Summary
Uncommon epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer (NSCLC) pose therapeutic challenge due to limited response to EGFR tyrosine kinase inhibitors (TKIs). This study presents preclinical evidence and mechanistic insights into the combination of lazertinib, a third-generation EGFR-TKI; and amivantamab, an EGFR-MET bispecific antibody, for treating NSCLC with uncommon EGFR mutations. The lazertinib-amivantamab combination demonstrates significant antitumor activity in patient-derived models with uncommon EGFR mutations either before treatment or after progressing on EGFR-TKIs. Lazertinib enhances the inhibitory capacity of amivantamab by increasing its on-target expression. Notably, the combination surpasses afatinib, a first-line treatment for uncommon EGFR mutations in NSCLC, in terms of in vivo efficacy. Promising clinical activity is also observed in two case studies of patients treated with this combination (NCT04077463). Our findings highlight the potential of the lazertinib-amivantamab combination as a therapeutic strategy for uncommon EGFR mutations, an area of unmet medical need, and support further clinical investigation.
Keywords: NSCLC, uncommon EGFR-mutatation, combination therapy, third-generation EGFR-TKI, EGFR-MET bispecific antibody
Graphical abstract

Highlights
-
•
Lazertinib-amivantamab combination shows potent activity in uncommon EGFR-mutant NSCLC
-
•
The combination can overcome resistance of uncommon EGFR mutations to EGFR-TKIs
-
•
Lazertinib enhances the on-target expression of amivantamab
-
•
High EGFR/MET expression associates with response to combination therapy
Oh et al. demonstrate promising preclinical activity of the lazertinib and amivantamab combination against uncommon EGFR mutations through multifaceted mechanisms, including cell-cycle arrest, apoptosis, and enhanced ADCC activity. This approach also shows significant efficacy in clinical cases, offering a potential therapeutic strategy to address an unmet medical need.
Introduction
Epidermal growth factor receptor (EGFR) mutations account for approximately 20% of non-small cell lung cancer (NSCLC) cases in Caucasians, while the incidence increases to over 50% in Asians.1,2 The majority of EGFR mutations are exon 19 deletions and exon 21 L858R point mutations, collectively referred to as common EGFR mutations, which account for 80%–90% of all EGFR mutations.3 EGFR tyrosine kinase inhibitors (EGFR-TKIs) have significantly improved the survival rates and clinical outcomes of patients harboring these EGFR mutations.4,5,6,7,8,9
Uncommon mutations represent the remaining EGFR mutations within exons 18 to 21, which generally exhibit lower response rates to EGFR-TKIs compared to common EGFR mutations. Excluding exon 20 insertions (ex20ins), the major uncommon EGFR mutations include G719X, S768I, and L861Q, which can coexist with other common EGFR mutations or with other uncommon EGFR mutations, forming compound mutations.10,11,12 Afatinib received the US Food and Drug Administration (FDA) approval for the treatment of NSCLC patients with uncommon EGFR mutations (G719X, S768I, and L861Q) based on a retrospective analysis of three prospective trials (LUX-Lung 2, 3, and 6).13,14 This was further supported by a large-scale pooled analysis and the ACHILLES/TORG1834 study.15,16,17 However, uncommon mutations that do not respond to afatinib still exist, and there is also no established treatment option for resistance following afatinib therapy. Recently, the UNICORN retrospective study reported the efficacy and safety of osimertinib in patients with previously untreated uncommon EGFR mutations, with high rates of systemic and intracranial disease control.18 Nevertheless, data regarding the clinical efficacy of osimertinib in NSCLC with uncommon EGFR mutations are still insufficient.
Currently, several clinical trials are ongoing to evaluate the efficacy of the combination of a third-generation EGFR-TKI, lazertinib, and an EGFRxMET bispecific antibody, amivantamab, in various clinical settings of EGFR-mutated advanced NSCLC. A recent phase 3 MARIPOSA study (NCT04487080) indicated that the combination of lazertinib and amivantamab demonstrated both efficacy and safety as a potential first-line treatment for EGFR-mutant NSCLC.19 Based on these results, the combination of lazertinib and amivantamab was approved in the US in 2024 as a first-line chemotherapy-free treatment for patients with EGFR-mutated advanced lung cancer.20 Additionally, results from cohort C of the CHRYSALIS-2 study (NCT04077463) showing promising clinical activity of the combination in patients with atypical (uncommon) EGFR mutations (excluding ex20ins) were presented at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting (2024 ASCO).21
In this study, we provide the first comprehensive evaluation of the antitumor activity and mechanism of action of the lazertinib and amivantamab combination in engineered Ba/F3 cell lines and patient-derived preclinical models harboring uncommon EGFR mutations. Furthermore, we provide clinical evidence of the combination therapy in patients with uncommon EGFR mutations, highlighting the translational significance of our findings.
Results
Combination therapy of lazertinib and amivantamab exhibits remarkable antitumor activity in Ba/F3 cells with certain uncommon EGFR mutations
To evaluate the combination efficacy of lazertinib (3rd-generation EGFR-TKI) and amivantamab (EGFR x MET bispecific antibody) on uncommon EGFR mutation, we constructed Ba/F3 cells with several uncommon EGFR mutations (e.g., G719S, S768I, L861Q, G719A/S768I, G719S/S768I, and S768I/L858R) (Table S1). Clinically available EGFR-TKIs, such as gefitinib,22 afatinib,22,23 osimertinib,23,24 and lazertinib,25 exhibited similar effect to previously reported effect in Ba/F3 cells harboring uncommon EGFR mutations (Figure 1A; Figure S1A). Treatment with amivantamab alone showed in vitro activity only against EGFRG719S Ba/F3 cells (Figures 1B and S1B). We demonstrated a significant synergistic effect of the combination of lazertinib and amivantamab compared to their individual treatments in Ba/F3 cells harboring EGFRG719S, EGFRS768I, and EGFRG719A/S768I (Figure 1C). Additionally, immunoblot analysis confirmed that combination treatment of lazertinib and amivantamab significantly reduced the phosphorylation of EGFR (pEGFR) in these Ba/F3 cells (Figure 1D). On the other hand, no synergistic effect was observed for combination therapy with amivantamab compared to lazertinib treatment alone in EGFRL861Q, EGFRS768I/L858R and EGFRG719S/S768I Ba/F3 cell lines (Figure S1C).
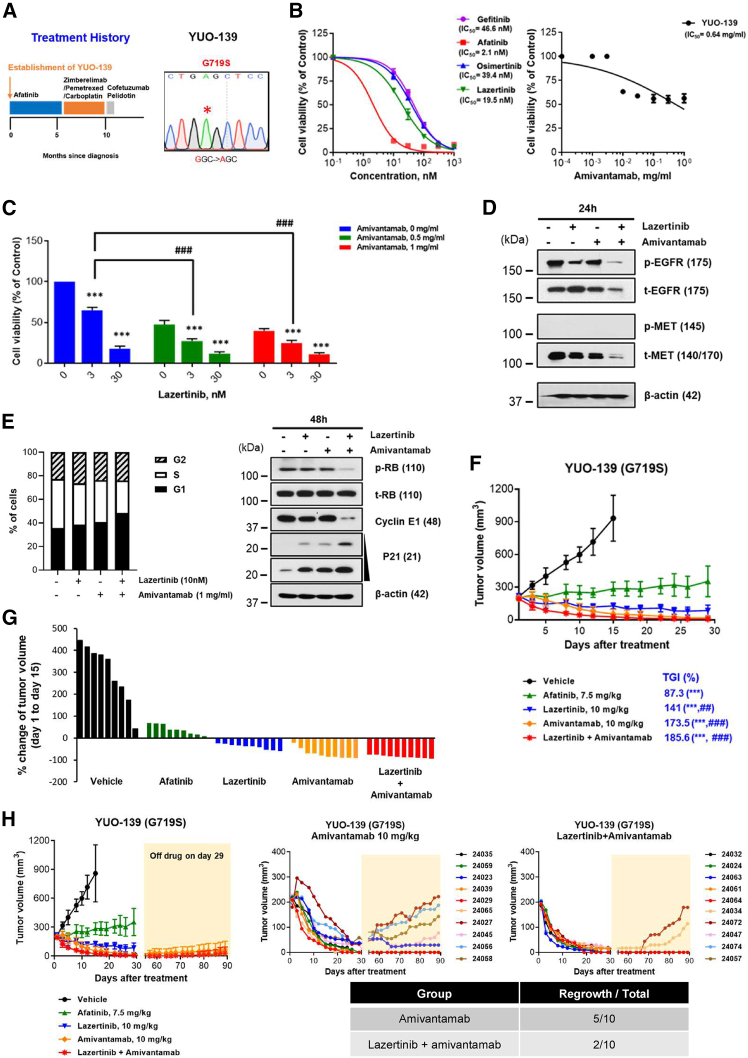
Figure 1.
Antitumor activity of lazertinib plus amivantamab in Ba/F3 cells with uncommon EGFR mutations
(A–C) Ba/F3 cells expressing uncommon EGFR mutations (G719S, S768I, and G719A/S768I) were seeded onto a 96-well plate and incubated with the EGFR-TKIs (gefitinib, afatinib, osimertinib, and lazertinib) and amivantamab. Cell viability was measured after 3 days of EGFR-TKIs and 5 days of amivantamab treatment via CellTiter-Glo. The curved graphs represent the cell viability results for a single drug (A and B), and the bar graphs show the cell viability for the combination of lazertinib and amivantamab (C). Data represent the means ± SE from three and more times of independent experiments. ANOVA with Tukey post hoc test: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 vs. lazertinib 0 nM in each group; ##p < 0.01, ###p < 0.001 vs. matched concentrate of lazertinib in amivantamab 0 mg/mL.
(D) Immunoblot analysis of Ba/F3 cells with uncommon EGFR mutations (G719S, S768I, and G719A/S768I) treated with lazertinib and amivantamab for 72 h. Ba/F3 EGFRG719S cells were treated with lazertinib at 3 nM and amivantamab 0.5 mg/mL, while Ba/F3 EGFRS768I and EGFRG719A/S768I cells were treated with lazertinib at 100 nM and amivantamab at 1 mg/ml.
(E) Ba/F3 EGFRG719S cells (5 × 106 in 100 μL PBS) were subcutaneously implanted into the flanks of 6-week-old female BALB/c nude mice. Mice were randomized once tumor volume reached 200 mm3 and then treated with afatinib, lazertinib, amivantamab, and lazertinib plus amivantamab. Tumor growth curves represent the response to the indicated drugs over a period of 27 days. Data represent the means ± SD (n = 10/group). Kruskal-Wallis with Dunn’s post hoc test: ∗∗p < 0.01, ∗∗∗p < 0.001 vs. vehicle; #p < 0.05, ###p < 0.001 vs. afatinib; §§§p < 0.001 vs. lazertinib.
(F) Waterfall plot representing the percentage of tumor volume change in mice after 4 weeks of treatment with the indicated drugs.
(G) Representative images of IHC staining for pEGFR and tEGFR of Ba/F3 EGFRG719S tumor sections. The scale bar indicates 50 μm.
We further evaluated the in vivo activity of the combination of lazertinib and amivantamab in EGFRG719S mutant Ba/F3 xenograft model using afatinib, which is approved for patients with uncommon EGFR mutations, as a positive control. The combination therapy of lazertinib and amivantamab induced a robust tumor regression with a 175.1% tumor growth inhibition (TGI) value. In contrast, monotherapies with afatinib, lazertinib, and amivantamab showed TGI values of 21.3%, 26.3%, and 75.2%, respectively (Figures 1E, 1F, and S2). Moreover, immunohistochemical (IHC) analysis revealed that amivantamab and the combination treatment of lazertinib with amivantamab significantly reduced pEGFR compared to the other groups (Figure 1G). Collectively, these results suggest that combination of lazertinib and amivantamab has antitumor efficacy in certain uncommon EGFR-mutant cells.
Combination therapy of lazertinib and amivantamab exhibits in vitro and in vivo activity in the EGFR-TKI-naive YUO-139 patient-derived organoid model with EGFRG719S
Next, we evaluated the antitumor efficacy of combination treatment with lazertinib and amivantamab in patient-derived preclinical models with uncommon EGFR mutations. The YUO-139, an organoid established from EGFR-TKI-naive patients with EGFRG719S mutation (Figure 2A; Figure S3), exhibited IC50 values of 46.6 nM for gefitinib, 2.1 nM for afatinib, 39.4 nM for osimertinib, and 19.5 nM for lazertinib (Figure 2B). Notably, when combined with amivantamab at a concentration of 0.5 mg/mL, lazertinib showed improved anti-proliferative efficacy at a concentration of 3 nM, which was approximately 6 times lower than the IC50 value observed for individual treatments (Figure 2C). Given that amivantamab targets EGFR and/or MET, we evaluated the effect of the combination of lazertinib and amivantamab on EGFR as well as MET. Immunoblot analysis revealed that the combination of lazertinib and amivantamab more robustly suppressed pEGFR/tEGFR and tMET compared to either agent alone (Figure 2D). Baseline pMET was not detected, likely due to the treatment-naive status of the model and its origin from an uncommon EGFR mutation.
Figure 2.
Antitumor activity of lazertinib plus amivantamab in the EGFR-TKI-naive YUO-139 PDO model with EGFRG719S
(A) Clinical treatment history of patient harboring EGFRG719S. The YUO-139 model was established from pleural effusion at the indicated time point (left panel). The harboring mutation of YUO-139 was confirmed by Sanger sequencing (right panel).
(B and C) YUO-139 organoids were seeded onto a 96-well plate and treated with EGFR-TKIs (gefitinib, afatinib, osimertinib, and lazertinib) and amivantamab at the indicated concentrations. Cell viability was measured using CellTiter-Glo 3D after 3 and 5 days of monotherapy with EGFR-TKIs and amivantamab, respectively, and after 10 days of treatment with lazertinib plus amivantamab. The curved graphs represent the cell viability results for a single drug (B), and the bar graphs show the cell viability for the combination of lazertinib and amivantamab (C). Data represent the means ± SE from three and more times of independent experiments. ANOVA with Tukey post hoc test: ∗∗∗p < 0.001 vs. lazertinib 0 nM in each group; ###p < 0.001 vs. matched concentrate of lazertinib in amivantamab 0 mg/mL.
(D) YUO-139 organoids were treated with lazertinib at 10 nM or/and amivantamab at 1 mg/mL for 24 h. Expression of pEGFR/tEGFR and pMET/tMET was evaluated through immunoblot assay.
(E) Left, YUO-139 organoids were treated with lazertinib or/and amivantamab with the indicated concentrations for 72 h. The bar graphs showed the percentage of cells in each phase of the cell cycles. Right, YUO-139 organoids were treated with lazertinib at 10 nM or/and amivantamab at 1 mg/mL for 48 h. Expression of pRB/tRB, Cyclin E1, and p21 was evaluated through immunoblot assay.
(F) Tumor growth curves of YUO-139 xenografts in response to the indicated drugs over a period of 29 days. Data represent the means ± SD (n = 10/group). Kruskal-Wallis with Dunn post hoc test: ∗∗∗p < 0.001 vs. vehicle; ##p < 0.01, ###p < 0.001 vs. afatinib.
(G) Waterfall plot representing the percentage of tumor volume change in mice after 2 weeks of treatment with the indicated drugs.
(H) For the amivantamab and lazertinib plus amivantamab groups in (F), the drug was discontinued on day 29 of treatment and tumor growth was further monitored until day 90. The upper panel shows the average growth curves for each of the two groups, and the lower panel shows the individual mouse tumor growth curves for amivantamab (left) and lazertinib + amivantamab (right).
Next, we investigated whether the anti-proliferative effects of lazertinib plus amivantamab were associated with cell-cycle arrest (Figure 2E). The combination therapy caused a marked increase in the G1 phase population, along with a reduction in the S phase, compared to either lazertinib or amivantamab monotherapy. This was aligned with the decreased expression of pRB and cyclin E1, both of which are key regulators of G1-S phase progression, and the increased expression of p21, a cyclin-dependent kinase inhibitor that promotes G1 arrest (Figure 2E).
To further evaluate the in vivo efficacy of lazertinib plus amivantamab in the YUO-139, we generated a xenograft model. Amivantamab alone and in combination with lazertinib demonstrated excellent antitumor efficacy with TGI values of 173.5% and 185.6%, respectively, whereas single treatment with afatinib and lazertinib yielded TGI values of 87.3% and 141%, respectively. (Figures 2F, 2G, and S4). Interestingly, despite the similar tumor regression between the monotherapy with amivantamab and the combination therapy with lazertinib, the combination therapy showed better durable responses (Figure 2H). After discontinuation of the drug on day 29, among 10 mice treated with amivantamab alone, tumors regrew in 5 mice. In contrast, among 10 mice treated with the combination of lazertinib and amivantamab, tumors regrew in only 2 mice, while the remaining 8 mice showed sustained inhibition for over 90 days. Collectively, upfront combined therapy of lazertinib and amivantamab demonstrated potent TGI based on in vitro and in vivo models of uncommon EGFR mutations.
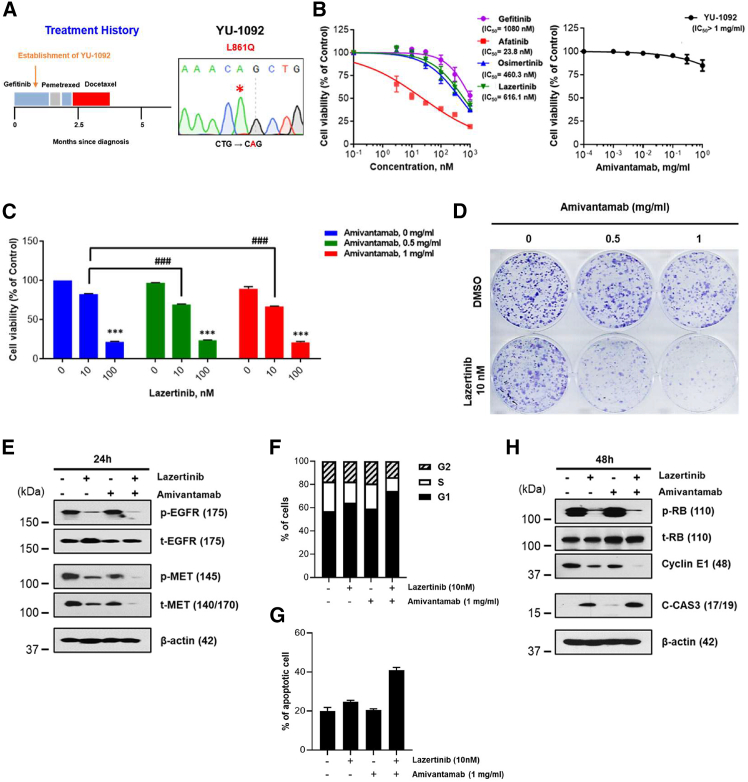
Combination therapy of lazertinib and amivantamab exhibits in vitro activity in patient-derived preclinical models with uncommon EGFR mutations previously treated with EGFR-TKIs
We established two patient-derived cells (PDCs), YU-1092 (EGFRL861Q) and YU-1099 (EGFRG719C/S768I), from patients who experienced disease progression after gefitinib treatment. Additionally, we generated a patient-derived organoid (PDO), YUO-076 (EGFRG719S/S768I) from a patient who had a history of afatinib and erlotinib use but discontinued due to side effects. The detailed clinical annotations and genetic profiles of these models are summarized in Figures 3A, S3, S5A, and S6A. While YU-1092 cells did not respond to monotherapy with any inhibitors except afatinib, a synergistic effect was observed when 10 nM lazertinib and 0.5 mg/mL amivantamab were combined (Figures 3B and 3C). Moreover, long-term combination analysis using colony formation revealed that lazertinib, when combined with an equal concentration of 0.5 mg/mL of amivantamab, displayed improved anti-proliferative efficacy at a concentration of 10 nM (Figure 3D). This combination treatment also showed synergistic inhibitory effects on pEGFR/tEGFR and pMET/tMET compared to each treatment alone (Figure 3E). In contrast to the absence of pMET in YUO-139, the pMET observed in YU-1092 may be induced by a well-known compensatory mechanism between EGFR and MET signaling in response to TKI treatment. Consistent with the cell-cycle arrest observed in YUO-139, the combination of lazertinib and amivantamab in YU-1092 led to a notable increase in the G1 phase population and a decrease in the S phase, which was accompanied by decreased expression of pRB and cyclin E1 (Figures 3F and 3H). Moreover, the combination therapy significantly increased apoptosis compared to either treatment alone. The total apoptosis rate (early and late) was synergistically enhanced with the combination therapy, accompanied by the upregulation of pro-apoptotic proteins such as cleaved caspase-3 (Figures 3G and 3H).
Figure 3.
In vitro activity of lazertinib plus amivantamab in YU-1092 PDC harboring EGFRL861Q
(A) Clinical treatment history of patient harboring EGFRL861Q. YU-1092 cells were established from pleural effusion at the indicated time point (left panel). EGFRL861Q mutation in YU-1092 cells was confirmed by Sanger sequencing (right panel).
(B and C) Cell viability was measured after 3 days of EGFR-TKIs and 5 days of amivantamab and lazertinib plus amivantamab treatment via CellTiter-Glo. The curved graphs represent the cell viability results for a single drug (B), and the bar graphs show the cell viability for the combination of lazertinib and amivantamab (C). Data represent the means ± SE from three and more times of independent experiments. ANOVA with Tukey post hoc test: ∗∗p < 0.01, ∗∗∗p < 0.001 ∗∗∗ vs. lazertinib 0 nM in each group; ###p < 0.001 vs. matched concentrate of lazertinib in amivantamab 0 mg/mL.
(D) Colony formation analysis of YU-1092 cells in response to single or combined treatment with lazertinib and amivantamab. Fresh medium containing the drug at the indicated concentration was replaced every 3 days and treated for 14 days.
(E) YU-1092 cells were treated with lazertinib at 100 nM and amivantamab at 1 mg/mL for 24 h. Expression of pEGFR/tEGFR and pMET/tMET was evaluated through immunoblot assay.
(F and G) YU-1092 cells were treated with lazertinib or/and amivantamab at the indicated concentrations for 72 h. The bar graphs showed the percentage of cells in each phase of the cell cycle (F) and the percentage of apoptotic cells (G).
(H) YU-1092 cells were treated with lazertinib at 100 nM and amivantamab at 1 mg/mL for 48 h. Expression of pRB/tRB, cyclin E1, and cleaved caspase-3 was evaluated through immunoblot assay.
On the other hand, YU-1099 exhibited resistance not only to all EGFR-TKI monotherapies but also to the combination treatment of lazertinib and amivantamab (Figures S5B–S5D). In the case of YUO-076, it showed sensitivity to all EGFR-TKI monotherapies except for amivantamab, but the combination treatment of lazertinib and amivantamab did not exhibit a synergistic effect (Figures S6B and S6C).
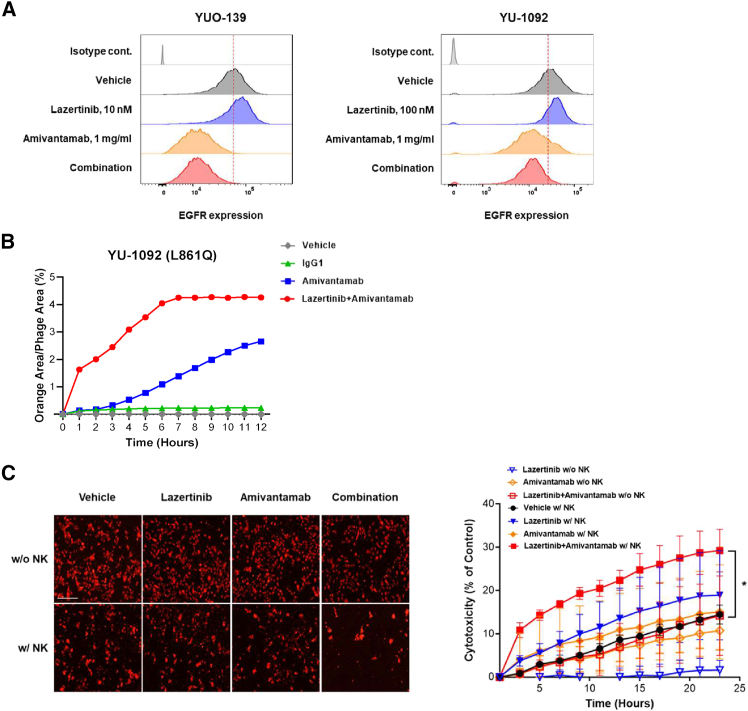
Lazertinib enhances the potential of amivantamab through EGFR upregulation and synergistic antibody-dependent cell-mediated cytotoxicity activity
To explore the mechanisms underlying the synergistic effects of lazertinib and amivantamab, we investigated the intrinsic changes in YUO-139 and YU-1092 following lazertinib and/or amivantamab treatment. We observed that lazertinib treatment led to an upregulation of EGFR surface expression in both models, whereas treatment with amivantamab alone or in combination with lazertinib resulted in reduced EGFR (Figure 4A; Figure S7). Additionally, antibody internalization analysis showed that the combination of lazertinib and amivantamab induced faster and more sustained internalization in YU-1092 compared to amivantamab alone (Figure 4B). This suggests that lazertinib may enhance the inhibitory capacity of amivantamab by increasing the availability of its target on the cancer cell surface.
Figure 4.
Lazertinib enhances the efficacy of amivantamab
(A) Surface expression of EGFR was measured in YUO-139 and YU-1092 cells after 72 h of treatment with lazertinib or/and amivantamab at indicated concentrations.
(B) Real-time measurement of pHrodo internalization as measured by normalized red fluorescent area (%) over 12 h for drug treated YU-1092 cell lines.
(C) The representative image at the 24-h time point shows tumor cells stained with CellTracker Red CMTPX dye (left). ADCC-mediated cytotoxicity was assessed in YU-1092 cells, with or without NK cells treated with lazertinib (10 nM) and/or amivantamab (1 mg/mL) for 24 h (right). Data represent the means ± SE from two times of independent experiments. (ANOVA with Tukey post hoc test: ∗p < 0.05 vs. amivantamab w/NK cell group) The scale bar indicates 250 μm.
Based on our previous report on the antibody-dependent cell-mediated cytotoxicity (ADCC) efficacy of amivantamab,26 next we assessed whether the combination with lazertinib enhances the ADCC activity of amivantamab. First, we determined the optimal effector (ET) ratio for the ADCC activity of amivantamab in uncommon EGFR mutant cells (Figure S8) and then evaluated the ADCC activity of lazertinib and/or amivantamab at an ET ratio with minimal ADCC activity from amivantamab alone. Live-cell imaging revealed that the combination therapy demonstrated significantly enhanced ADCC activity compared to amivantamab monotherapy in a time-dependent manner (Figure 4C). These results suggested that lazertinib treatment induces the actionable targets of amivantamab on the cancer cell surface to magnify the on-target inhibitory potency of amivantamab.
Combination therapy of lazertinib and amivantamab overcomes resistance of uncommon EGFR mutations to various EGFR-TKIs
Next, we assessed the antitumor efficacy of lazertinib plus amivantamab in the YU-1092 xenograft model. As shown in Figures 5A, 5B, and S9, the combination therapy of lazertinib and amivantamab showed the most potent antitumor effect with a TGI value of 158.4%, while the single treatment of gefitinib, afatinib, and lazertinib failed to inhibit tumor growth. IHC analysis also revealed a significant reduction in pEGFR expression with amivantamab and lazertinib plus amivantamab treatments compared to other treatment groups (Figure 5C). Interestingly, while amivantamab monotherapy showed no in vitro activity, it induced an 85.3% TGI value in in vivo assays, delaying tumor growth. This antitumor efficacy observed in vivo may be attributed to the ADCC effect of amivantamab, as mentioned earlier. Therefore, we assessed the tumor infiltration of effector cells, including macrophages and natural killer (NK) cells, which are key components of the ADCC mechanism. As shown in Figure 5D, compared to other treatments, significant infiltration of macrophages and NK cells was observed in both the amivantamab monotherapy and the lazertinib plus amivantamab combination therapy groups. We further confirmed the antitumor efficacy of lazertinib plus amivantamab in the YHIM-1008 (EGFRG719C/S768I) patient-derived xenograft (PDX) model established from patients with disease progression following erlotinib and sequential afatinib treatments (Figures 5E and S3). YHIM-1008 tumors exhibited initial regression with all tested drugs except for amivantamab (Figure 5F). However, rapid tumor regrowth was observed in the osimertinib, lazertinib, and afatinib groups after discontinuation of drug treatment. In contrast, the combination of lazertinib and amivantamab showed delayed tumor growth until the end of the experiment (Figure 5F). Previously, preclinical data suggested that MET amplification/overexpression serves as a bypass resistance mechanism to all EGFR-TKIs27,28,29,30 and that TKI enhances the tumor cell surface receptor and antibody-mediated ADCC.31,32,33 On the basis of these studies, IHC analysis was performed on the expression of surface EGFR/MET in tumor sections of YHIM-1008 (Figure 5G). We found that expression levels of MET increased dramatically in the lazertinib treatment group compared to control groups. These results indicate that amivantamab monotherapy failed to suppress YHIM-1008 tumors due to low basal MET expression, but combination therapy with lazertinib enhanced antitumor efficacy due to increased MET expression caused by lazertinib. In addition, when lazertinib and amivantamab were treated in combination, the penetration of macrophages and NK cells was increased (Figure 5H). Taken together, these results suggest that the combination treatment strategy of lazertinib and amivantamab may be an alternative treatment option for uncommon EGFR-mutated NSCLC patients with prior TKI therapy experience.
Figure 5.
In vivo activity of lazertinib plus amivantamab in patient-derived preclinical models with uncommon EGFR mutations resistant to EGFR-TKIs
(A) Tumor growth curves of YU-1092 xenografts in response to the indicated drugs over a period of 19 days. Data represent the means ± SD (n = 10/group). (Kruskal-Wallis with Dunn post hoc test: ∗∗p < 0.01, ∗∗∗p < 0.001 vs. vehicle; #p < 0.05 vs. afatinib.).
(B) Waterfall plot representing the percentage of tumor volume change in mice after 19 days of treatment with the indicated drugs.
(C) Representative images of IHC staining for pEGFR/tEGFR. The scale bar indicates 50 μm.
(D) Representative images of IHC staining for mF4/80 and mNKp46 (left panel) of YU-1092 tumor sections. The graph represents the quantification of macrophage and NK cell infiltration of tumor microenvironment (right panel). The scale bar indicates 20 μm. Data represent the means ± SD (n = 4/group). (ANOVA with Tukey post hoc test: ∗∗∗p < 0.001 vs. vehicle).
(E) Clinical treatment history of patient harboring EGFRG719S/L861Q. YHIM-1008 PDXs were established from pleural effusion at the indicated time point.
(F) Tumor growth curves of YHIM-1008 xenografts showing response to indicated drugs. Except for amivantamab alone, the remaining treatment groups stopped the drug on day 29 and were further monitored for tumor growth until day 80. Data represent the means ± SD (n = 7/group). (Kruskal-Wallis with Dunn post hoc test: ∗∗∗p < 0.001 vs. vehicle at day 29 of treatment; ###p < 0.001 vs. osimertinib and lazertinib, respectively, at the end of the experiment after drug discontinuation; §p < 0.05 vs. afatinib at the end of the experiment after drug discontinuation.).
(G) Representative images of IHC staining for pEGFR/tEGFR and pMET/tMET. The scale bar indicates 50 μm.
(H) Representative images of IHC staining for mF4/80 and mNKp46 (upper panel) of YHIM-1008 tumor sections from mice sacrificed on day 11. The scale bar indicates 20 μm. The graph represents the quantification of macrophage and NK cell infiltration in the tumor microenvironment (lower panel). Data represent the means ± SD (n = 4/group). (ANOVA with Tukey post hoc test: ∗∗∗p < 0.001 vs. vehicle).
Combination therapy of lazertinib and amivantamab displays clinical activity in a lung cancer patient with uncommon EGFR mutations
In cohort C of the ongoing CHRYSALIS-2 study of lazertinib and amivantamab in patients with advanced NSCLC (NCT04077463) with uncommon EGFR mutations, promising clinical activity has been observed. A 55-year-old female Asian treatment-naive patient harboring the EGFRG719S/S768I mutation achieved a partial response with a 45% tumor reduction (Figure 6A). Primary lung cancer in the left lower lobe and bilateral lung metastases showed marked regression following 3 months of treatment. This patient is progression free for 16 months with lazertinib and amivantamab without significant adverse events, and the response is ongoing. Additionally, a 67-year-old male Asian patient with the EGFRL861Q mutation, who had previously received afatinib treatment, also demonstrated clinical benefit from the combination of lazertinib and amivantamab. This patient experienced a 38% tumor reduction, with shrinkage observed in the metastatic paratracheal lymph node and bilateral lung metastases after 3 months of treatment (Figure 6B). The patient was progression free for 18 months, further highlighting the durable clinical benefit of this combination therapy in patients with uncommon EGFR mutations.
Figure 6.
Clinical activity of lazertinib plus amivantamab in NSCLC patients harboring uncommon EGFR mutations
(A and B) Radiologic response following 240 mg lazertinib and 1,050 mg amivantamab treatment in a 55-year-old patient with the EGFRG719S/S768I mutation (A) and a 67-year-old patient with the EGFRL861Q mutation (B). Arrows indicate the tumor lesion.
High expression of EGFR/MET is associated with responsiveness to the combination of lazertinib and amivantamab
Recent studies have reported that high EGFR and/or MET expression may serve as a potential predictive biomarker for the combination therapy of amivantamab and lazertinib in patients with osimertinib-relapsed, EGFR-mutant advanced NSCLC.34 Based on this, we investigated the correlation between basal EGFR and/or MET expression and the efficacy of the combination therapy in in vitro models. Considering the sample types, we first distinguished between PDC and PDO models and compared EGFR and MET surface expression between the responsive and non-responsive groups against lazertinib plus amivantamab. As shown in Figure 7A, the mean fluorescence intensity (MFI) values for EGFR and MET were 902 vs. 260 and 49 vs. 16 in responder YUO-139 compared to non-responder YUO-076, respectively. Similarly, in responder YU-1092 versus non-responder YU-1099, the values were 66,700 vs. 11,542 and 185 vs. 117, respectively. Although we were unable to define a specific threshold, these findings suggest that higher EGFR and/or MET expression may be associated with better responses to the combination therapy. Indeed, in the non-responder YU-1099, amivantamab monotherapy showed minimal internalization, suggesting the importance of adequate target expression for amivantamab activity (Figure S10). Furthermore, IHC analysis of pre-treatment tumor tissue from a 55-year-old patient who responded to the combination therapy showed an H-score of 236 for EGFR and 195 for MET, indicating high levels of EGFR and MET expression, which may have contributed to the observed clinical response (Figure 7B). Unfortunately, pre-treatment tissue from the second patient was not available for analysis. Collectively, these results provide a rationale for further exploration of these biomarkers in larger clinical cohorts.
Figure 7.
High expression of EGFR/MET is associated with responsiveness to the combination of lazertinib and amivantamab
(A) Surface expression of EGFR and MET was evaluated through flow cytometry analysis. Bar graphs represent the MFI of MET, and linear graphs represent the MFI of EGFR. Left panel is group of PDO, and right panel is PDC group.
(B) Representative images of IHC staining for tEGFR and tMET of treatment-naive patients with EGFRG719S/S768I mutation tumor sections. The scale bar indicates 20 μm. The graph represents the pathological visual score of EGFR and MET in tumor tissue. Scores were assigned as follows: +0 (no expression), +1 (low expression), +2 (moderate expression), and +3 (high expression).
Discussion
We demonstrated the potent antitumor activity of the lazertinib and amivantamab combination in engineered Ba/F3 cell lines and patient-derived preclinical models harboring uncommon EGFR mutations. Notably, the combination therapy exhibited superior in vivo efficacy compared to afatinib, both in treatment-naive models and those that had progressed on EGFR-TKIs. These preclinical findings were further supported by the promising clinical responses observed in patients with uncommon EGFR mutations enrolled in the ongoing CHRYSALIS-2 study.
Data on the efficacy of EGFR-TKIs in NSCLC patients with uncommon EGFR mutations remain limited. First-generation EGFR-TKIs have demonstrated lower response rates and shorter progression-free survival (PFS) compared to those in patients with common mutations.35,36 In contrast, the second-generation EGFR-TKI afatinib showed efficacy in patients with uncommon mutations (G719X, S768I, and L861Q), with a median PFS (mPFS) of 10.7 months and an overall response rate (ORR) of 71.1% in a pooled analysis of the LUX-Lung 2, 3, and 6 trials, leading to its FDA and European Medicines Agency (EMA) approval.13,14 The ACHILLES/TORG1834 trial provided the first randomized evidence that afatinib achieved superior PFS compared to platinum-based chemotherapy.16,17 Similarly, osimertinib, a third-generation EGFR-TKI, showed an ORR of 55% and an mPFS of 9.4 months in the UNICORN phase 2 trial,37 with comparable results in other studies.18,38 However, due to the heterogeneity of uncommon EGFR mutations, uncertainty remains regarding the optimal treatment strategy for these patients. Studies show that the efficacy of both afatinib and osimertinib varies depending on the mutation subtype, and the presence of compound mutations further complicates treatment approaches.11,12,37 Thus, the need for alternative treatment strategies for this diverse patient population is highlighted.
Recent results from cohort C of the CHRYSALIS-2 study (NCT04077463) demonstrated promising clinical efficacy of lazertinib plus amivantamab, with an ORR of 51% in patients with uncommon EGFR mutations, excluding ex20ins.21 Notably, treatment-naive patients showed an ORR of 55% and an mPFS of 19.5 months. We provided the first evidence of clinically meaningful and durable antitumor activity of the combination in two cases within this cohort. Interim data from a phase 2 trial of lazertinib monotherapy (ORR 44.1%, PFS 7.69 months)39 suggest that the combination is more favorable. However, the CHRYSALIS-2 Cohort C results were derived from a non-randomized, single-arm trial without a control group, limiting the ability to draw definitive conclusions regarding the comparative efficacy of the combination therapy. Additionally, caution is needed when interpreting comparisons between different cohorts due to potential differences in patient populations and treatment histories. In this context, our preclinical study provides compelling evidence that the combination of lazertinib and amivantamab exhibits superior antitumor activity compared to monotherapies with afatinib, lazertinib, or amivantamab in various preclinical models derived from treatment-naive patients or those with disease progression following EGFR-TKI therapy (Figures 2, 3, and 5). Mechanistically, the combined approach of lazertinib and amivantamab synergistically induced cell-cycle arrest and apoptosis compared to either monotherapy, in addition to suppressing EGFR and MET signaling. Interestingly, lazertinib monotherapy upregulated surface expression of EGFR or MET, depending on the tumor model, which was strongly suppressed by combination with amivantamab. Furthermore, the combination enhanced amivantamab internalization and ADCC activity compared to amivantamab alone. These results suggest that lazertinib increases the expression of actionable targets for amivantamab, thereby enhancing its antitumor efficacy. This is consistent with other studies showing that TKIs can elevate surface receptor expression, improving antibody-mediated ADCC in tumor cells.31,32,33 Consistent with the findings from Cho et al.,34 our study found significantly higher MFI values for EGFR and MET in responder samples (YUO-139 and YU-1092) compared to non-responders (YUO-076 and YU-1099). High EGFR/MET expression was also observed in pre-treatment tumor tissues from treatment-naive patients who later showed promising clinical responses to the combination therapy. These findings suggest that, unlike afatinib or osimertinib, whose efficacy is difficult to predict depending on mutation subtypes, lazertinib plus amivantamab may offer a more reliable, biomarker-driven approach to identifying responders. However, our preclinical findings are based on a limited number of models, and the extent to which these results generalize to a broader spectrum of uncommon EGFR mutations remains uncertain. Further investigation is needed to determine the efficacy of the combination across diverse mutation subtypes and to elucidate whether EGFR/MET expression universally serves as a predictive biomarker for response. Moreover, while the combination has shown efficacy and safety as a first-line treatment for EGFR-mutant NSCLC,20 its long-term safety and durability require confirmation in larger, multi-cohort clinical trials.
In summary, this study represents the first preclinical study demonstrating the potential of lazertinib plus amivantamab as a novel therapeutic option for uncommon EGFR mutations, either in treatment-naive patients or in those with disease progression after afatinib therapy. Our preclinical findings strongly support the results observed in cohort C of the CHRYSALIS-2 study, addressing the limitations of single-cohort study and warranting further exploration in a larger cohort. A phase 2 clinical trial of the combination therapy is currently in the planning stages, which will be crucial in providing robust evidence of its efficacy and safety in a broader patient population, including a randomized controlled trial comparing the combination therapy to the current standard of care, afatinib.
Limitations of the study
This study demonstrates the antitumor efficacy of the combination of lazertinib and amivantamab in NSCLC with uncommon EGFR mutations through diverse mechanisms, including EGFR downstream signaling inhibition, cell-cycle arrest, apoptosis, and enhanced ADCC activity. However, there are several limitations to consider. First, the preclinical studies were conducted using a limited set of model systems. Consequently, the generalizability of the efficacy of combination therapy to a broader range of uncommon and personalized EGFR mutations remains uncertain. Although EGFR/MET expression has been suggested to be associated with responsiveness to the combination, this finding may be mutation specific. Experimental evidence assessing the efficacy of the combination across diverse mutation patterns is lacking. Additionally, the clinical evidence presented in this study relies on a small cohort of patients. Larger and more comprehensive clinical trials are necessary to confirm the observed efficacy and validate the findings across a more diverse patient population. These limitations highlight the need for future research to expand the scope of preclinical models, investigate additional mutation patterns, and evaluate the combination therapy’s safety and efficacy in diverse and larger patient populations.
Resource availability
Lead contact
Further information and requests for resource and reagent should be directed to the lead contact, Mi Ran Yun (fortune@yuhs.ac).
Materials availability
PDCs, PDOs, and PDXs generated in this study will be made available from the lead contact upon reasonable request and subject to a material transfer agreement. Distribution may require payment for processing and shipping.
Data and code availability
-
•
The whole-exome sequencing data have been deposited in the Sequence Read Archive (SRA) under the following project accession numbers: PRJNA1095562 and PRJNA1095016. The individual sequencing runs are available under the following SRR accession number: SRR28543204, SRR28520122, SRR28543206, SRR28543205, and SRR28543203. These data are publicly available.
-
•
The code used for generating OncoPrint plot data has been deposited at Zenodo (https://doi.org/10.5281/zenodo.14435819) and is publicly available as of the date of publication. For further details or inquiries regarding the code, please contact the lead contact.
-
•
Additional information required to reanalyze the data presented in this paper can be provided by the lead contact upon request.
Acknowledgments
We thank all the patients who donated samples for this study. This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (no. 2022R1A2C3005817 to B.C.C.), the Dongin Sports research grant of Yonsei University College of Medicine (6-2018-0115 to B.C.C.), the NRF grant funded by the Korean government (no. 2018R1D1A1B07050233 to M.R.Y.), and a faculty research grant of Yonsei University College of Medicine (6-2018-0158 to M.H.H.). We also express our gratitude to the Heo Ji-Young Scholarship Foundation for their financial support.
Author contributions
S.Y.O. and S.P. performed all experiments, collected and analyzed the data, and contributed to writing the manuscript. S.L. participated in the patient case study and manuscript writing. E.J.L. established the Ba/F3 cell line. T.H.K. participated in all animal experiments. S.-J.C. conducted all whole-exome sequencing analyses. S.P. and J.H.K. performed all immunohistochemistry analyses. S.M.L., J.B.L., and B.C.C. discussed the data and reviewed the manuscript. M.H.H. and M.R.Y. conceptualized the project, designed the experiments, supervised data collection and analysis, and wrote the manuscript.
Declaration of interests
B.C.C. declares the following competing interests: royalties from Champions Oncology, Crown Bioscience, Imagen, and PearlRiver Bio GmbH under licensing contracts for PDX, PDO, and PDC (not patent-related); research funding from GI Innovation, AstraZeneca, Champions Oncology, CJ Bioscience, Cyrus Therapeutics, Janssen, MSD, Dong-A ST, Yuhan, ImmuneOncia, Therapex Co., J INTS Bio, and Vertical Bio AG; consulting roles with BeiGene, Novartis, AstraZeneca, Boehringer Ingelheim, Roche, Bristol Myers Squibb (BMS), CJ Bioscience, Cyrus Therapeutics, Ono Pharmaceutical, Yuhan, Pfizer, Eli Lilly, Janssen, Takeda, MSD, Gilead, Amgen, Daiichi Sankyo, Regeneron, Sanofi, AnHeart Therapeutics, Seagen, Harpoon Therapeutics, GSK, and ArriVent; employment with Yonsei University Health System; advisory board memberships at KANAPH Therapeutics Inc., BridgeBio Therapeutics, Cyrus Therapeutics, Guardant Health, J INTS Bio, and Therapex Co., Ltd; invited speaker engagements for ASCO, AstraZeneca, Guardant, Roche, ESMO, IASLC, Korean Cancer Association, Korean Society of Medical Oncology, Korean Society of Thyroid-Head and Neck Surgery, Korean Cancer Study Group, Novartis, MSD, The Chinese Thoracic Oncology Society, Pfizer, and Zailab; stock holdings in TheraCanVac Inc., Gencurix Inc., BridgeBio Therapeutics, KANAPH Therapeutics Inc., Cyrus Therapeutics, Interpark Bio Convergence Corp., and J INTS Bio; and roles as founder of DAAN Biotherapeutics and board member of J INTS Bio.
M.H.H. declares the following competing interests: honoraria from AstraZeneca, Amgen, BMS, MSD, Ono Pharmaceutical, Takeda, and Roche; consulting or advisory roles for AstraZeneca, BMS, MSD, Pfizer, Takeda, Roche, and Yuhan; investigator or co-investigator roles in clinical trials for AbbVie, AstraZeneca, BMS, IMPACT Therapeutics, Ignyta, Loxo Oncology, Merck Serono, MSD, Novartis, ORIC, Roche, Pfizer, and Yuhan; and research support from AstraZeneca, MSD, Novartis, and Yuhan.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-Phospho-EGFR (Tyr1068) | Cell Signaling Technology | Cat#2234 RRID:AB_331701 |
| Rabbit monoclonal anti EGFR | Cell Signaling Technology | Cat #4267 RRID:AB_2895042 |
| Rabbit monoclonal anti-Phospho-Met (Tyr1234/1235) | Cell Signaling Technology | Cat #3077 RRID:AB_2143884 |
| Rabbit monoclonal anti Met | Cell Signaling Technology | Cat #8198 RRID:AB_10858224 |
| Rabbit monoclonal anti-Phospho-Rb (Ser807/811) | Cell Signaling Technology | Cat #8516 RRID:AB_11178658 |
| Mouse monoclonal anti RB | Cell Signaling Technology | Cat #9309 RRID:AB_823629 |
| Rabbit monoclonal anti-Cyclin E1 | Cell Signaling Technology | Cat #20808 RRID:AB_2783554 |
| Rabbit monoclonal anti p21 | Cell Signaling Technology | Cat #2947 RRID:AB_823586 |
| Rabbit polyclonal anti-Cleaved caspase-3 | Cell Signaling Technology | Cat #9661 RRID:AB_2341188 |
| Mouse monoclonal anti-β-actin-Peroxidase | Sigma-Aldrich | Cat A3854 RRID:AB_262011 |
| Rabbit monoclonal anti EGFR | Abcam | Cat ab52894 RRID:AB_869579 |
| Rabbit monoclonal anti F4/80 | Cell Signaling Technology | Cat #70076 RRID:AB_2799771 |
| Goat polyclonal anti-NKp46/NCR1 | R&D System | Cat AF2225 RRID:AB_355192 |
| FITC anti human c-MET monoclonal antibody | Invitrogen | Cat #11-858-42 |
| APC anti human EGFR antibody |
BioLegend | Cat #352906 RRID:AB_11150410 |
| FITC Annexin V Apoptosis Detection Kit 1 |
BD Biosciences | Cat #556547 RRID:AB_2869082 |
| Rabbit anti-TTF1 monoclonal antibody (EP1584Y) | Abcam | Cat. ab76013 RRID:AB_1310784 |
| p53 antibody | Leica Biosystems | Cat #NCL-p53-CM5p RRID:AB_563933 |
| Horseradish Peroxidase (HPR) | DaKo (Agilent Technologies | Cat K4003 |
| Boost IHC Detection reagent (HPR, Rabbit) | Cell Signaling Technology | Cat 8114 |
| Monoclonal mouse anti-human Calretinib (concentrate), clone DAK-Calret 1 |
Dako (Agilent Technologies | Cat GA777 |
| Biological samples | ||
| Treatment naive EGFR G719S/S768I tumor |
Yonsei University Severance Hospital |
IRB no. 4-2016-0788 |
| Previously treated with afatinib EGFR L861Q tumor |
Yonsei University Severance Hospital |
IRB no. 4-2016-0788 |
| Chemicals, peptides, and recombinant proteins | ||
| CellTiter-Glo 2.0 | Promega | Cat G9243 |
| Gefitinib | Selleck-Chemicals | Cat S1025 |
| Afatinib | Selleck-Chemicals | Cat S1011 |
| Osimertinib | Selleck-Chemicals | Cat S7297 |
| Lazertinib | Selleck-Chemicals | Cat S8724 |
| Amivantamab | Janssen Pharmaceuticals | N/A |
| IgG1 controls | Janssen Pharmaceuticals | N/A |
| Matrigel | Corning | Cat 356231 |
| Dispase | Invitrogen | Cat 17105041 |
| Glutamax™ (100X) | Gibco | Cat 35050-061 |
| Advanced DMEM/F12 | Gibco | Cat 12634-010 |
| RPMI medium | Cytiva | Cat SH30027.01 |
| Trypsin-EDTA (0.05%), phenol red | Cytiva | Cat SH30042.01 |
| N-acetylcysteine amide | Sigma-Aldrich | Cat A9165 |
| B27 supplement | Gibco | Cat 17504001 |
| R-Spondin1 | GBCC | Cat GBC-hR1 |
| Noggin | GBCC | Cat GBC-hNG |
| FGF7 | GBCC | Cat GBC-hF7 |
| FGF10 | GBCC | Cat GBC-hF10 |
| HEPES | Gibco | Cat 15630-080 |
| Antibiotic-Antimycotic | Gibco | Cat 15240096 |
| FabFluor-pH Orange | Sartorius | Cat 4812 |
| Propidium Iodide (P4170) | Sigma-Aldrich | Cat P4170 |
| Y-27632 | Sigma-Aldrich | Cat SCM075 |
| Nicotinamide | Sigma-Aldrich | Cat N0636 |
| SB202190 | Sigma-Aldrich | Cat S7067 |
| A83-01 | Tocris | Cat 2939 |
| Crystal violet solution | Sigma-Aldrich | Cat V5265 |
| SuperSignal West Pico PLUS chemiluminescent Substrate | Thermo Fisher Scientific | Cat 34578 |
| Bio-Rad Protein Assay Dye Reagent Concentrate |
Bio-Rad | Cat 5000006 |
| Primocin | Invitrogen | Cat ant-pm |
| puromycin | Gibco | Cat A1113803 |
| IL3 | Gibco | Cat #213-13 |
| Cell Titer Glo-3D | Promega | Cat G9681 |
| Critical commercial assays | ||
| Myco-ReadTM Mycoplasma Detection Kit |
Biomax | Cat SM0172 |
| Deposited data | ||
| Raw data of whole-exome sequencing of YHIM-1008 | Sequenced Read Acrchive | PRJNA1095562, SRR28543203 |
| Raw data of whole-exome sequencing of YU-1099 | Sequenced Read Acrchive | PRJNA1095562, SRR28543206 |
| Raw data of whole-exome sequencing of YUO-076 | Sequenced Read Acrchive | PRJNA1095562, SRR28543205 |
| Raw data of whole-exome sequencing of YUO-139 | Sequenced Read Acrchive | PRJNA1095562, SRR28543204 |
| Raw data of whole-exome sequencing of YU-1092 | Sequenced Read Acrchive | PRJNA1095016, SRR28520122 |
| Experimental models: Cell lines | ||
| Ba/F3 (hEGFR L861Q) | Crown Bioscience Inc. | RRID: CVCL_UA18 |
| Ba/F3 | Dana-Farber Cancer Institute |
RRID: CVCL_0161 |
| Ba/F3 (hEGFR) | This paper | N/A |
| Ba/F3 (hEGFR G719S) | This paper | N/A |
| Ba/F3 (hEGFR S768I) | This paper | N/A |
| Ba/F3 (hEGFR G719A/S768I) | This paper | N/A |
| Ba/F3 (hEGFR S768I/L858R) | This paper | N/A |
| Ba/F3 (hEGFR G719S/S768I) | This paper | N/A |
| YU-1092 | This paper | N/A |
| YU-1099 | This paper | N/A |
| YUO-076 | This paper | N/A |
| YUO-139 | This paper | N/A |
| YHIM-1008 | This paper | N/A |
| Experimental models: Organisms/strains | ||
| Mouse: Nu/nu mice | Orient bio | RRID:IMSR_CRL:088 |
| Recombinant DNA | ||
| pLVX-puro | Addgene | RRID:Addgene_125939 |
| pLVX-puro-hEGFR | This paper | N/A |
| pLVX-puro-hEGFR G719S | This paper | N/A |
| pLVX-puro-hEGFR S768I | This paper | N/A |
| pLVX-puro-hEGFR G719A/S768I | This paper | N/A |
| pLVX-puro-hEGFR L861Q | This paper | N/A |
| pLVX-puro-hEGFR S768I/L858R | This paper | N/A |
| pLVX-puro-hEGFR G719S/S768I | This paper | N/A |
| Software and algorithms | ||
| FlowJo v10.8.1 | BD Biosciences | RRID:SCR_008520 |
| GraphPad Prism v8.0.2 | GraphPad Software LLC | RRID:SCR_002798 |
| IncuCyte 2020B | Sartorius | https://www.satorius.com |
| Phenochart v1.1.0 | PerkinElmer | RRID:SCR_019156 |
| Burrows-Wheeler Aligner (BWA) | BWA GitHub | RRID:SCR_010910 |
| Genome Analysis Toolkit (GATK) | Broad Institute | RRID:SCR_001876 |
| Mutect2 | Broad Institute | Included in GATK |
| Oncotator | Oncotator GitHub | RRID:SCR_005183 |
| CNVkit | CNVkit GitHub | RRID:SCR_015821 |
| Oncoprint plot of patient-derived models | This paper | https://doi.org/10.5281/zenodo.14435819 |
| Other | ||
| BD FACS Verse I | BD Biosciences | https://www.bdbiosiences.com |
| IncuCyte SX5 Live-Cell Analysis System |
Sartorius | https://www.satorius.com |
| Phenoimager HT | Akoya Biosciences | https://www.akoyabio.com |
| Illumina HiSeq 2500 platform | Illumina | https://www.illumina.com |
| Illumina NovaSeq 6000 platform | Illumina | https://www.illumina.com |
Experimental model and study participant details
Ba/F3 cell lines
Ba/F3 cells harboring human EGFR (hEGFR) L861Q were purchased from Crown Bioscience Inc. The other Ba/F3 cells tested were transduced with lentiviral particles containing human wild-type or mutated EGFR sequences in pLVX-puro vector. The transduced Ba/F3 cells were selected with 1 μg/mL of puromycin. Cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics, in a 37°C incubator with 5% CO2. Cells were replaced with new medium every two days and passaged once or twice a week depending on the growth rate of the cell lines. The EGFR wild-type Ba/F3 cell lines were cultured in growth medium supplemented with IL3 (1 ng/mL) and puromycin (0.5 μg/mL). All cell lines were regularly tested for Mycoplasma contamination using the Myco-ReadTM Mycoplasma Detection Kit (Biomax, Korea) and confirmed to be negative.
Patient-derived cells
Patient-derived cells (PDCs, YU-1092 and YU-1099) were also derived from EGFR-mutant NSCLC patients under the same protocol approved by the institutional Review Board of Severance Hospital (IRB no. 4-2016-0788) with written informed consent obtained from all participants. PDCs were maintained and passaged using the same the Ba/F3 cell culture methods.
Patient-derived organoids
Patient-derived organoids (PDOs, YUO-076 and YUO-139) obtained from EGFR-mutant NSCLC patients at Yonsei University Severance Hospital (Seoul, Republic of Korea). The study was conducted in accordance with guidelines approved by the institutional Review Board of Severance Hospital (IRB no. 4-2016-0788) and all patients have provided written informed consent. For passaging, organoids were collected, mechanically sheared with needle, and washed with cold PBS. A pellet was resuspended in the cold Matrigel and seeded in 24-well plates at ratios of 1:2 or 1:4.
Mouse model
All experiments involving mice were conducted in accordance with protocols approved by the institutional Animal Care and Use Committee (IACUC) and Animal Research Committee at Yonsei University College of Medicine (approval number: 2022-0180). Female 6-week-old nu/nu mice were used for all experiments. Mouse cages were limited to maximum of 5 animals per cage and checked daily for cage cleanliness and sufficient food/water. The housing environment was maintained under controlled conditions with 100% HEPA-filtered air and a minimum of 10–15 air changes per hour. The temperature and relative humidity were kept at 22 ± 2°C and 55% ± 5%, respectively. A 12-h light/dark cycle was provided, with lights on from 08:00 to 20:00, and the light intensity was maintained between 150 and 300 lux. Noise levels were controlled under 50 phon, and ammonia concentrations were maintained below 20 ppm.
Patients
The clinical study (NCT04077463) was approved by the ethical review boards of all participating institutions and conducted in accordance with Good Clinical Practice guidelines and the ethical principles outlined in the Declaration of Helsinki. All patient with EGFR-mutant NSCLC were approved by the institutional Review Board of Severance Hospital (IRB no. 4-2020-0758). The first patient is a 55-year-old Asian female, treatment-naïve, diagnosed with stage IV NSCLC and harboring an EGFR G719S/S768I mutation. The second patient is a 67-year-old male of Asian ethnicity, previously treated with afatinib, diagnosed with stage IV NSCLC and harboring an EGFR L861Q mutation.
Method details
Establishment of patient-derived cells
Patient-derived cells (PDCs, YU-1092 and YU-1099) were established from malignant effusions of patients, as described previously.40 Briefly, malignant effusion samples were centrifuged at 500 g for 5 min before cell pellets were suspended in HBSS. The cells were then separated by density gradient centrifugation using Ficoll-Paque PLUS (GE Healthcare Bio-Sciences, Uppsala, Sweden). Isolated cells were washed and seeded with RPMI 1640 medium, which contains 10% of fetal bovine serum and 1X antibiotic-antimycotic. Flow cytometric staining of EpCAM confirmed PDCs with over 99% cancer cell purity.
Establishment of patient-derived organoids
Patient-derived organoids (PDOs, YUO-076 and YUO-139) were established from malignant effusions of patients as described previously.41 In brief, malignant effusion samples were centrifuged at 500 g for 5 min to collect cell pellet. Red blood cells in the cell pellet were removed through hypotonic lysis using sterile MilliQ H2O (Merck Millipore), followed by the addition of Advanced DMEM/F12 medium (Gibco). The solution was then filtered through a 100 μm strainer to remove debris and centrifuged at 500 × g for 5 min. Filtered cells were suspended into cold Matrigel and then seeded into 24-well culture plates (Corning). After 20 min incubation at 37°C, add to solidified Matrigel cells with Complete organoid medium [DMEM/F12 medium, and supplemented with 20% conditioned R-spondin1 medium, 1X antibiotic-antimycotic, and 10 mM HEPES, 1X GlutaMax] in a 37°C incubator with 5% CO2. IHC tumor purity of the organoids was confirmed by positive staining for Calretinin, Thyroid Transcription Factor 1 (TTF-1), and p53.
Establishment of patient-derived xenograft
Patient-derived xenograft (PDX) model YHIM-1008 was established as previously described.42 To generate YU-1092, YUO-139 PDC and PDO-derived tumor xenograft models, cells (5 × 106 in 100 μL) were implanted subcutaneously into the flanks of 6-week-old female nu/nu mice. Once the tumor size reached 150-200mm2, the mice were randomly allocated and assigned to the respective treatment groups as follows: vehicle, osimertinib (25 mg/kg, oral daily), lazertinib (10 mg/kg, oral daily), afatinib (7.5 mg/kg, oral daily), gefitinib (30 mg/kg, oral daily), amivantamab (10 mg/kg, subcutaneous (SC), twice per week), and combination with either lazertinib or amivantamab. The tumor size was measured with an electronic caliper. And calculated according to the equation for a prolate spheroid: tumor volume = 0.532 × (large diameter) × (small diameter).2 Body weight of tumor bearing mice was monitored every other of the experiment.
Whole-exome sequencing (WES)
Genomic DNA purity and concentration were assessed through PicoGreen dsDNA assay (Invitrogen) and agarose gel electrophoresis. The genomic fragment library was constructed using the SureSelect v6 kit (Agilent Technologies) and subsequently subjected to sequencing on the Illumina HiSeq 2500 platform and NovaSeq6000, employing targeted sequencing to capture 171 cancer-related genes. The resulting reads were aligned to the human genome reference (hg19) using Burrows-Wheeler Alignment (BWA, RRID:SCR_010910). Followed by analysis with the Genome Analysis Toolkit. Somatic mutations were identified using Mutect2 and annotated using Oncotator (RRID:SCR_005183). Copy number variation (CNV) was performed using CNVkit (RRID:SCR_015821), with copy number (CN) > 5 as amplification and CN < 1 as deletion.
Cell viability assay
Cells were plated onto 96-well plates at a density of 2×103 cells per well. After allowing the cell to attach, they were exposed to different drug concentrations for 72 or 120 h. Cells viability was measured using Cell Titer Glo (Promega) following the provided protocol. For organoids, Dispase treatment was performed for 30 min, followed by pellet collection. The pellets were then washed with organoid medium devoid of 20% conditioned R-spondin1 medium and filtered through a strainer (pluriSelect). Organoids ranging in size from 20 to 70 μm were seeded with 5% Matrigel onto 96-well ULA (Ultra-Low Attachment) plates at a density of 2×103 organoids per well. After allowing them to attach, the organoids were exposed to various drug concentrations for the indicated times. Cell viability was assessed using Cell Titer Glo-3D (Promega) as per the manufacturer’s instructions. Survival graphs were generated using Prism 8 software.
Colony formation assays
For colony formation assays, cells were seeded at a density of 3X103 cells per well on 6-well culture plates. Cells were incubated overnight and exposed to the indicated drugs for 14 days. The medium containing drugs was refreshed every 3 days. The cells were fixed with 4% paraformaldehyde (PFA) and stained with crystal violet solution (Sigma).
Immunoblot analysis
Samples were subjected to centrifugation at 1300 rpm for 20 min at 4°C. The resulting supernatants (cytosolic fraction) were transferred to new tubes. Protein concentration was determined using a Bradford assay (Bio-rad). Equal amounts of protein were then separated via SDS-PAGE and transferred onto a nitrocellulose membrane. Immunoblots were visualized using SuperSignal West Pico Chemiluminescent substrate (Thermo Fisher Scientific).
Immunohistochemistry
Immunohistochemistry (IHC) was carried out on 4-μm-thick formalin-fixed, paraffin-embedded (FFPE) tissue sections. The slides were subjected to baking, deparaffinization in xylenen, and a series of graded alcohol washes. Antigen retrieval was performed by exposing the slides to 1 mM EDTA at pH8.0 for 30 s at 125°C. subsequently the slides were pretreated with Peroxidase Block (Dako, USA) for 5 min and then rinsed with 50 nM Tris-CI at pH7.4. Slides were blocked using normal goat serum (Dako, USA) and subsequently incubated for 1 h with primary antibody. Following this, the slides were washed with 50 mM Tris-Ci at pH7.4 and exposed to the Signal stain boost IHC detection reagent for 30 min. After further washing, immunoperoxidase staining was developed using a 3,30-diaminobenzidine (DAB) chromogen (Dako, USA) for 5 min. Slides were then counterstained with hematoxyline, dehydrated through graded alcohol and xylene, mounted, and covered with slips.
Flow cytometry
For cell cycle analysis, cells were resuspended in 1.5 mL of PBS and then treated with 3.5 mL of ice-cold ethanol for fixation. After fixation and permeabilization for at least 1 h at 4°C, the cells were washed, resuspended in 100 μg/mL of RNAse A, and stained with propidium iodide (Sigma) at a final concentration of 50 μg/mL. Cells were then filtered through a strainer to remove clumps.
For apoptosis analysis, cells ware treated with lazertinib, amivantamab, or lazertinib plus amivantamab for 72 h. Apoptosis levels were assessed using the FITC Annexin V Apoptosis Detection Kit 1 according to the manufacturer’s protocol.
To measure the expression of MET and EGFR on the cell surface, we used FITC anti human c-MET fluorescence and APC anti-human EGFR fluorescence, respectively.
These measurements were performed using the BD FACS Verse equipment and subsequently analyzed with the Flow Jo 10 software.
ADCC assays
The ADCC assay was conducted using the IncuCyte Live-Cell Analysis System (Sartorius, Germany). Human Peripheral Blood Mononuclear Cells (PBMCs) were isolated from healthy donors and used for the extraction of NK (natural killer) cells as effector cells. Cancer cells were labeled with CellTracker Red CMTPX dye and co-cultured with NK cells, at an E:T (effector-to-target) ratio ranging from 4:1 to 16:1. Imaging was conducted hourly for 24 h to monitor real-time cellular interaction and cytotoxic effects. All procedures were performed following the manufacturer’s instructions.
Antibody internalization assay
Cells were plated onto 96-well plates (2X104cells/well) and attached for 24h in incubation at 37°C. Mix antibody and FabFluor-pH orange antibody labeling dye at a molecular ratio of 1:3 in media and incubated for 15 min to allow conjugation. Add antibody-FabFluor mixture to cell plate and scanned for orange fluorescence. This was done 20X magnification every 15 min for up to 12 h. Images were analyzed using the integrated, automated incucyte software for the following metrics phase confluence and orange fluorescence object area.
Quantification and statistical analysis
Statistical analyses were performed using GraphPad Prism software (version 8). Cell line experiments were independently repeated at least 3 times, with technical triplicate in each condition. The data are expressed as the mean ± standard error of the mean (SEM). In animal studies, mice were grouped randomly once a tumor reached a size of approximately 200 mm3. The number of mice per group was 7–10. The data is presented as the means ± standard deviation (SD). Between-group differences were assessed using the Kruskal-Wallis test with Dunn’s post-hoc analysis or one-way ANOVA with Tukey’s post-hoc test, as applicable. A significance level of p < 0.05 was considered.
Additional resources
This clinical trials has been registered on https://clinicaltrials.gov (NCT04077463).
Published: January 27, 2025
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2025.101929.
Contributor Information
Min Hee Hong, Email: minhee_hong@yuhs.ac.
Mi Ran Yun, Email: fortune@yuhs.ac.
Supplemental information
References
- 1.Kris M.G., Johnson B.E., Berry L.D., Kwiatkowski D.J., Iafrate A.J., Wistuba I.I., Varella-Garcia M., Franklin W.A., Aronson S.L., Su P.F., et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Travis W.D., Brambilla E., Nicholson A.G., Yatabe Y., Austin J.H.M., Beasley M.B., Chirieac L.R., Dacic S., Duhig E., Flieder D.B., et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J. Thorac. Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 3.Harrison P.T., Vyse S., Huang P.H. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Semin. Cancer Biol. 2020;61:167–179. doi: 10.1016/j.semcancer.2019.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y.L., Cheng Y., Zhou X., Lee K.H., Nakagawa K., Niho S., Tsuji F., Linke R., Rosell R., Corral J., et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:1454–1466. doi: 10.1016/S1470-2045(17)30608-3. [DOI] [PubMed] [Google Scholar]
- 5.Soria J.C., Ohe Y., Vansteenkiste J., Reungwetwattana T., Chewaskulyong B., Lee K.H., Dechaphunkul A., Imamura F., Nogami N., Kurata T., et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 6.Mok T.S., Wu Y.L., Thongprasert S., Yang C.H., Chu D.T., Saijo N., Sunpaweravong P., Han B., Margono B., Ichinose Y., et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 7.Rosell R., Carcereny E., Gervais R., Vergnenegre A., Massuti B., Felip E., Palmero R., Garcia-Gomez R., Pallares C., Sanchez J.M., et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 8.Cheng Y., He Y., Li W., Zhang H.L., Zhou Q., Wang B., Liu C., Walding A., Saggese M., Huang X., et al. Osimertinib Versus Comparator EGFR TKI as First-Line Treatment for EGFR-Mutated Advanced NSCLC: FLAURA China, A Randomized Study. Target. Oncol. 2021;16:165–176. doi: 10.1007/s11523-021-00794-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramalingam S.S., Yang J.C.H., Lee C.K., Kurata T., Kim D.W., John T., Nogami N., Ohe Y., Mann H., Rukazenkov Y., et al. Osimertinib As First-Line Treatment of EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018;36:841–849. doi: 10.1200/JCO.2017.74.7576. [DOI] [PubMed] [Google Scholar]
- 10.Gristina V., Malapelle U., Galvano A., Pisapia P., Pepe F., Rolfo C., Tortorici S., Bazan V., Troncone G., Russo A. The significance of epidermal growth factor receptor uncommon mutations in non-small cell lung cancer: A systematic review and critical appraisal. Cancer Treat Rev. 2020;85 doi: 10.1016/j.ctrv.2020.101994. [DOI] [PubMed] [Google Scholar]
- 11.Passaro A., Mok T., Peters S., Popat S., Ahn M.J., de Marinis F. Recent Advances on the Role of EGFR Tyrosine Kinase Inhibitors in the Management of NSCLC With Uncommon, Non Exon 20 Insertions, EGFR Mutations. J. Thorac. Oncol. 2021;16:764–773. doi: 10.1016/j.jtho.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Pretelli G., Spagnolo C.C., Ciappina G., Santarpia M., Pasello G. Overview on Therapeutic Options in Uncommon EGFR Mutant Non-Small Cell Lung Cancer (NSCLC): New Lights for an Unmet Medical Need. Int. J. Mol. Sci. 2023;24:8878. doi: 10.3390/ijms24108878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J.C.H., Sequist L.V., Geater S.L., Tsai C.M., Mok T.S.K., Schuler M., Yamamoto N., Yu C.J., Ou S.H.I., Zhou C., et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16:830–838. doi: 10.1016/S1470-2045(15)00026-1. [DOI] [PubMed] [Google Scholar]
- 14.US Food & Drug Administration. FDA broadens afatinib indication to previously untreated, metastatic NSCLC with other non-resistant EGFR mutations. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-broadens-afatinib-indication-previously-untreated-metastatic-nsclc-other-non-resistant-egfr.
- 15.Yang J.C.H., Schuler M., Popat S., Miura S., Heeke S., Park K., Märten A., Kim E.S. Afatinib for the treatment of NSCLC harboring uncommon EGFR muta- tions: a database of 693 cases. J. Thorac. Oncol. 2020;15:803–815. doi: 10.1016/j.jtho.2019.12.126. [DOI] [PubMed] [Google Scholar]
- 16.Miura S., Tanaka H., Misumi T. LBA66 Afatinib versus chemotherapy for treatment-naïve non-small cell lung cancer with a sensitizing uncommon epidermal growth factor receptor mutation: a phase III study (ACHILLES/TORG1834) Presented at 2023 European Society of Medical Oncology; October. 2023;34:S1310–S1311. [Google Scholar]
- 17.Luo F.X., Ou S.H.I. “ACHILLES” Heel No More? Afatinib at 40 Mg Once Daily is Superior to Platinum-Based Chemotherapy in EGFR Uncommon (G719X, S768I, and L861Q) Mutations (ACHILLES/TORG1834) Lung Cancer. 2024;15:69–73. doi: 10.2147/LCTT.S461758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bar J., Peled N., Schokrpur S., Wolner M., Rotem O., Girard N., Aboubakar Nana F., Derijcke S., Kian W., Patel S., et al. UNcommon EGFR Mutations: International Case Series on Efficacy of Osimertinib in Real-Life Practice in First-LiNe Setting (UNICORN) J. Thorac. Oncol. 2023;18:169–180. doi: 10.1016/j.jtho.2022.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Cho B.C., Lu S., Felip E., Spira A.I., Girard N., Lee J.-S., Lee S.-H., Ostapenko Y., Danchaivijitr P., Liu B. Amivantamab plus Lazertinib in Previously Untreated EGFR-Mutated Advanced NSCLC. N Engl J Med. 2024;391:1486–1498. doi: 10.1056/NEJMoa2403614. [DOI] [PubMed] [Google Scholar]
- 20.US Food & Drug Administration. FDA approves lazertinib with amivantamab-vmjw for non-small lung cancer. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lazertinib-amivantamab-vmjw-non-small-lung-cancer.
- 21.Cho B.C., Wang Y., Felip E., Cui J., Spira A.I., Neal J.W., Baik C., Marmarelis M.E., Ichihara E., Lee J.S., et al. 2024. Amivantamab plus lazertinib in atypical EGFR-mutated advanced non-small cell lung cancer (NSCLC): Results from CHRYSALIS-2. [Google Scholar]
- 22.Kobayashi Y., Mitsudomi T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: Perspectives for individualized treatment strategy. Cancer Sci. 2016;107:1179–1186. doi: 10.1111/cas.12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banno E., Togashi Y., Nakamura Y., Chiba M., Kobayashi Y., Hayashi H., Terashima M., de Velasco M.A., Sakai K., Fujita Y., et al. Sensitivities to various epidermal growth factor receptor-tyrosine kinase inhibitors of uncommon epidermal growth factor receptor mutations L861Q and S768I: What is the optimal epidermal growth factor receptor-tyrosine kinase inhibitor? Cancer Sci. 2016;107:1134–1140. doi: 10.1111/cas.12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Floc'h N., Lim S., Bickerton S., Ahmed A., Orme J., Urosevic J., Martin M.J., Cross D.A.E., Cho B.C., Smith P.D. Osimertinib, an Irreversible Next-Generation EGFR Tyrosine Kinase Inhibitor, Exerts Antitumor Activity in Various Preclinical NSCLC Models Harboring the Uncommon EGFR Mutations G719X or L861Q or S768I. Mol. Cancer Ther. 2020;19:2298–2307. doi: 10.1158/1535-7163.MCT-20-0103. [DOI] [PubMed] [Google Scholar]
- 25.Yun J., Hong M.H., Kim S.Y., Park C.W., Kim S., Yun M.R., Kang H.N., Pyo K.H., Lee S.S., Koh J.S., et al. YH25448, an Irreversible EGFR-TKI with Potent Intracranial Activity in EGFR Mutant Non-Small Cell Lung Cancer. Clin. Cancer Res. 2019;25:2575–2587. doi: 10.1158/1078-0432.CCR-18-2906. [DOI] [PubMed] [Google Scholar]
- 26.Yun J., Lee S.H., Kim S.Y., Jeong S.Y., Kim J.H., Pyo K.H., Park C.W., Heo S.G., Yun M.R., Lim S., et al. Antitumor activity of amivantamab (JNJ-61186372), an EGFR–MET bispecific antibody, in diverse models of EGFR exon 20 insertion–driven NSCLC. Cancer Discov. 2020;10:1194–1209. doi: 10.1158/2159-8290.CD-20-0116. [DOI] [PubMed] [Google Scholar]
- 27.Chmielecki J., Mok T., Wu Y.L., Han J.Y., Ahn M.J., Ramalingam S.S., John T., Okamoto I., Yang J.C.H., Shepherd F.A., et al. Analysis of acquired resistance mechanisms to osimertinib in patients with EGFR-mutated advanced non-small cell lung cancer from the AURA3 trial. Nat. Commun. 2023;14:1071. doi: 10.1038/s41467-023-35962-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu H.A., Arcila M.E., Rekhtman N., Sima C.S., Zakowski M.F., Pao W., Kris M.G., Miller V.A., Ladanyi M., Riely G.J. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin. Cancer Res. 2013;19:2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chabon J.J., Simmons A.D., Lovejoy A.F., Esfahani M.S., Newman A.M., Haringsma H.J., Kurtz D.M., Stehr H., Scherer F., Karlovich C.A., et al. Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nat. Commun. 2016;7 doi: 10.1038/ncomms11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bean J., Brennan C., Shih J.Y., Riely G., Viale A., Wang L., Chitale D., Motoi N., Szoke J., Broderick S., et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc. Natl. Acad. Sci. USA. 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maruyama T., Mimura K., Izawa S., Inoue A., Shiba S., Watanabe M., Kawaguchi Y., Inoue M., Nogata H., Inoue S., et al. Lapatinib enhances herceptin-mediated antibody-dependent cellular cytotoxicity by up-regulation of cell surface HER2 expression. Anticancer Res. 2011;31:2999–3005. [PubMed] [Google Scholar]
- 32.Scaltriti M., Verma C., Guzman M., Jimenez J., Parra J.L., Pedersen K., Smith D.J., Landolfi S., Ramon y Cajal S., Arribas J., Baselga J. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28:803–814. doi: 10.1038/onc.2008.432. [DOI] [PubMed] [Google Scholar]
- 33.Collins D.M., Madden S.F., Gaynor N., AlSultan D., Le Gal M., Eustace A.J., Gately K.A., Hughes C., Davies A.M., Mahgoub T., et al. Effects of HER Family-targeting Tyrosine Kinase Inhibitors on Antibody-dependent Cell-mediated Cytotoxicity in HER2-expressing Breast Cancer. Clin. Cancer Res. 2021;27:807–818. doi: 10.1158/1078-0432.CCR-20-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho B.C., Kim D.W., Spira A.I., Gomez J.E., Haura E.B., Kim S.W., Sanborn R.E., Cho E.K., Lee K.H., Minchom A., et al. Amivantamab plus lazertinib in osimertinib-relapsed EGFR-mutant advanced non-small cell lung cancer: a phase 1 trial. Nat. Med. 2023;29:2577–2585. doi: 10.1038/s41591-023-02554-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirano T., Yasuda H., Tani T., Hamamoto J., Oashi A., Ishioka K., Arai D., Nukaga S., Miyawaki M., Kawada I., et al. In vitro modeling to determine mutation specificity of EGFR tyrosine kinase inhibitors against clinically relevant EGFR mutants in non-small-cell lung cancer. Oncotarget. 2015;6:38789–38803. doi: 10.18632/oncotarget.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe S., Minegishi Y., Yoshizawa H., Maemondo M., Inoue A., Sugawara S., Isobe H., Harada M., Ishii Y., Gemma A., et al. Effective- ness of gefitinib against non-small-cell lung cancer with the uncommon EGFR mutations G719X and L861Q. J. Thorac. Oncol. 2014;9:189–194. doi: 10.1097/JTO.0000000000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okuma Y., Kubota K., Shimokawa M., Hashimoto K., Kawashima Y., Sakamoto T., Wakui H., Murakami S., Okishio K., Hayashihara K., et al. First-Line Osimertinib for Previously Untreated Patients With NSCLC and Uncommon EGFR Mutations The UNICORN Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2024;10:43–51. doi: 10.1001/jamaoncol.2023.5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho J.H., Lim S.H., An H.J., Kim K.H., Park K.U., Kang E.J., Choi Y.H., Ahn M.S., Lee M.H., Sun J.M., et al. Osimertinib for Patients With Non-Small-Cell Lung Cancer Harboring Uncommon EGFR Mutations: A Multicenter, Open-Label, Phase II Trial (KCSG-LU15-09) J. Clin. Oncol. 2020;38:488–495. doi: 10.1200/JCO.19.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park S., Ahn H., Lee S., Min Y., Jung H., Sun J.M., Lee S.H., Ahn J., Ahn M.J., Lee J., et al. MA13.11 Lazertinib for Patients with NSCLC Harboring Uncommon EGFR Mutations: A Single-Arm, Phase II Multi-Center Trial. J. Thorac. Oncol. 2023;18:S148–S149. [Google Scholar]
- 40.Kim S.Y., Lee J.Y., Kim D.H., Joo H.S., Yun M.R., Jung D., Yun J., Heo S.G., Ahn B.C., Park C.W., et al. Patient-Derived Cells to Guide Targeted Therapy for Advanced Lung Adenocarcinoma. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-56356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim S.Y., Kim S.M., Lim S., Lee J.Y., Choi S.J., Yang S.D., Yun M.R., Kim C.G., Gu S.R., Park C., et al. Modeling Clinical Responses to Targeted Therapies by Patient-Derived Organoids of Advanced Lung Adenocarcinoma. Clin. Cancer Res. 2021;27:4397–4409. doi: 10.1158/1078-0432.CCR-20-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang H.N., Choi J.W., Shim H.S., Kim J., Kim D.J., Lee C.Y., Hong M.H., Park S.Y., Park A.Y., Shin E.J., et al. Establishment of a platform of non-small-cell lung cancer patient-derived xenografts with clinical and genomic annotation. Lung Cancer. 2018;124:168–178. doi: 10.1016/j.lungcan.2018.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The whole-exome sequencing data have been deposited in the Sequence Read Archive (SRA) under the following project accession numbers: PRJNA1095562 and PRJNA1095016. The individual sequencing runs are available under the following SRR accession number: SRR28543204, SRR28520122, SRR28543206, SRR28543205, and SRR28543203. These data are publicly available.
-
•
The code used for generating OncoPrint plot data has been deposited at Zenodo (https://doi.org/10.5281/zenodo.14435819) and is publicly available as of the date of publication. For further details or inquiries regarding the code, please contact the lead contact.
-
•
Additional information required to reanalyze the data presented in this paper can be provided by the lead contact upon request.