Summary
Inflammation is critical for obesity and obesity-induced insulin resistance (IR). In this study, we reveal the function and mechanism of acarbose on adipose tissue macrophage (ATM)-mediated inflammation in obesity and obesity-induced IR. First, acarbose enhances the abundance of propionic acid-producing Parasutterella, therefore indirectly inhibiting the survival and proinflammatory function of M1-like ATMs via GPR43. Most interestingly, acarbose can directly inhibit M1-like ATM-mediated inflammation through GPR120. Diet-induced obese mice exhibit nitrobenzoxadiazoles (NBD) fluorescence-labeled ATMs, but lean mice that also orally received NBD fluorescence-labeled acarbose do not exhibit NBD fluorescence-labeled ATMs. This direct inhibition of macrophages by acarbose is validated in mouse and human macrophages in vitro. In conclusion, our study reveals that acarbose directly and indirectly inhibits proinflammatory macrophage phenotype, which contributes to the improvement of obesity and obesity-induced IR. The understanding of the immune regulatory effects of acarbose may extend its potential for further therapeutic applications.
Keywords: obesity, acarbose, Parasutterella excrementihominis, propionic acid, adipose tissue macrophages
Graphical abstract

Highlights
-
•
Acarbose increases propionic acid-producing gut Parasutterella in obese mice
-
•
Propionic acid inhibits M1-like adipose tissue macrophage via GPR43
-
•
Parasutterella administration reduces obesity and adipose inflammation in mice
-
•
Acarbose directly inhibits M1-like macrophage proinflammation through GPR120
Li et al. identify that acarbose enhances the abundance of propionic acid-producing Parasutterella, therefore indirectly inhibiting M1-like adipose tissue macrophages (ATMs) via GPR43 in diet-induced obese mice. Meanwhile, acarbose directly inhibits M1-like ATM proinflammation through GPR120, thus leading to a reduction in adipose tissue inflammation overall.
Introduction
Obesity is the most prevalent chronic disease worldwide and is caused by excessive accumulation or abnormal distribution of body fat.1 Obesity and its complications, including insulin resistance (IR), type 2 diabetes, cardiovascular diseases, and fatty liver disease, impose a considerable economic burden and constitute major contributors to a decline in both quality of life and life expectancy.2,3
Increasing evidence has shown that chronic low-grade inflammation in adipose tissues is critical for the progression of obesity-induced IR.4,5 Macrophages comprise a majority of immune cells in adipose tissue and regulate both tissue homeostasis in the lean state and metabolic dysregulation in obesity.6 Macrophages can polarize to different phenotypes according to microenvironment variation and regulate inflammatory responses by releasing inflammatory cytokines and other mediators. The quantity of adipose tissue macrophages (ATMs) increases in obesity and they participate in inflammatory pathways that are activated in individuals with obesity.7 Diet-induced obesity leads to a shift in the activation state of ATMs from an M2-polarized state (anti-inflammatory macrophage phenotype) in lean animals that may protect adipocytes from inflammation to an M1-polarized state (proinflammatory macrophage phenotype) that contributes to IR.8
Gut microbiota is also an important factor in various metabolic syndromes, such as obesity and metabolic dysfunction-associated steatotic liver disease, and can regulate the immune-inflammatory response through multiple biological axes. During metabolic disorder process, the intestinal homeostasis is destroyed, presenting as alterations in the composition and proportion of gut microbiota and its metabolites.9 Furthermore, a large number of inflammatory factors and chemokines are released into the blood through the damaged gut barrier, aggravating systemic chronic inflammation response and the process of metabolic diseases.10
Acarbose (ACA) is an alpha-glucosidase inhibitor that has been used to alleviate obesity-induced IR and type 2 diabetes11 with additional cardiovascular benefits12 and weight-loss effects.13,14 The therapeutic effects of ACA include lowering postprandial blood glucose and insulin levels15 and positively regulating the gut microbiota by increasing the relative abundances of Lactobacillus and Bifidobacterium.16 A recent study indicated that oral administration of ACA can attenuate the severity of psoriasis with local and systemic anti-inflammatory and immune modulation effects.17 In this study, we aim to determine whether ACA has immune-regulating effects in the development and progression of obesity and obesity-induced IR. We hypothesize that ACA could modulate gut microbiota and its metabolites, thereby influencing the immune microenvironment in obese mice and ultimately suppressing obesity and IR.
Results
ACA ameliorated high-fat diet-induced obesity and rebalanced macrophage homeostasis in adipose tissue
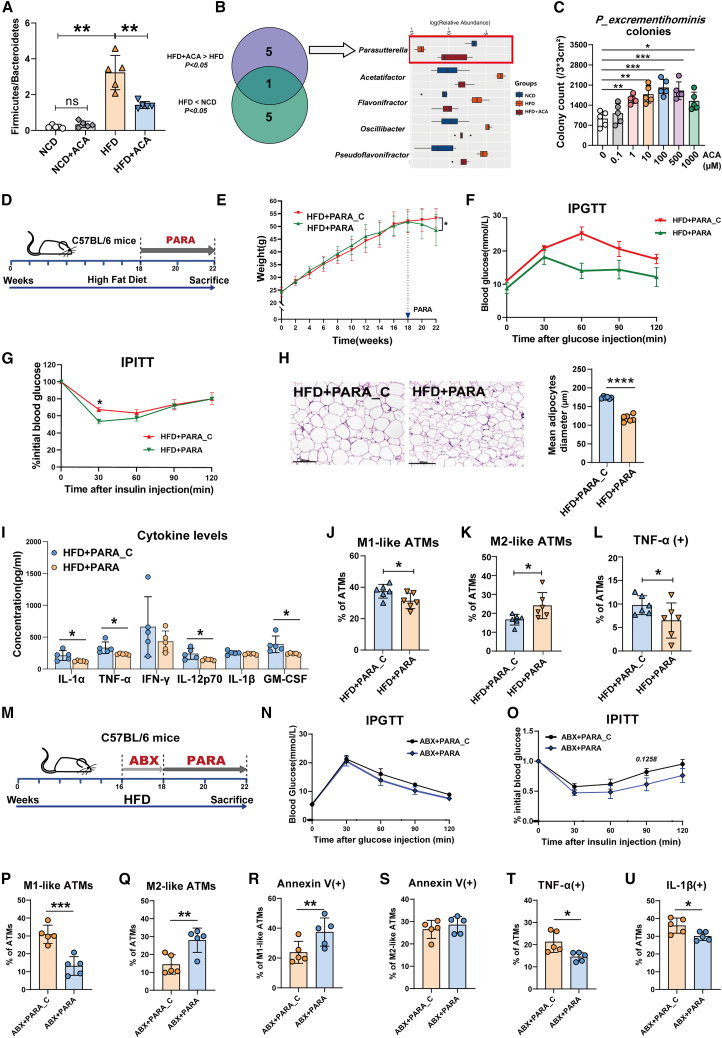
To explore the protective effect of ACA against obesity and obesity-induced IR, C57BL/6 mice were fed a high-fat diet (HFD) or normal control diet (NCD) for 18 weeks and then provided drinking water supplemented with ACA for another 4 weeks (Figure 1A). As expected, although food intake was not different (Figure 1B), the ACA-treated mice gained significantly less weight (Figure 1C), had significantly lower fasting glucose (Figure S1A), and improved glucose tolerance (Figures 1D and S1B) and insulin sensitivity (Figure 1E) compared to age-matched HFD-fed mice.
Figure 1.
ACA ameliorated HFD-induced obesity and rebalanced macrophage homeostasis in adipose tissue
(A) Schema of experiment procedure for ACA treatment on NCD- and HFD-fed mice (n = 6 per group).
(B) Food intake from each group of mice over 72 h during ACA or vehicle supplementation (n = 6 per group).
(C) Body weight in each group of mice (n = 6 per group).
(D) Intraperitoneal glucose tolerance test (IPGTT) in each group (n = 6 per group).
(E) Intraperitoneal insulin tolerance test (IPITT) in HFD-fed mice (n = 6 per group).
(F) Representative H&E staining images of adipose paraffin sections (scale bar: 200 μm, left) and the average length of adipocyte diameter (right) in 4 groups of mice (n = 5 per group).
(G) Mouse serum inflammatory cytokine levels (n = 5 per group).
(H) The percentage of ATMs relative to CD45+ cells (n = 6 per group).
(I) The percentage of M1-like (left) and M2-like (right) ATMs (n = 6 per group).
(J and K) Statistical analysis of Annexin V+ M1-like (J) and M2-like (K) ATMs (n = 6 per group).
(L) The percentage of TNF-α+ ATMs (n = 6 per group).
(M) The experiment procedure of ABX and ACA treatment on HFD-fed mice (n = 6 per group).
(N) Body weight from each group of mice (n = 6 per group).
(O) IPGTT results (n = 6 per group).
(P) IPITT results (∗: HFD group vs. HFD + ACA group; #: HFD + ABX group vs. HFD + ABX + ACA group, n = 6 per group).
(Q) Flow cytometric analysis of the percentage of gonadal M1-like and M2-like ATMs (n = 6 per group).
(R and S) Statistical analysis of Annexin V+ M1-like (R) and M2-like (S) ATMs (n = 6 per group).
(T) The percentage of TNF-α+ ATMs relative to total ATMs (n = 6 per group). (B–F), (H–L), and (N–T) were performed by one-way ANOVA with a post hoc test; (G) was performed by Student’s t test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001; ns, not significant. Data are displayed as the mean ± SD. See also Figure S1.
Administration of ACA significantly decreased the size of adipocytes, which were enlarged by HFD in gonadal white adipose tissues (GWATs) (Figure 1F). Compared with mice fed an HFD, mice that received ACA showed significantly decreased serum levels of interleukin (IL)-1α, IL-1β, and IL-6 (Figure 1G). The immune cells in adipose tissue were detected via flow cytometry, and the gating strategies were shown in Figures S1C and S1D. HFD-fed mice had more GWAT macrophage (F4/80+CD11b+) infiltration than NCD-fed mice, while ACA administration diminished the percentage of ATMs in adipose tissue CD45+ cells (Figure 1H). Inflammatory M1-like ATMs (CD11b+F4/80+CD11chighCD206−) accumulated in obese mice, while alternatively, anti-inflammatory M2-like ATMs (CD11b+F4/80+CD11clowCD206+) were more abundant in healthy/lean mice. ACA resulted in decreased M1-like ATMs but increased M2-like ATMs (Figure 1I). Apoptosis of M1-like but not M2-like ATMs was increased in ACA-treated HFD mice (Figures 1J and 1K). The typical fluorescence-activated cell sorting data of the apoptosis level of M1- and M2-like ATMs in different groups were provided (Figure S1E). M1-like macrophages also expressed high levels of CD86, major histocompatibility complex (MHC) II, and tumor necrosis factor alpha (TNF-α), whereas M2-like macrophages expressed high levels of CD206 and IL-10. To further investigate the impact of ACA on these macrophage populations, we also classified M1-like ATMs as CD11b+F4/80+CD86+CD206− or CD11b+F4/80+MHC II+CD206− and M2-like ATMs as CD11b+F4/80+CD86−CD206+ or CD11b+F4/80+MHC II−CD206+. Similarly, treatment with ACA significantly decreased the proportion of M1-like ATMs (CD86+CD206− or MHC II+CD206−) and increased the proportion of M2-like ATMs (CD86−CD206+ or MHC II−CD206+, Figures S1F and S1G). Reduced TNF-α and increased IL-10 secretion were also observed in ATMs (Figures 1L, S1H, and S1I).
To further investigate whether ACA could affect macrophage infiltration into adipose tissue, we examined the effects of ACA on adipose tissue chemokines. qPCR analysis of adipose tissue demonstrated that while the levels of the chemokines Ccl2, Ccl7, and Ccl13 were significantly increased in obesity, ACA stimulation did not affect their expression (Figure S1J; Table S1). Furthermore, flow cytometry analysis of ATMs after ACA administration revealed no change in macrophage CCR2 expression (Figure S1K). This suggested that ACA primarily affects the proportion of ATMs by influencing macrophage survival rather than through chemotactic migration. In addition, ACA-treated mice exhibited a significant change in macrophages in adipose tissue, while other immune cells, such as B, CD4+ T, CD8+ T, natural killer (NK), NKT, neutrophils, and regulatory T cells, were not significantly changed (Figures S1L and S1M).
We also found that the proportion of mesenteric ATMs was significantly reduced by ACA than the control group (Figure S1N). The percentage of proinflammatory M1-like mesenteric ATMs significantly decreased, while the proportion of anti-inflammatory M2-like ATMs accumulated in ACA-treated HFD-fed mice (Figure S1O). Annexin V staining showed that the apoptosis of M1-like but not M2-like mesenteric ATMs increased significantly after ACA treatment (Figure S1P). TNF-α secretion was also reduced after ACA administration (Figure S1Q). Increased apoptotic pararenal M1-like ATMs and reduced pararenal ATM TNF-α secretion were also observed (Figures S1R–S1U). These results suggested that ACA could inhibit ATM proinflammation and improve obesity and obesity-induced IR.
Gut microbiota was involved in the regulation of ACA on diet-induced obesity and macrophage-mediated inflammation
The gut microbiota is known to influence host metabolism and obesity. To assess the involvement of the gut microbiota in inhibiting ATM proinflammation by ACA, mice fed an HFD for 16 weeks were administered an antibiotic cocktail (ABX) for another 2 weeks followed by 4 weeks of ACA treatment (Figure 1M). The ACA- or ABX-treated mice had significantly lower body weight (Figure 1N) and fasting glucose (Figure S1V) and improved glucose tolerance (Figures 1O and S1W) and insulin sensitivity (Figure 1P) compared with HFD-fed mice. However, although the combination of ABX and ACA treatment showed further improvements in fasting glucose, glucose tolerance, and insulin sensitivity compared with ABX intervention alone, the improvements were not significant. The inhibitory effects of ACA on proinflammatory macrophages were also monitored. As shown in Figures 1Q–1T, ACA but not ABX treatment decreased the percentage of M1-like ATMs, promoted M1-like ATM apoptosis, and reduced ATM TNF-α secretion. However, compared with mice that received ABX alone, mice that received ABX and ACA sequential treatment showed weakened inhibition of M1-like ATMs. These results suggested that the gut microbiota was involved in ACA alleviation of obesity and macrophage proinflammation.
Parasutterella was increased after ACA treatment in obese mice and negatively correlated with macrophage proinflammatory responses
To investigate the changes in the gut microbiome affected by ACA, we performed 16S rRNA gene microbiome sequencing on mouse fecal samples from different groups. The analysis of genus-level operational taxonomic unit (OTU) profiles demonstrated that ACA treatment led to a significant increase in the OTU number in HFD-fed mice, whereas no significant changes in OTU number were observed in NCD-fed mice (Figure S2A). The analysis results of α diversity in 4 different groups showed that ACA reversed HFD-induced alterations in community diversity but not in NCD-fed mice (Figures S2B and S2C). The analysis at the phylum level showed that the ratio of Firmicutes to Bacteroidetes was increased in HFD-fed mice, which is in line with other studies,18,19 while ACA lowered the ratio (Figures 2A and S2D). The plot from the principal component analysis showed that the gut microbiota composition was substantially reshaped after ACA treatment (Figure S2E). Based on the genus level, a variety of probiotics, including Parasutterella, decreased in HFD-fed mice (Figure S2F), while only the abundance of Parasutterella was significantly increased after ACA intervention (Figures 2B and S2G). It has been reported that Parasutterella was associated with obesity, diabetes, and other diseases.20,21 Furthermore, analysis of the effect of Parasutterella on metabolism and inflammation suggested that the abundance of Parasutterella was negatively correlated with body weight, fasting blood glucose, the proportion of M1-like ATMs, and TNF-α level (Figures S2H–S2K) but positively correlated with the apoptosis of M1-like ATMs and the proportion of M2-like ATMs (Figures S2L and S2M).
Figure 2.
Parasutterella-mediated ACA indirect inhibition of inflammation in HFD-fed mice
(A) The ratio of Firmicutes and Bacteroidetes abundance (n = 5 per group).
(B) Significantly differential gut bacterial genera (n = 5 per group).
(C) Statistical analysis of average PARA (P_excrementihominis) colony counts with different concentrations of ACA stimulation (per 3 × 3 cm2) (n = 5 per group).
(D) Schema of experiment procedure of PARA administration in HFD-fed mice.
(E) Mouse body weights (n = 6 per group).
(F) IPGTT results (n = 6 per group).
(G) IPITT results (n = 6 per group).
(H) Representative H&E staining images of adipose tissue (scale bar: 200 μm, left) and the mean length of adipocyte diameter (right, n = 6 per group).
(I) Mouse multiple inflammatory cytokine levels (n = 5 per group).
(J and K) Flow cytometric analysis of the percentage of M1-like (J) and M2-like (K) ATMs (n = 6 per group).
(L) The percentage of TNF-α+ ATMs (n = 6 per group).
(M) Schema of experiment procedure of PARA administration in ABX-treated HFD-fed mice.
(N) IPGTT results (n = 5 per group).
(O) IPITT results (n = 5 per group).
(P and Q) Flow cytometric analysis of the percentage of M1-like (P) and M2-like (Q) ATMs (n = 5 per group).
(R and S) Statistical analysis of Annexin V+ M1-like (R) and M2-like (S) ATMs (n = 5 per group).
(T and U) The percentage of TNF-α+ (T) and IL-1β+ (U) ATMs (n = 5 per group). (A) was performed by Kruskal-Wallis test; (C) was performed by one-way ANOVA with a post hoc test; (E–L) and (N–U) were performed by Student’s t test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001; ns, not significant. Data are displayed as the mean ± SD. See also Figure S2.
The genus Parasutterella contains two type of strains, Parasutterella excrementihominis YIT11859 and Parasutterella secunda YIT12071, which were first isolated from human feces. 16S rRNA gene sequence similarities indicated that the Parasutterella sequences from mice are most closely related to Parasutterella excrementihominis.22 To deeply and directly analyze the impact of ACA on Parasutterella, different concentrations of ACA were added during the culture of Parasutterella excrementihominis (PARA, JCM #15078) in vitro. As shown in Figures 2C and S2N, with increasing ACA concentration, both the number and size of the colonies increased.
PARA administration reduced obesity, improved glucose metabolism, and limited adipose inflammation in HFD-fed mice
To further confirm the contribution of Parasutterella, mice were fed an HFD for 18 weeks and then administered PARA or heat-inactivated PARA (PARA_C) by gavage for 4 weeks (Figure 2D). The PARA-treated mice gained significantly less weight (Figure 2E) and had lower blood glucose (Figure S2O), better glucose tolerance (Figures 2F and S2P), and higher insulin sensitivity (Figure 2G) than the PARA_C-treated mice. H&E staining showed that PARA administration significantly decreased adipocyte size compared with that in the PARA_C group (Figure 2H). Proinflammatory cytokines in mouse serum, including IL-1α, TNF-α, IL-12P70, and granulocyte-macrophage colony-stimulating factor, were significantly decreased (Figure 2I). PARA treatment also resulted in a decrease in M1-like ATMs but an obvious increase in M2-like ATMs in the adipose tissue of HFD-fed mice, as detected by flow cytometry (Figures 2J and 2K). Reduced TNF-α secretion was observed in HFD-fed mouse ATMs after PARA supplementation (Figure 2L).
To verify whether PARA could colonize in intestine, we repeated the aforementioned experiments in ABX-pretreated HFD-fed mice (Figure 2M). Compared with the control group, PARA-treated group of mice showed relatively lower weight gain (Figure S2Q) and improved glucose tolerance (Figures 2N and S2R) and insulin sensitivity (Figure 2O). The proportion of gonadal M1-like ATMs was significantly decreased (Figure 2P), and the proportion of M2-like ATMs was markedly increased (Figure 2Q). PARA treatment increased M1-like ATM apoptosis (Figures 2R and 2S). TNF-α (Figure 2T) and IL-1β (Figure 2U) expression were also decreased. The 16S rRNA gene microbiome sequencing of fecal samples was performed in ABX-pretreated HFD-fed mice. PARA treatment significantly elevated gut microbiota diversity (Figure S2S) and induced a different gut microbiota composition (Figure S2T). Although the abundance of Parasutterella genus was not detected in fecal samples, PARA supplementation was able to promote the abundance of other beneficial bacteria, such as g_Bacteroides and g_Mucispirillum (Figure S2U). Meanwhile, we also analyzed the changes in microbiome composition in HFD-fed mice after administering PARA. As shown in Figure S2V, the administration of PARA did not lead to its colonization in the mouse gut, but increased the levels of certain beneficial bacteria. These results suggested that PARA administration was able to suppress the inflammatory response of ATMs and reduce the occurrence of obesity and obesity-induced IR. This improvement might be mainly through the regulation of the overall microbial community dynamics.
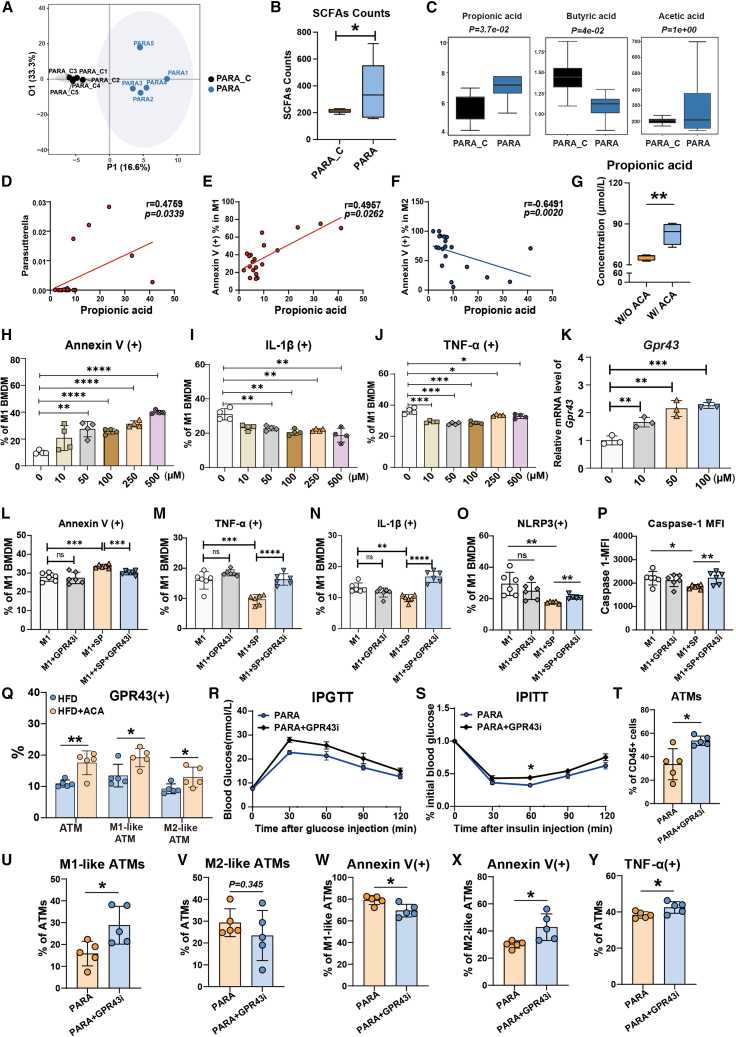
PARA administration increased serum propionic acid levels in HFD-fed mice
To further explore the possible mediators of PARA’s influence on inflammation, we investigated serum metabolites between the PARA and PARA_C-treated mice. These two groups of mice had completely different blood serum metabolites (Figures 3A and S3A). Short-chain fatty acids (SCFAs), in particular, were significantly increased in mouse serum compared with the control PARA_C group (Figures 3B and S3B). PARA led to a prominent increase in propionic acid (PA) levels and a reduction in butyric acid levels, while acetic acid levels did not change significantly (Figure 3C). Meanwhile, serum metabolite analysis indicated that ACA administration also caused elevated PA levels in HFD-fed mice (Figures S3C and S3D). Further correlation analysis verified that the level of PA was positively correlated with Parasutterella abundance (Figure 3D) and M1-like ATM apoptosis (Figure 3E) but negatively correlated with M2-like ATM apoptosis (Figure 3F). Furthermore, we also cultured PARA in vitro with ACA (100 μM) stimulation for 3 days. Supernatant metabolomic testing revealed that ACA-stimulated PARA could produce a higher level of PA (Figure 3G). These results suggested that PARA could produce PA, which may be involved in ACA in improving obesity and adipose tissue inflammation.
Figure 3.
PA balanced pro- and anti-inflammatory macrophages via GPR43
(A) Orthogonal partial least squares discriminant analysis (OPLS-DA) score plot between the serum metabolites of PARA_C and PARA-treated HFD-fed mice (n = 5 per group, samples with hemolysis during blood collection were excluded).
(B) Statistical analysis of SCFA counts in mouse serum (n = 5 per group).
(C) Statistical analysis of serum PA (left), butyric acid (middle), and acetic acid (right) in mice (n = 5 per group).
(D–F) Spearman’s correlation analysis between PA concentration and Parasutterella abundance (D), percentage of Annexin V+ M1-like ATMs (E), and the percentage of Annexin V+ M2-like ATMs (F) (n = 20).
(G) Concentration of PA in the supernatant of PARA with or without ACA (100 μM) stimulation (n = 5 per group).
(H–J) Statistical analysis of Annexin V+ (H), IL-1β+ (I), and TNF-α+ (J) M1 BMDMs after 48 h of stimulation with SP (n = 4 per group).
(K) Relative mRNA expression of Gpr43 in M1 BMDMs (n = 3 per group).
(L) Flow cytometric analysis of apoptosis of M1 BMDMs (n = 6 per group).
(M and N) Flow cytometric analysis of TNF-α+ (M) and IL-1β+ (N) M1 BMDMs (n = 6 per group).
(O and P) Flow cytometric analysis of NLRP3+ (O) and caspase 1+ (P) M1 BMDMs (n = 6 per group).
(Q) Flow cytometric analysis of GPR43 expression level in ATMs (left), M1-like ATMs (middle), and M2-like ATMs (right, n = 5 per group). After 16 weeks of HFD feeding, mice were administrated with GPR43 inhibitor (GPR43i, GLPG-0974) or vehicle weekly for 5 weeks. Meanwhile, PARA bacteria gavage was given for 4 weeks at the 17th week of HFD-fed mice, then sacrificed these HFD-fed mice at the 21st week.
(R) IPGTT results (n = 5 per group).
(S) IPITT results (n = 5 per group).
(T–V) Flow cytometric analysis of the percentage of ATMs (T), M1-like (U), and M2-like (V) ATMs (n = 5 per group).
(W and X) Statistical analysis of Annexin V+ M1-like (W) and M2-like (X) ATMs (n = 5 per group).
(Y) The percentage of TNF-α+ ATMs (n = 5 per group). (B), (C), (G), and (Q–Y) were performed by Student’s t test; (D–F) was performed by Spearman’s correlation analysis; (H–P) was performed by one-way ANOVA with a post hoc test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001; ns, not significant. Data are displayed as the mean ± SD. See also Figure S3; Table S1.
PA inhibited the survival and proinflammatory function of M1-polarized macrophages through GPR43
To further verify the relationship between PA and macrophages, we next tested the effect of PA on bone-marrow-derived macrophages (BMDMs) in vitro. BMDMs were first polarized to M1 BMDMs and then stimulated with different concentrations of sodium propionate (SP). After 48 h, the effects of SP on the survival and proinflammatory functions of M1-polarized BMDMs were observed. We found that SP significantly induced M1-polarized BMDM apoptosis in a dose-dependent manner (Figure 3H). In addition, SP reduced M1-polarized BMDM secretion of proinflammatory cytokines, such as IL-1β and TNF-α (Figures 3I and 3J). With increased SP concentration, the expression levels of some proinflammatory genes, such as Nos2 and Il1b, and anti-apoptosis genes, such as Bcl2, accordingly decreased (Figure S3E). SP also induced human M1-polarized macrophage apoptosis and inhibited TNF-α secretion (Figures S3F and S3G). These results indicated that PA played an important role in inhibiting the proinflammatory effects of M1-polarized macrophages.
It has been reported that PA is capable of binding and activating GPR43 to play a role in immune regulation.23 Therefore, we detected the mRNA level of Gpr43 via quantitative reverse-transcription PCR and found that SP caused increased Gpr43 gene expression in BMDMs in a dose-dependent manner (Figure 3K; Table S1). Furthermore, a GPR43 inhibitor (GPR43i, GLPG-0974) weakened the effect of SP on M1-polarized macrophages (Figures 3L–3N), which was consistent with the mRNA results (Figures S3H and S3I). SP stimulation also inhibited NLRP3 (Figure 3O), caspase-1 (Figure 3P), and apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC, Figure S3J) expression in M1-polarized BMDMs, but the effects were reversed by GPR43i pretreatment. These results suggested that PA mainly played an important role in inhibiting macrophage proinflammatory phenotype and NLRP3 inflammasome through GPR43. Because of the significant correlation between PA and M2 macrophages, we also detected the influence of SP on M2-polarized macrophage survival and function. As shown in Figures S3K and S3L, SP inhibited M2-polarized BMDM apoptosis and promoted M2 BMDM survival and Gpr43 and Il10 expression.
Furthermore, our in vivo experiments demonstrated that both ACA and PARA treatment remarkably increased GPR43 expression on ATMs and M1-like ATMs (Figures 3Q, S3M, and S3N). To further investigate the role of GPR43 in vivo, GPR43i (GLPG-0974) was given to HFD-fed mice followed by PARA administration. GPR43 inhibition tended to weaken the effects of PARA on weight gain (Figure S3O), glucose tolerance, and IR (Figures 3R, 3S, and S3P). The proportion of ATMs and M1-like ATMs increased after GPR43i treatment (Figures 3T–3V). Meanwhile, GPR43i treatment inhibited M1-like ATM apoptosis, but increased M2-like ATM apoptosis (Figures 3W and 3X), accompanied by elevated ATM TNF-α secretion (Figure 3Y). Through the aforementioned data, we provided evidence that the Parasutterella induced by ACA could inhibit obesity and macrophage-mediated inflammation via its metabolite PA and GPR43.
ACA could directly inhibit macrophage proinflammation phenotype in vitro and in vivo
In this study, we also found that the regulatory effect of ACA on obesity and obesity-induced IR was not completely blocked after ABX treatment (Figures 1O and 1P) and still maintained the tendency to inhibit ATM proinflammation (Figures 1Q–1T). To further determine whether ACA could exert anti-inflammatory effects in a gut microbiota-independent manner, we stimulated BMDMs with ACA in vitro. BMDMs were differentiated into M1- or M2-polarized macrophages and then stimulated with ACA at different doses. Under ACA stimulation, M1-polarized, rather than M2-polarized BMDMs, had higher apoptosis and lower levels of TNF-α secretion in a dose-dependent manner (Figures 4A, 4B, and S4A). These results were also validated in human macrophages (Figures S4B and S4C). These results indicated that ACA could directly regulate the survival and proinflammatory function of M1-polarized macrophages in vitro.
Figure 4.
ACA directly inhibited macrophage-mediated proinflammation
(A and B) Statistical analysis of Annexin V+ (A) and TNF-α+ (B) M1 BMDMs after 48 h of ACA stimulation (n = 8 per group).
(C) The experiment procedure of NBD fluorescence-labeled ACA (ACA-NBD) administration to mice.
(D) The percentage of NBD+ ATMs (n = 5 per group).
(E and F) Statistical analysis of the percentage of M1-like (E) and M2-like (F) ATMs (n = 5 per group).
(G) The percentage of Annexin V+ ATMs (n = 5 per group).
(H) Volcano plot illustrating the significantly upregulated and downregulated genes in ACA-treated BMDMs (fold change > 1.5 or fold change < 0.67; p < 0.05, n = 3 per group).
(I and J) GO (I) and KEGG (J) enrichment pathway analyses based on significantly differentially expressed genes (p < 0.05).
(K) Heatmap showing significantly changed differentially expressed genes related to lysosome organization.
(L) Heatmap showing significantly changed differentially expressed genes related to the electronic transport chain.
(M) Flow cytometric analysis of the percentage of GPR120+ ATMs (left), GPR120+ M1-like ATMs (middle), and GPR120+ M2-like ATMs (right) in HFD-fed mice with or without ACA treatment (n = 5 per group).
(N) Statistical analysis of the percentage of GPR120+ ATMs between NBD+ and NBD− ATMs (n = 5 per group).
(O and P) Relative Gpr120 mRNA expression (O) and percentage of GPR120+ cells (P) in M1 BMDMs (n = 6 per group).
(Q and R) Statistical analysis of Annexin V+ (Q) and TNF-α+ (R) M1 BMDMs treated with ACA or negative control (FAM) after Gpr120 knockdown (n = 6 per group).
(S) Heatmap showing significantly changed differentially expressed genes related to mTOR signaling pathway (n = 3 per group).
(T) The relative AKT, phosphorus AKT, Sirt1, and AMPKα expression level of M1 BMDM after ACA (250 μM) stimulation (n = 6 per group).
(U) Flow cytometric analysis of relative phosphorus mTOR (p-mTOR) expression after Gpr120 knockdown (n = 5 per group).
(V–X) Statistical analysis of p-mTOR+ (V), Annexin V+ (W), and TNF-α+ (X) M1 BMDMs after ACA or mTOR activator (3BDO) treatment (n = 5 per group).
(Y) Mechanism of the direct and indirect regulation of ATMs by ACA in obesity. Created with BioRender.com. (A), (B), (Q), (R), and (V–X) were performed by one-way ANOVA with a post hoc test; (D–G), (M–P), and (T) were performed by Student’s t test; (U) was performed by Kruskal-Wallis test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001. Data are displayed as the mean ± SD. See also Figure S4; Table S1.
However, whether ACA directly regulates macrophages in vivo is still unknown. To further confirm the direct regulation of ACA on macrophage proinflammation, we prepared NBD fluorescence-labeled ACA (ACA-NBD). Mice fed an HFD or NCD for 16 weeks were intragastrically administered ACA-NBD solution (Figure 4C). After 2 h, the mice were sacrificed, and ATMs were detected. Compared with NCD-fed mice with ACA-NBD gavage, the ATMs of HFD-fed mice with ACA-NBD gavage presented greater NBD fluorescence enrichment (Figures 4D and S4D). Then, we further analyzed the NBD+ and NBD− ATMs by flow cytometry in HFD-fed mice. NBD+ ATMs contained a lower proportion of M1-like and a higher proportion of M2-like ATMs (Figures 4E and 4F). In addition to the cell proportion, NBD+ ATMs and NBD+ M1-like ATMs significantly increased apoptosis (Figure 4G).
To further confirm that the effects were not caused by NBD itself, we stimulated M1 BMDMs with NBD fluorescence alone (C-NBD) or ACA-NBD in vitro. As shown in Figures S4E and S4F, there was no difference in apoptosis between C-NBD+ or C-NBD− M1-polarized BMDMs; however, NBD+ BMDMs from mice treated with ACA-NBD had markedly increased apoptosis than NBD− M1-polarized BMDMs. These observations indicated that ACA may affect the inflammatory states of adipose tissue by directly regulating the macrophage pro- and anti-inflammatory balance.
ACA directly inhibited macrophage proinflammatory phenotype by regulating mitochondrial and lysosomal function via the GPR120/mTOR signaling pathway
To further reveal the mechanism of ACA in anti-inflammatory function, M1-polarized BMDMs treated with or without ACA in vitro for 48 h were compared using transcriptome sequencing. Ninety-two differentially expressed genes were found, of which 26 genes were significantly upregulated and 66 genes were significantly downregulated (Figure 4H). Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses indicated that the regulated genes in ACA-stimulated M1-polarized BMDMs were mainly involved in the immune response, inflammatory cell apoptotic process, oxidative stress, regulation of mammalian target of rapamycin (mTOR) signaling, phagosome and autophagy, especially lysosomal function (Figures 4I and 4J). We found that ACA positively regulated lysosome organization genes, such as Hexa, Grn, Atp6ap2, and Rab34 (Figures 4K and S4G), and electron transport chain genes, such as Atp1a3, Atp6v1c1, Atp5j2, and Atp6v0e (Figures 4L and S4H).
Among the 31 genes enriched in the “regulation of inflammatory response” pathway, we found that Ffar4 gene was the most upregulated gene (p value: 0.0011) (Figure 4H). Ffar4 gene could encode G protein-coupled receptor 120 (GPR120), which has been reported to be expressed in macrophages.24 Oligosaccharides or glucose could directly interact as ligands with G protein-coupled receptors.25,26
To investigate the specific role of GPR120 in ACA-mediated attenuation of the proinflammatory function of macrophages, we first analyzed GPR120 changes in vivo. GPR120 expression levels were markedly increased after ACA supplementation in both gonadal ATMs and M1-like ATMs (Figures 4M and S4I). Similarly, the NBD+ ATMs and M1-like ATMs expressed higher levels of GPR120 than the NBD− group in the gonadal adipose tissue of HFD-fed mice (Figure 4N). In vitro ACA stimulation also elevated GPR120 expression in M1-polarized BMDMs (Figures 4O and 4P; Table S1). As expected, NBD fluorescence alone failed to enhance GPR120 expression in M1-polarized BMDMs (Figure S4J). Furthermore, we transfected M1-polarized BMDMs with small interfering RNA (siRNA) to knock down Gpr120 expression, and the interference efficiency of different Gpr120 siRNAs was shown in Figure S4K. GPR120 knockdown reversed the ACA-induced increase in apoptosis and decrease in TNF-α secretion in M1-polarized BMDMs (Figures 4Q and 4R). These data suggested that ACA regulated the survival and proinflammatory function of macrophages via GPR120.
GO enrichment analysis indicated that ACA may affect the mTOR signaling pathway in M1-polarized BMDMs. ACA-related mTOR genes were significantly regulated (Figures 4S and S4L). We examined the protein expression of mTOR signaling pathway and found that ACA supplementation significantly inhibited total and phosphorus AKT expression, while promoted Sirt1 and AMPKα expression (Figures 4T and S4M) in M1-polarized BMDMs. ACA also suppressed phosphorus mTOR protein expression in M1-polarized BMDMs, whereas this inhibition was reversed after Gpr120 knockdown (Figure 4U). Next, we introduced the mTOR activator 3BDO to investigate its relationship with ACA. When mTOR signaling was activated, the ability of ACA to inhibit M1-polarized macrophage proinflammatory function was downregulated (Figures 4V–4X). The aforementioned results illustrated that ACA affected the downstream mTOR signaling pathway mainly through GPR120. Similarly, ACA elevated GPR120 levels and suppressed mTOR signaling levels in human M1-polarized macrophages (Figures S4N and S4O). These data suggested that ACA regulated the survival and proinflammatory function of macrophages via the GPR120/mTOR pathway.
Discussion
ACA can regulate glucose metabolism, impacting carbohydrate absorption and ultimately suppressing weight gain.27 Our research also observed weight reduction in HFD-fed mice treated with ACA. Moreover, this study reveals the function and mechanism of ACA on ATM-mediated inflammation in obesity and obesity-induced IR. First, our study showed that ACA could enhance the abundance of PA-producing Parasutterella, therefore indirectly inhibiting the survival and proinflammatory function of M1-like ATMs in vivo. Most interestingly, we found that ACA directly inhibited ATM proinflammatory phenotype (Figure 4Y), leading to a reduction in adipose tissue inflammation overall.
Results from ABX-treated HFD-fed mice with ACA supplementation indicated that ACA could influence adipose tissue inflammation by regulating gut microbiota. Although PARA administration alone reduced obesity, improved glucose metabolism, and limited adipose inflammation in HFD-fed mice, these benefits were more likely based on PARA promoting metabolite production and influencing the entire bacterial community.
Parasutterella’s role in diseases was controversial. It had been reported that Parasutterella was abundant in patients with Crohn’s disease28 and was scarce in pediatric patients with inflammatory bowel disease (IBD) with good response to treatment.29 Jonathan P.J. et al. pointed out that IBD progressors had a depletion of Parasutterella, which was negatively associated with IBD clinical progression.30 Studies also showed that the abundance of g_Parasutterella or s_Parasutterella excrementihominis in human feces was negatively correlated with BMI, waistline, and other clinical indices.31,32,33 Patients with type 2 diabetes mellitus (T2DM) had a relatively lower abundance of Parasutterella, while diabetic patients who took ACA orally had a higher abundance of Parasutterella,34,35 which was consistent with our findings in mice. However, recent studies suggest that Parasutterella may increase with weight gain and negatively impact insulin sensitivity. This effect might be linked to its role in carbohydrate metabolism, especially when more carbohydrates are present in the gut, as observed in a Yersinia pseudotuberculosis model of transient gut infection.36 Meanwhile, the Bacteroidetes genus expansion after ACA treatment might also contribute to disease protection by ACA.
Gut microbiota-derived metabolites influence a plethora of immune cells and thus have critical roles in the maintenance of immune homeostasis.37 Among various metabolites, PA, one type of SCFAs, was significantly elevated in PARA-treated mice and associated with the inhibitory effect of PARA on M1-like ATMs.
SCFA supplementation, especially PA, could reduce body weight and improve IR.38,39,40 In humans, dietary PA could also improve glucose tolerance and insulin sensitivity in healthy volunteers.41 Recently, there was a Mendelian randomization analysis presented that the concentration of fecal PA was causally associated with an increased incidence of T2DM. The researchers hypothesized that increased fecal PA levels were more likely to be the result of reduced absorption of PA to circulation, which was more consistent with the beneficial effects of PA on energy balance and metabolic homeostasis. Treatment of human adipose tissue explants with PA resulted in a significant downregulation of inflammatory cytokines and chemokines.42 PA could also attenuate atherosclerosis by affecting intestinal cholesterol metabolism in an immune-dependent manner.43 These reports were consistent with our findings that PA played an important role in inhibiting the proinflammatory effect of M1-like ATMs.
Most interestingly, our in vitro and in vivo experiments revealed that ACA could directly regulate the survival and proinflammatory function of M1-like ATMs. Moreover, HFD-fed obese mice exhibited NBD fluorescence-labeled ATMs, but NCD-fed lean mice that orally received ACA-NBD did not exhibit NBD fluorescence-labeled ATMs. Monocyte recruitment through chemokines is important in populating adipose tissue with macrophages during obesity.44 Since ACA is rarely absorbed into the bloodstream, it raises the possibility of circulating macrophage migration from the gut to adipose tissue. Huang et al. also reported that anti-inflammatory macrophages in the lamina propria, which were induced by REG3γ-associated Lactobacillus, may migrate into adipose tissues and are involved in resistance against HFD-mediated obesity.45 Moreover, ACA exhibits more pronounced effects in vivo compared to in vitro. ACA induced significantly more apoptosis in M1-like ATMs in vivo than in LPS/IFNγ-induced macrophages in vitro. This suggests that ACA may have a stronger immunomodulatory effect circulating macrophages, which migrate from the gut to adipose tissue, compared to BMDMs.
Previous works have reported that polysaccharides or oligosaccharides can work through multiple surface receptors on macrophages, such as Toll-like receptors, mannose receptors, Dectin-1, scavenger receptors,46,47 and G protein-coupled receptors.25,26 In this study, we demonstrated that ACA could directly upregulate macrophage GPR120 expression. In addition, ACA activated GPR120 on the membrane surface of M1 macrophages and enhanced intracellular lysosomal and mitochondrial function, thus accelerating M1 macrophage apoptosis.
In conclusion, our study reveals the underlying mechanisms of ACA in controlling obesity and obesity-induced IR. ACA directly and indirectly inhibits proinflammatory macrophage phenotype, which contributes to the improvement of obesity and obesity-induced IR. The understanding of the immune regulatory effects of ACA may extend its potential for further therapeutic applications.
Limitations of the study
Firstly, after the stimulation of ACA, some metabolites also showed significant alterations, such as indole-3-PA and 3-hydroxybutyric acid. Therefore, we cannot rule out the possibility that other metabolites play important roles in ACA influencing ATM inflammatory responses through PARA. Secondly, the administration of PARA did not lead to its colonization in murine gut, but increased the abundance of certain beneficial bacteria. Further experiments are needed to reveal the regulation of ACA on these beneficial bacteria. Thirdly, the mechanism of ACA’s direct effects on macrophages has been explored mainly through in vitro experiments, which lacks further in vivo experiments. At the same time, the clinical testing and verification of Parasutterella and PA before and after ACA oral treatment need further supplement.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed and will be fulfilled by the lead contact, Dong Zhang (zhangd@ccmu.edu.cn).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
Raw data of deidentified RNA-seq have been deposited at GEO as GEO: GSE232578. 16S rRNA gene microbiome sequencing data have been deposited at NCBI as PRJNA1181246. Raw metabolomics data have been deposited at MetaboLights as MTBLS11553. The aforementioned data are publicly available as of the date of publication. All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report any original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
Grants from the National Natural Science Foundation of China (no. 82270606 and 82370578), R&D Program of Beijing Municipal Education Commission (no. KZ202210025036), Chinese Institutes for Medical Research, Beijing (grant no. CX24PY16), Beijing Municipal Administration of Hospitals’ Ascent Plan (no. DFL20220103), Reform and Development Program of Beijing Institute of Respiratory Medicine (Ggyfz202403), and the Youth Beijing Scholar (no. 035) supported this work.
Author contributions
All listed authors participated meaningfully in the study and have seen and approved the submission of this manuscript. X.L. and S.Z. participated in performing the research, analyzing the data, and initiating the original draft of the article. Haozhe Xu, Zihan Zhang, X.H., Y.W., H.J., X.D., Hufeng Xu, and M.L. participated in performing the research. D.Z. and G.S. established the hypotheses, supervised the studies, analyzed the data, and co-wrote the manuscript. S.W. and Zhongtao Zhang participated in the review and editing of the manuscript.
Declaration of interests
X.L., S.Z., G.S., and D.Z. are inventors of a Chinese patent application for the prevention and treatment of obesity and obesity-induced IR using Parasutterella excrementihominis.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| PE/Cy7 anti-CD11b (clone M1/70) | Thermo Fisher Scientific | Cat# 25-0112-82; RRID: AB_469588 |
| FITC anti-CD11b (clone M1/70) | Thermo Fisher Scientific | Cat# 11-0112-82; RRID: AB_464935 |
| PE anti-CD11b (clone M1/70) | Biolegend | Cat# 101208; RRID: AB_312791 |
| BV421 anti-CD11b (clone M1/70) | Biolegend | Cat# 101223; RRID: AB_755985 |
| APC anti-F4/80 (clone BM8) | Biolegend | Cat# 123116; RRID: AB_893481 |
| PE anti-F4/80 (clone BM8) | Biolegend | Cat# 123110; RRID: AB_893486 |
| APC anti-CD206 (clone C068C2) | Biolegend | Cat# 141707; RRID: AB_10896057 |
| PE/Cy7 anti-CD206 (clone C068C2) | Biolegend | Cat# 141719; RRID: AB_2562247 |
| FITC anti-CD206 (clone C068C2) | Biolegend | Cat# 141703; RRID: AB_10900988 |
| BV421 anti-CD11c (clone N418) | Biolegend | Cat# 117321; RRID: AB_755987 |
| FITC anti-CD11c (clone N418) | Biolegend | Cat#,117305; RRID: AB_313774 |
| APC anti-CD11c (clone N418) | Biolegend | Cat#,117309; RRID: AB_313778 |
| PerCP/Cyanine5.5 anti-Ly-6G (clone 1A8) | Biolegend | Cat#,127653; RRID: AB_2616998 |
| BV785 anti-Ly-6G (clone 1A8) | Biolegend | Cat#,1276453; RRID: AB_2566317 |
| APC anti-CD3 (clone 145-2C11) | Thermo Fisher Scientific | Cat# 17-0031-83; RRID: AB_469316 |
| PE/Cy7 anti-CD3 (clone 145-2C11) | Thermo Fisher Scientific | Cat# 25-0031-82; RRID: AB_469572 |
| PE/Cy7anti-CD4 (clone GK1.5) | Thermo Fisher Scientific | Cat# 25-0041-82; RRID: AB_469576 |
| PerCP/Cyanine5.5 anti-CD4 (clone GK1.5) | Thermo Fisher Scientific | Cat# 45-0042-82; RRID: AB_1107001 |
| FITC anti-CD4 (clone GK1.5) | Thermo Fisher Scientific | Cat# 11-0041-85; RRID: AB_464893 |
| PE anti-CD8 (clone 5H10) | Thermo Fisher Scientific | Cat#: MCD0804; RRID: AB_10373415 |
| FITC anti-CD8 (clone 5H10) | Thermo Fisher Scientific | Cat#: 53-0081-82; RRID: AB_469897 |
| FITC anti-CD45 (clone 30-F11) | Thermo Fisher Scientific | Cat#: 12-0451-85; RRID: AB_465051 |
| PE anti-CD45 (clone 30-F11) | Thermo Fisher Scientific | Cat#: 12-0451-82; RRID: AB_465668 |
| APC/Cy7 anti-CD45 (clone 30-F11) | Biolegend | Cat#: 103115; RRID: AB_312980 |
| PE anti-NK1.1 (clone PK136) | Thermo Fisher Scientific | Cat#: 12-5941-83; RRID: AB_466051 |
| BV605 anti-NK1.1 (clone PK136) | Biolegend | Cat#: 108739; RRID: AB_ 2562273 |
| PE anti-TNFα (clone MP6-XT22) | Thermo Fisher Scientific | Cat#: 12-7321-82; RRID: AB_466199 |
| PE anti-IL1β (clone NJTEN3) | Thermo Fisher Scientific | Cat#: 12-7114-82; RRID: AB_10732630 |
| NLRP3 Recombinant Rabbit Monoclonal Antibody (clone SC06-23) |
Thermo Fisher Scientific | Cat#: MA5-32255; RRID: AB_2809541 |
| Caspase 1 Recombinant antibody (clone 1018) | Proteintech | Cat#: 81482-1-RR |
| ASC/TMS1Rabbit mAb (clone D2W8U) | Cell Signaling Technology | Cat#: 67824T |
| Phospho-mTOR Monoclonal Antibody, PE (clone MRRBY) | Thermo Fisher Scientific | Cat#: 12-9718-42; RRID: AB_2572724 |
| AKT Monoclonal antibody (clone 2C5D1) | Proteintech | Cat#: 60203-2-Ig |
| Phospho-AKT Recombinant antibody (clone 2E17) | Proteintech | Cat#: 80455-1-RR |
| Recombinant Anti-SIRT1 antibody (clone EPR18239) | Abcam | Cat#: ab189494 |
| AMPK Alpha 1 Monoclonal antibody (clone 1H8E1) | Proteintech | Cat#: 66536-1-Ig |
| FFAR2 Rabbit PolyAb | Proteintech | Cat#: 19952-1-AP; RRID: AB_2878628 |
| Rabbit anti-GPR120 Polyclonal Antibody | Absin | Cat#: abs131314 |
| CoraLite647-conjugated AffiniPure F(ab')2 Fragment Goat Anti-Rabbit IgG (H + L) |
Proteintech | Cat#: SA00014-9 |
| Annexin V-PE kit | BD Pharmingen | Cat#: 559763 |
| 7-AAD Viability Staining Solution | Biolegend | Cat#: 420404 |
| PE anti-Gr1 (clone RB6-8C5) | Biolegend | Cat#: 108407; RRID: AB_313372 |
| PE anti-TER119 (clone TER-119) | Biolegend | Cat#: 116207; RRID: AB_313708 |
| PE anti-CD45/B220 (clone RA3-6B2) | Biolegend | Cat#: 103207; RRID: AB_312992 |
| Biological samples | ||
| Human PBMC | Biobank Resource of Beijing Friendship Hospital | ID: 2017-P2-131-03 |
| Chemicals, peptides, and recombinant proteins | ||
| Phorbol 12-myristate 13-acetate | Sigma-Aldrich | Cat#: 16561-29-8 |
| Collagenase type II | Sigma-Aldrich | Cat#: 9001-12-1 |
| DNase I | Roche | Cat#: 11284932001 |
| Percoll | GE Health | Cat#: 17-0891-09 |
| Permeabilization Buffer 10× | Thermo Fisher Scientific | Cat#: 00-8333-56 |
| Fixation/Permeabilization Concentrate | Thermo Fisher Scientific | Cat#: 00-5123-43 |
| Fixation/Permeabilization Diluent | Thermo Fisher Scientific | Cat#: 00-5223-56 |
| Fixation Buffer | BioLegend | Cat#: 420801 |
| Dimethyl sulfoxide (DMSO) | Sigma-Aldrich | Cat#: 67-68-5 |
| Granulocyte-Macrophage Colony-Stimulating Factor, (GM-CSF) |
PeproTech | Cat#: PMC2011 |
| Lipopolysaccharide (LPS) | Sigma-Aldrich | Cat#: 437627 |
| Interferon-γ (IFN-γ) | PeproTech | Cat#: 315-05 |
| Interleukin-4 (IL-4) | PeproTech | Cat#: 214-14 |
| Acarbose | MCE | Cat#: 56180-94-0 |
| Sodium propionate | MCE | Cat#: 56180-94-0 |
| GLPG0974(GPR43 inhibitor) | MCE | Cat#: 1391076-61-1 |
| Ampicillin | INALCO | Cat#: 1758-9314 |
| Neomycin sulfate | INALCO | Cat#: 1758-9329 |
| Vancomycin HCI | Lablead | Cat#: 1404-93-9 |
| Metronidazole | Sigma-Aldrich | Cat#: 443-48-1 |
| Critical commercial assays | ||
| PrimeScript RT Master Mix | TaKaRa | Cat#: RR036A |
| qPCR SYBR Green Master Mix | Applied Biosystems | Cat#: K0253 |
| Direct Human Monocyte Isolation Kit | STEMCELL | Cat#: 19669 |
| Deposited data | ||
| Raw and analyzed data | This paper | GEO: GSE232578 |
| 16S rRNA gene microbiome sequencing | This paper | NCBI: PRJNA1181246 |
| Raw metabolomics data | https://www.ebi.ac.uk/metabolights/MTBLS11553 | MetaboLights: MTBLS11553 |
| Experimental models: Organisms/strains | ||
| Parasutterella excrementihominis | Japan Collection of Microorganisms | JCM #15078 |
| Oligonucleotides | ||
| See Table S1 for gene primer sequences | This paper | N/A |
| Software and algorithms | ||
| FlowJo software version 10.5.2 | Treestar | https://www.flowjo.com/ |
| GraphPad Prism 9.0 | GraphPad software | https://www.graphpad.com |
| SPSS software | SPSS Inc | https://www.ibm.com/spss |
| ImageJ | National Institutes of Health | https://imagej.net/ij/ |
| BioRender | BioRender | https://www.biorender.com/ |
| Other | ||
| ABI 7500 sequence detection system | Applied Biosystems | N/A |
| BD FACS Aria II | BD Biosciences | N/A |
| Gentle-MACS dissociator | Miltenyi Biotec | Cat#: 130-093-235 |
| Diet: Normal Control Diet (NCD) | Research Diets | Cat#: D12450B |
| Diet: High fat Diet (HFD) | Research Diets | Cat#: D12492 |
Experimental model and study participant details
Animals
Eight-week-old male C57BL/6 mice were purchased from HFK Laboratory (Beijing, China). These mice were fed either a normal control diet (NCD, D12450B, Research Diets, USA) or a high-fat diet (HFD, D12492, Research Diets, USA). The HFD is widely used to make experimental animal models for the research of obesity, type 2 diabetes and MASLD. The mice were maintained in a pathogen-free, temperature-controlled environment under a 12-h light/dark cycle. Animal studies were performed in compliance with the ethical guidelines for animal studies and were approved by the Institutional Animal Care and Ethics Committee of Beijing Friendship Hospital (ID: 20–2038).
For the acarbose treatment experiment, the mice were fed an HFD and drinking water with vehicle or 3.0 mg/mL acarbose (Shanghai Yiyan Biotech, Shanghai, China) for 4 weeks 3.0 mg/mL acarbose in water was equal to 500 mg/kg/d in mice. For the gut microbiota experiment, after 14 days of antibiotic cocktail (vancomycin 0.5 mg/mL, neomycin 1 mg/mL, ampicillin 1 mg/mL and metronidazole 1 mg/mL) administration in drinking water, the microbiota-depleted mice were treated with acarbose (500 mg/kg/d) under HFD treatment for 4 weeks. To avoid the reversal of gut microbiota, antibiotics (vancomycin 5 mg/d, neomycin 5 mg/d, ampicillin 5 mg/d and metronidazole 5 mg/d) were orally administered to each mouse for 3 days after 2 weeks of acarbose treatment.
To transplant Parasutterella excrementihominis (P_excrementihominis) (JCM #15078), HFD-fed mice were given 0.3 mL of a bacterial suspension of P_excrementihominis at a concentration of 108 colony forming units (CFU)/mL (HFD+PARA group) or the same dose of heat-killed P_excrementihominis and sterile water as the control (HFD+PARA_C group) by gavage for 4 weeks.
Human macrophages isolation
Purified CD14+ macrophages were isolated directly from human whole blood by immunomagnetic negative selection (EasySep Direct Human Monocyte Isolation Kit, Cat#19669). After that, cells were broadcasted to 24-well plates with 100 ng/mL human GM-CSF stimulation. After 5–7 days, human macrophages were polarized to M1 macrophages (LPS, human IFN-γ) and M2 macrophages (human IL-4). 12 healthy human subjects, aged 18–45, were recruited from the physical examination center of Beijing Friendship Hospital. Both male and female subjects were included, and all samples were deidentified. Ethics approval was obtained from the Human Ethics Committee of Beijing Friendship Hospital (ID: 2017-P2-131-03).
Parasutterella excrementihominis culture and counting
Parasutterella excrementihominis (JCM #15078, Japan) was purchased from the Japan Collection of Microorganisms (JCM). The activated strain was unpacked in an anaerobic glove box, cultured anaerobically at 37°C for 48 h, and recorded as the P1 generation. Part of the colony was picked and transferred to a fresh plate, anaerobic culture was continued at 37°C for 24–37 h, and the result was recorded as the P2 generation. The collected bacteria were washed with pretreated saline (sterilized and placed in an anaerobic glove box for at least 48 h), shaken and mixed to make the bacterial suspension. Determine approximate concentrations based on McFarlane turbidity. The 10 mL system was serially diluted 105 times, 200 μL of which was pipetted onto the prepared plate (special medium containing different concentrations of ACA). Parasutterella excrementihominis was cultured in anaerobic conditions for 72 h at 37°C in the presence or absence of acarbose (0.1, 1, 10, 100, 500 and 1000 μM).
Bacterial dishes were photographed on the same light and color setting. Randomly select 3 fields of view (Size: 3-centimeter-long, 3-centimeter-wide) for each picture. ImageJ software (National Institutes of Health, USA) was used to automatically calculate the number of colonies in the images with same size. The prepared pictures were opened and converted to type 8-bit grayscale to create binary images. Then set the threshold to ensure that all the bacterial colonies were retained. The positive count value obtained was the number of colonies on this prepared picture. The average of 3 count values was the number of bacterial colonies in this dish. Each group had 5 replicate samples. One-way ANOVA was used in multiple comparisons. p values <0.05 were considered significant.
Method details
Glucose tolerance test and insulin tolerance test
Glucose tolerance tests (GTTs) were performed after 16 h of fasting. Blood glucose concentrations were measured with a glucometer (Terumo Corporation, Japan), and blood samples were collected from the tail tip at 0, 30, 60, 90 and 120 min after intraperitoneal injection of glucose (Sigma, CAS#50997) (1.5 g/kg body weight). For the insulin tolerance tests (ITTs), insulin (Novo Nordisk, 202106ABF1) (0.75 units/kg body weight) was administered via intraperitoneal injection after 4 h of fasting. All of the ITT and GTT tests were performed at the indicated times.
Histological analysis
Mouse adipose tissues were fixed in 4% paraformaldehyde for 24 h, and paraffin sections were cut into 4-μm sections and stained by hematoxylin and eosin (H&E). H&E staining was commissioned by Servicebio Technology (Wuhan, China). At least three discontinuous sections were evaluated for each sample in over three samples per group. The length measurements of adipose tissue were taken at ten different views, and the mean values were used to assess variation.
Isolation of adipose immune cells by enzymatic digestion
After the mice were anesthetized, the adipose tissues were resected from the mice and dissociated into fine pieces. Then, the tissue pieces were treated with 0.5 mg/mL type II collagenase (Sigma) in digestion buffer (HBSS with 1 mg/mL BSA, 0.001% DNase I) and mechanically ground by a gentleMACS Octo Dissociator (Miltenyi Biotec, Bergisch-Gladbach, Germany). After that, the tissue suspension continued to be incubated in a 37°C shaking incubator for 30 min. The suspensions were passed through a 70-μm filter (BD Biosciences, Falcon) and centrifuged at 50 × g for 5 min to obtain the supernatant, which was then centrifuged at 500 × g for 5 min. The remaining single-cell suspensions were washed and counted for FACS staining.
Flow cytometry analysis of adipose immune cells
The isolation and examination of adipose immune cells were described previously. Immune cells were harvested and analyzed to determine the expression levels of various cell surface and intracellular markers. The conjugated surface marker antibodies were incubated for 30 min at 4°C. For intracellular antigen detection, cells were fixed and permeabilized with the corresponding fixation buffer after surface staining. For the detection of intracellular cytokines, cells were stimulated with cell activation cocktail, which composed of PMA, ionomycin, and Brefeldin A in DMSO (500×, BioLegend), for 6 h. Then, cells were surface stained, fixed and subsequently permeabilized using commercially available Perm/Wash buffers (BioLegend). Intracellular cytokines were quantified using flow cytometry for intracellular capture. All samples were acquired on a FACS Aria II flow cytometer (BD Biosciences, CA, USA), and data were analyzed using FlowJo software version V10 (Tree Star, OR, USA).
16S rRNA gene microbiome sequencing and metabolomics sequencing
Fecal samples were collected from live mice, snap-frozen and stored at −80°C, and subjected to identification of the composition of gut microbiota using 16S rRNA Gene Microbiome Sequencing (Metabo-Profile, Shanghai, China). The serum samples from the HFD-fed, acarbose treatment HFD-fed mice, PARA administration HFD-fed mice and inactive PARA administration HFD-fed mice were subjected to identification and quantification of metabonomic sequencing using a Q300 Metabolite Array Kit (Metabo-Profile, Shanghai, China). The significant differential serum metabolites between two groups were analyzed by unidimensional test method. And the threshold was set as: p value <0.05 with an absolute value of log2FC (FC: Fold Change) > = 0.
Bone marrow-derived macrophage (BMDM) experiment
Bone marrow cells were collected from the tibia and femurs of C57BL/6 mice. After erythrocytes were lysed, the cells were resuspended and incubated with an antibiotic cocktail (Ter119, B220, Ly6G). Then, the positive cells were removed by magnetic bead-based selection. The remaining macrophages were seeded in six-well plates and cultured with 20 ng/mL GM-CSF (murine Granulocyte-Macrophage Colony-Stimulating Factor, Peprotech, #315-02) at 37°C/5% CO2 for 5 days (7×104 cells per well). Mature bone marrow-derived macrophages (BMDMs) were used for subsequent experiments. BMDMs were induced to differentiate into M1 macrophages by 100 ng/mL LPS (E. coli serotype 0111: B4, Sigma) and 20 ng/mL IFN-γ (murine IFN-γ, PeproTech, #315-05) and into M2 macrophages by 20 ng/mL IL-4 (murine IL-4, PeproTech, #214-14) for 24 h. After inducing M1 macrophages, sodium propionate (Sigma, P5436) or acarbose (MCE, 56180-94-0) was added to the culture medium for the next 48 h of stimulation. Then, the cell suspension was collected for analysis. A receptor inhibitor assay was performed as follows. Prior to LPS and IFN-γ stimulation, macrophages were incubated with GPR43i (GLPG0974, 0.1 μM) for 3 h. Next, the unmatured monocytes were differentiated into M1 macrophages and stimulated with sodium propionate. Based on initial experiments, the acarbose concentration of 250 μM and the sodium propionate concentration of 50 μM were chosen for the subsequent transcription sequencing experiment and pathway tests.
Real-time qPCR analysis
Total RNA was isolated from adipose tissues and BMDMs using TRI Reagent (Sigma) and reverse transcribed into cDNA with PrimeScript RT Master Mix (TAKARA). Real-time qPCR was performed by the 7500 Fast Real-time System (Applied Biosystems, CA, USA) using SYBR Green Master Mix (Applied Biosystems). Amplicon expression in each sample was normalized to GAPDH expression. The relative gene expression value was subsequently quantified using the 2−ΔΔCt method. Genes and primer sequences are shown in Table S1.
Synthesis of NBD fluorescence-labeled acarbose
Five hundred milligrams of acarbose powder was dissolved in 5 mL of DMSO. Then, NBD-Cl (1.5 eq.) and triethylamine (4.5 eq.) to completely dissolve, react at room temperature for 24 h and concentrate the solution by rotary evaporation under reduced pressure. After that, glacial ether was precipitated and washed twice with dichloromethane. When glacial ether was solidified and dried, NBD fluorescence-labeled acarbose (NBD-ACA) was obtained (Ruixi Technologies, Xi’an, China).
Transcriptome sequencing analysis
M1 BMDMs were stimulated with acarbose (250 μM) for 48 h, and then total RNA was isolated. Transcriptome sequencing libraries were generated using the NEBNext Ultra RNA Library Prep Kit for Illumina (NEB, USA) following the manufacturer’s recommendations and sequenced on an Illumina HiSeq platform (Illumina, San Diego, CA). Sequences were aligned to the reference genome with TopHat and processed with Cufflinks, which quantified each transcript in each sample using reference annotations produced by the University of California Santa Cruz UCSC. Differentially expressed genes with a fold change ≥1.5 or ≤0.67 and p value <0.05 between acarbose-treated or nonacarbose-treated M1 macrophages were submitted to Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses, which used unbiased methods to assess pathway enrichment. The mRNA sequencing data described in this study were uploaded to the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (accession No. GSE232578).
Quantification and statistical analysis
Statistical analysis was performed using SPSS Statistics (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY, USA) and Prism 9.0 software (GraphPad Software, San Diego, CA, USA). Each experiment was performed at least in triplicate and the results are shown as mean ± standard deviation (SD). The normal distribution of variables was tested with the Shapiro–Wilk test. Differences between two groups were compared by t test for normal variables and Kruskal–Wallis test for nonnormal variables. One-way ANOVA with a post hoc test for normal variables and the Kruskal‒Wallis test for nonnormal variables were used in multiple comparisons. p values <0.05 were considered significant. The sample distribution was determined using a Kolmogorov‒Smirnov normality test. Correlation analysis of the gut microbiome and host metabolome was investigated using nonparametric Spearman’s test.
Published: December 31, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2024.101883.
Contributor Information
Guangyong Sun, Email: sungy@ccmu.edu.cn.
Dong Zhang, Email: zhangd@ccmu.edu.cn.
Supplemental information
References
- 1.Bluher M. Obesity: global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019;15:288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 2.Di Angelantonio E., Bhupathiraju S., Wormser D., Gao P., Kaptoge S., Berrington de Gonzalez A., Cairns B., Huxley R., Jackson C., et al. Global BMI Mortality Collaboration Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–786. doi: 10.1016/S0140-6736(16)30175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afshin A., Forouzanfar M.H., Reitsma M.B., Sur P., Estep K., Lee A., Marczak L., Mokdad A.H., Moradi-Lakeh M., et al. GBD 2015 Obesity Collaborators Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olefsky J.M., Glass C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 5.Rohm T.V., Meier D.T., Olefsky J.M., Donath M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55:31–55. doi: 10.1016/j.immuni.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caslin H.L., Bhanot M., Bolus W.R., Hasty A.H. Adipose tissue macrophages: Unique polarization and bioenergetics in obesity. Immunol. Rev. 2020;295:101–113. doi: 10.1111/imr.12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lumeng C.N., Bodzin J.L., Saltiel A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh R., Zogg H., Wei L., Bartlett A., Ghoshal U.C., Rajender S., Ro S. Gut Microbial Dysbiosis in the Pathogenesis of Gastrointestinal Dysmotility and Metabolic Disorders. J. Neurogastroenterol. 2021;27:19–34. doi: 10.5056/jnm20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tilg H., Zmora N., Adolph T.E., Elinav E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 2020;20:40–54. doi: 10.1038/s41577-019-0198-4. [DOI] [PubMed] [Google Scholar]
- 11.Jia W., Weng J., Zhu D., Ji L., Lu J., Zhou Z., Zou D., Guo L., Ji Q., Chen L., et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes. Metab. Res. Rev. 2019;35 doi: 10.1002/dmrr.3158. [DOI] [PubMed] [Google Scholar]
- 12.Hanefeld M., Schaper F. Acarbose: oral anti-diabetes drug with additional cardiovascular benefits. Expert Rev. Cardiovasc Ther. 2008;6:153–163. doi: 10.1586/14779072.6.2.153. [DOI] [PubMed] [Google Scholar]
- 13.Wang N., Zhang J.P., Xing X.Y., Yang Z.J., Zhang B., Wang X., Yang W.Y. MARCH: factors associated with weight loss in patients with newly diagnosed type 2 diabetes treated with acarbose or metformin. Arch. Med. Sci. 2019;15:309–320. doi: 10.5114/aoms.2018.75255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang F., Xu S., Tang L., Pan X., Tong N. Acarbose With Comparable Glucose-Lowering but Superior Weight-Loss Efficacy to Dipeptidyl Peptidase-4 Inhibitors: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 2020;11:288. doi: 10.3389/fendo.2020.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alssema M., Ruijgrok C., Blaak E.E., Egli L., Dussort P., Vinoy S., Dekker J.M., Denise Robertson M. Effects of alpha-glucosidase-inhibiting drugs on acute postprandial glucose and insulin responses: a systematic review and meta-analysis. Nutr. Diabetes. 2021;11:11. doi: 10.1038/s41387-021-00152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu Y., Wang X., Li J., Zhang Y., Zhong H., Liu R., Zhang D., Feng Q., Xie X., Hong J., et al. Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat. Commun. 2017;8:1785. doi: 10.1038/s41467-017-01682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen H.H., Chao Y.H., Chen D.Y., Yang D.H., Chung T.W., Li Y.R., Lin C.C. Oral administration of acarbose ameliorates imiquimod-induced psoriasis-like dermatitis in a mouse model. Int. Immunopharmacol. 2016;33:70–82. doi: 10.1016/j.intimp.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 19.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 20.Blasco-Baque V., Coupé B., Fabre A., Handgraaf S., Gourdy P., Arnal J.F., Courtney M., Schuster-Klein C., Guardiola B., Tercé F., et al. Associations between hepatic miRNA expression, liver triacylglycerols and gut microbiota during metabolic adaptation to high-fat diet in mice. Diabetologia. 2017;60:690–700. doi: 10.1007/s00125-017-4209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bush J.R., Alfa M.J. Increasing levels of Parasutterella in the gut microbiome correlate with improving low-density lipoprotein levels in healthy adults consuming resistant potato starch during a randomised trial. BMC Nutr. 2020;6 doi: 10.1186/s40795-020-00398-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagai F., Morotomi M., Sakon H., Tanaka R. Parasutterella excrementihominis gen. nov., sp nov., a member of the family Alcaligenaceae isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2009;59:1793–1797. doi: 10.1099/ijs.0.002519-0. [DOI] [PubMed] [Google Scholar]
- 23.Kim M.H., Kang S.G., Park J.H., Yanagisawa M., Kim C.H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145:396–406.e1. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 24.Oh D.Y., Talukdar S., Bae E.J., Imamura T., Morinaga H., Fan W., Li P., Lu W.J., Watkins S.M., Olefsky J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemaire K., Van de Velde S., Van Dijck P., Thevelein J.M. Glucose and sucrose act as agonist and mannose as antagonist ligands of the G protein-coupled receptor Gpr1 in the yeast Saccharomyces cerevisiae. Mol. Cell. 2004;16:293–299. doi: 10.1016/j.molcel.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Foata F., Sprenger N., Rochat F., Damak S. Activation of the G-protein coupled receptor GPR35 by human milk oligosaccharides through different pathways. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-73008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer S., Patzak A., Rietzsch H., Schwanebeck U., Köhler C., Wildbrett J., Fuecker K., Temelkova-Kurktschiev T., Hanefeld M. Influence of treatment with acarbose or glibenclamide on insulin sensitivity in type 2 diabetic patients. Diabetes Obes. Metab. 2003;5:38–44. doi: 10.1046/j.1463-1326.2003.00239.x. [DOI] [PubMed] [Google Scholar]
- 28.Rausch P., Ellul S., Pisani A., Bang C., Tabone T., Marantidis Cordina C., Zahra G., Franke A., Ellul P. Microbial Dynamics in Newly Diagnosed and Treatment Naive IBD Patients in the Mediterranean. Inflamm. Bowel Dis. 2023;29:1118–1132. doi: 10.1093/ibd/izad004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoyhtya M., Korpela K., Saqib S., Junkkari S., Nissila E., Nikkonen A., Dikareva E., Salonen A., de Vos W.M., Kolho K.L. Quantitative Fecal Microbiota Profiles Relate to Therapy Response During Induction With Tumor Necrosis Factor alpha Antagonist Infliximab in Pediatric Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2023;29:116–124. doi: 10.1093/ibd/izac182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobs J.P., Goudarzi M., Lagishetty V., Li D., Mak T., Tong M., Ruegger P., Haritunians T., Landers C., Fleshner P., et al. Crohn's disease in endoscopic remission, obesity, and cases of high genetic risk demonstrates overlapping shifts in the colonic mucosal-luminal interface microbiome. Genome Med. 2022;14:91. doi: 10.1186/s13073-022-01099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kreutzer C., Peters S., Schulte D.M., Fangmann D., Türk K., Wolff S., van Eimeren T., Ahrens M., Beckmann J., Schafmayer C., et al. Hypothalamic Inflammation in Human Obesity Is Mediated by Environmental and Genetic Factors. Diabetes. 2017;66:2407–2415. doi: 10.2337/db17-0067. [DOI] [PubMed] [Google Scholar]
- 32.Liu R., Hong J., Xu X., Feng Q., Zhang D., Gu Y., Shi J., Zhao S., Liu W., Wang X., et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017;23:859–868. doi: 10.1038/nm.4358. [DOI] [PubMed] [Google Scholar]
- 33.Zeng Q., Li D., He Y., Li Y., Yang Z., Zhao X., Liu Y., Wang Y., Sun J., Feng X., et al. Discrepant gut microbiota markers for the classification of obesity-related metabolic abnormalities. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-49462-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X., Fang Z., Zhang C., Xia H., Jie Z., Han X., Chen Y., Ji L. Effects of Acarbose on the Gut Microbiota of Prediabetic Patients: A Randomized, Double-blind, Controlled Crossover Trial. Diabetes Ther. 2017;8:293–307. doi: 10.1007/s13300-017-0226-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X., Ren H., Zhao C., Shi Z., Qiu L., Yang F., Zhou X., Han X., Wu K., Zhong H., et al. Metagenomic analysis reveals crosstalk between gut microbiota and glucose-lowering drugs targeting the gastrointestinal tract in Chinese patients with type 2 diabetes: a 6 month, two-arm randomised trial. Diabetologia. 2022;65:1613–1626. doi: 10.1007/s00125-022-05768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siqueira M.K.D., Andrade-Oliveira V., Stacy A., Guimaraes J.P.T., Alberca-Custodio R.W., Castoldi A., Santos J.M., Davoli-Ferreira M., Menezes-Silva L., Turato W.M., et al. Infection-elicited microbiota promotes host adaptation to nutrient restriction. Proc. Natl. Acad. Sci. USA. 2023;120 doi: 10.1073/pnas.2214484120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang W., Cong Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell. Mol. Immunol. 2021;18:866–877. doi: 10.1038/s41423-021-00661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.den Besten G., Bleeker A., Gerding A., van Eunen K., Havinga R., van Dijk T.H., Oosterveer M.H., Jonker J.W., Groen A.K., Reijngoud D.J., Bakker B.M. Short-Chain Fatty Acids Protect Against High-Fat Diet-Induced Obesity via a PPARgamma-Dependent Switch From Lipogenesis to Fat Oxidation. Diabetes. 2015;64:2398–2408. doi: 10.2337/db14-1213. [DOI] [PubMed] [Google Scholar]
- 39.Lin H.V., Frassetto A., Kowalik E.J., Jr., Nawrocki A.R., Lu M.M., Kosinski J.R., Hubert J.A., Szeto D., Yao X., Forrest G., Marsh D.J. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Vadder F., Kovatcheva-Datchary P., Goncalves D., Vinera J., Zitoun C., Duchampt A., Bäckhed F., Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 41.Venter C.S., Vorster H.H., Cummings J.H. Effects of dietary propionate on carbohydrate and lipid metabolism in healthy volunteers. Am. J. Gastroenterol. 1990;85:549–553. [PubMed] [Google Scholar]
- 42.Al-Lahham S., Roelofsen H., Rezaee F., Weening D., Hoek A., Vonk R., Venema K. Propionic acid affects immune status and metabolism in adipose tissue from overweight subjects. Eur. J. Clin. Invest. 2012;42:357–364. doi: 10.1111/j.1365-2362.2011.02590.x. [DOI] [PubMed] [Google Scholar]
- 43.Haghikia A., Zimmermann F., Schumann P., Jasina A., Roessler J., Schmidt D., Heinze P., Kaisler J., Nageswaran V., Aigner A., et al. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur. Heart J. 2022;43:518–533. doi: 10.1093/eurheartj/ehab644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bai Y., Sun Q. Macrophage recruitment in obese adipose tissue. Obes. Rev. 2015;16:127–136. doi: 10.1111/obr.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang Y.G., Qi H.B., Zhang Z.Q., Wang E.L., Yun H., Yan H., Su X.M., Liu Y.Q., Tang Z.Z., Gao Y.H., et al. Gut REG3γ-Associated Induces Anti-inflammatory Macrophages to Maintain Adipose Tissue Homeostasis. Front. Immunol. 2017;8:1063. doi: 10.3389/fimmu.2017.01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pujari R., Banerjee G. Impact of prebiotics on immune response: from the bench to the clinic. Immunol. Cell Biol. 2021;99:255–273. doi: 10.1111/imcb.12409. [DOI] [PubMed] [Google Scholar]
- 47.He Y., Lawlor N.T., Newburg D.S. Human Milk Components Modulate Toll-Like Receptor-Mediated Inflammation. Adv. Nutr. 2016;7:102–111. doi: 10.3945/an.115.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Raw data of deidentified RNA-seq have been deposited at GEO as GEO: GSE232578. 16S rRNA gene microbiome sequencing data have been deposited at NCBI as PRJNA1181246. Raw metabolomics data have been deposited at MetaboLights as MTBLS11553. The aforementioned data are publicly available as of the date of publication. All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report any original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.