvaa181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heusch G., Andreadou I., Bell R., et al. Health position paper and redox perspectives on reactive oxygen species as signals and targets of cardioprotection. Redox Biol. 2023;67 doi: 10.1016/j.redox.2023.102894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreno-Arciniegas A, Cadiz L, Galan-Arriola C, Clemente-Moragon A, Ibanez B. Cardioprotection strategies for anthracycline cardiotoxicity. Basic Res Cardiol.https://doi.org/10.1007/s00395-024-01078-6. [DOI] [PMC free article] [PubMed]
- 13.Packer M. Critical reanalysis of the mechanisms underlying the cardiorenal benefits of SGLT2 inhibitors and reaffirmation of the nutrient deprivation signaling/autophagy hypothesis. Circulation. 2022;146:1383–1405. doi: 10.1161/CIRCULATIONAHA.122.061732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avula V., Sharma G., Kosiborod M.N., et al. SGLT2 inhibitor use and risk of clinical events in patients with cancer therapy-related cardiac dysfunction. JACC Heart Fail. 2024;12:67–78. doi: 10.1016/j.jchf.2023.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Abdel-Qadir H., Carrasco R., Austin P.C., et al. The association of sodium-glucose cotransporter 2 inhibitors with cardiovascular outcomes in anthracycline-treated patients with cancer. JACC CardioOncol. 2023;5:318–328. doi: 10.1016/j.jaccao.2023.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gongora C.A., Drobni Z.D., Quinaglia Araujo Costa Silva T., et al. Sodium-glucose co-transporter-2 inhibitors and cardiac outcomes among patients treated with anthracyclines. JACC Heart Fail. 2022;10:559–567. doi: 10.1016/j.jchf.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatti AW, Patel R, Dani SS, al. e. SGLT2i and primary prevention of cancer therapy–related cardiac dysfunction in patients with diabetes. JACC CardioOncol.https://doi.org/10.1016/j.jaccao.2024.08.001. [DOI] [PMC free article] [PubMed]
- 18.Daniele A.J., Gregorietti V., Costa D., Lopez-Fernandez T. Use of Empagliflozin in the Prevention of Cardiotoxicity: the EMPACARD - PILOT trial. Cardiooncology. 2024;10:58. doi: 10.1186/s40959-024-00260-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh C.M., Cho S., Jang J.Y., Kim H., Chun S., Choi M., Park S., Ko Y.G. Cardioprotective potential of an SGLT2 inhibitor against doxorubicin-induced heart failure. Korean Circ J. 2019;49:1183–1195. doi: 10.4070/kcj.2019.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salerno M., Sharif B., Arheden H., et al. Recent advances in cardiovascular magnetic resonance: techniques and applications. Circ Cardiovasc Imaging. 2017;10 doi: 10.1161/CIRCIMAGING.116.003951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreno-Arciniegas A., Lopez A., Kelm M., et al. Rationale and design of RESILIENCE: a prospective randomized clinical trial evaluating remote ischaemic conditioning for the prevention of anthracycline cardiotoxicity. Eur J Heart Fail. 2024;26(10):2213–2222. doi: 10.1002/ejhf.3395. [DOI] [PubMed] [Google Scholar]
- 22.Ozcan M., Guo Z., Valenzuela Ripoll C., et al. Sustained alternate-day fasting potentiates doxorubicin cardiotoxicity. Cell Metab. 2023;35(6):928–942.e4. doi: 10.1016/j.cmet.2023.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo Z., Valenzuela Ripoll C., Picataggi A., et al. Apolipoprotein M attenuates anthracycline cardiotoxicity and lysosomal injury. JACC Basic Transl Sci. 2023;8(3):340–355. doi: 10.1016/j.jacbts.2022.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans S., Ma X., Wang X., et al. Targeting the autophagy-lysosome pathway in a pathophysiologically relevant murine model of reversible heart failure. JACC Basic Transl Sci. 2022;7(12):1214–1228. doi: 10.1016/j.jacbts.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolb H., Kempf K., Rohling M., Lenzen-Schulte M., Schloot N.C., Martin S. Ketone bodies: from enemy to friend and guardian angel. BMC Med. 2021;19:313. doi: 10.1186/s12916-021-02185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lau K.T.K., Ng L., Wong J.W.H., et al. Repurposing sodium-glucose co-transporter 2 inhibitors (SGLT2i) for cancer treatment—a review. Rev Endocr Metab Disord. 2021;22:1121–1136. doi: 10.1007/s11154-021-09675-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.