Summary
Anti-PD-1 therapy, effective in patients with various advanced tumors, still encounters the challenge of insensitivity in most patients. Here, we demonstrate that PD-L1 on tumor cell-derived extracellular vesicles (TEVs) is critical for anti-PD-1 therapy resistance. Reducing endogenous and transferring exogenous TEVs abrogates and induces anti-PD-1 therapy resistance, respectively. Notably, PD-L1 is sorted onto TEVs via the endosomal sorting complex required for transport after ubiquitination by UBE4A and gradually upregulated on TEVs with tumor progression. During progression, increased MFGE8 from tumor cells promotes self αv integrin signaling activation, enabling themselves to upregulate UBE4A, thereby increasing PD-L1 on TEVs and enhancing their immunosuppressive abilities. Translationally, anti-MFGE8-neutralizing antibodies effectively downregulate UBE4A and TEV PD-L1, thereby negating anti-PD-1 therapy resistance. Furthermore, serum MFGE8 and PD-L1+ EV levels of tumor patients correlate positively, and high levels of both indicate poor prognosis after anti-PD-1 therapy. Thus, MFGE8 is a promising target for overcoming resistance and predicting responsiveness to anti-PD-1 therapy.
Keywords: extracellular vesicles, PD-L1, MFGE8, UBE4A, anti-PD-1 therapy resistance
Graphical abstract

Highlights
-
•
TEV PD-L1 contributes to αPD-1 therapy resistance
-
•
UBE4A-mediated PD-L1 ubiquitination induces EV sorting of PD-L1
-
•
MFGE8 from tumor cells upregulates UBE4A by activating self αv integrin signaling
-
•
MFGE8-blocking antibodies overcome αPD-1 therapy resistance by reducing TEV PD-L1
Wang et al. show that TEVs PD-L1 mediates αPD-1 therapy resistance, and PD-L1 is sorted onto TEVs after ubiquitination by UBE4A. During progression, increased MFGE8 from tumor cells promotes self αv integrin signaling activation to upregulate UBE4A, thereby increasing PD-L1 on TEVs. αMFGE8-neutralizing antibodies (Abs) can reduce TEVs PD-L1, overcoming αPD-1 therapy resistance.
Introduction
Although immune checkpoint inhibitors anti-PD-1/anti-PD-L1 (αPD-1/αPD-L1) prolong the survival of various advanced tumor patients, the clinical response rate is low, ranging from 10% to 30%.1,2 Furthermore, some tumors, such as prostate and pancreatic cancer, are insensitive to αPD-1/αPD-L1 therapy.3,4 Therefore, it is crucial to decipher the mechanisms responsible for αPD-1/αPD-L1 therapy resistance.
Extracellular vesicles (EVs) are classified into ectosomes/microvesicles and exosomes. Ectosomes are vesicles formed by direct outward budding of the plasma membrane. Exosomes are vesicles produced by the endosomal pathway.5 Due to lacking specific markers, EVs are currently used to term ectosomes and exosomes collectively.6 EVs can communicate information between different cells because they carry parental components, including proteins, nucleic acids, lipids, and metabolites.5 Tumor cell-derived EVs (TEVs) are essential in suppressing antitumor immunity. TEVs with high Fas ligand (FasL) induce T cell apoptosis.7 TEVs also induce regulatory T (Treg) cells and myeloid-derived suppressor cells (MDSCs) through transforming growth factor β, inhibit M1-like macrophages, and promote M2-like macrophages, thereby suppressing antitumor immunity.8 Besides, TEVs bear abundant membrane PD-L1 and inhibit antitumor immunity through PD-L1. Melanoma cell-derived PD-L1+ EVs suppress antitumor CD8+ T cell responses, and patients with higher circulating EV PD-L1 are less responsive to αPD-1 therapy.9 Prostate and colon cancer EVs inhibit systemic antitumor immunity and immune memory formation through PD-L1. Furthermore, TEV PD-L1 likely mediates αPD-L1 therapy resistance.10 Although we previously reported that TEV PD-L1 contributes to αPD-L1-therapy resistance,11 TEV PD-L1’s role in αPD-1 therapy resistance remains unknown. But anyway, reducing TEV PD-L1 is a potential strategy to improve antitumor immunity and patient responses to αPD-1/αPD-L1 therapy. Nevertheless, how PD-L1 is sorted to TEVs remains unclear.
Proteins are sorted to EVs in an endosomal sorting complex required for transport (ESCRT)-dependent and ESCRT-independent manner. ESCRT machine includes ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III, four major complexes, and some auxiliary proteins. ESCRT-0 recognizes and binds ubiquitinated substrate proteins, sorting them to endosomal membranes. Then, ESCRT-I and ESCRT-II mediate the budding of endosomal membranes containing related proteins inward. Afterward, ESCRT-III snips the endosomal membrane, detaching and forming intraluminal vesicles containing associated proteins.12,13 ESCRT-independent sorting of EV proteins occurs primarily in a ceramide-dependent manner. First, proteins are sorted on endosomal membranes independent of ESCRT-0; later, ceramide mediates the inward budding of endosomal membranes containing associated proteins to form intraluminal vesicles.14 TEV PD-L1 was significantly reduced after ESCRT-0 subunit hepatocyte growth factor–regulated tyrosine kinase substrate (HRS) knockdown,9 suggesting an ESCRT-dependent sorting of PD-L1 to EVs. So, PD-L1 needs to be ubiquitinated because HRS is an ubiquitin (Ub) recognition receptor.15 However, which E3 Ub ligase-mediated PD-L1 modification induces PD-L1 recognition by HRS and subsequent EV sorting is unknown.
Here, we demonstrate that αPD-1 therapy-insensitive mouse tumor cells with reduced EV secretion become sensitized to αPD-1 therapy. Supplementation of EVs from tumor cells resistant rather than sensitive to αPD-1 therapy confers resistance to this therapy of mouse tumor, indicating that TEV PD-L1 mediates αPD-1 therapy resistance. Therefore, we surmise that reducing TEV sorting of PD-L1 likely overcomes αPD-1 therapy resistance and investigate the mechanisms for TEV sorting of PD-L1. We find that ubiquitin conjugation factor E4 A (UBE4A)-mediated PD-L1 ubiquitination promotes PD-L1 binding to HRS and triggers ESCRT-dependent TEV sorting of PD-L1. Silencing of UBE4A in tumor cells markedly reduces PD-L1 on TEVs, activates antitumor immunity, and delays tumor growth. Furthermore, tumor cells release increased milk fat globule-epidermal growth factor factor 8 (MFGE8) as the tumor progresses, which enhances Ube4a transcription and induces themselves to produce TEVs with more PD-L1 and stronger immunosuppressive abilities by activating αv integrin signaling. Through developing an MFGE8-neutralizing monoclonal antibody (mAb), we verify that MFGE8 blockade abrogates αPD-1 therapy resistance, concomitantly downregulates UBE4A expression in tumor tissues (TTs), and reduces PD-L1 on EVs from TTs (TT-EVs). Collectively, our results provide an effective strategy to overcome αPD-1 therapy resistance.
Results
TEV PD-L1 mediates αPD-1 therapy resistance
To verify whether TEV PD-L1 mediates αPD-1 therapy resistance, we inhibited EV production by knocking out Rab27a16,17 in αPD-1 therapy-resistant B16F10 melanoma cells and 4T1 breast cells (B16F10 Rab27a−/− and 4T1 Rab27a−/−) (Figures S1A–S1E). αPD-1 therapy resistance in B16F10 and 4T1 tumors was eliminated in B16F10 Rab27a−/− and 4T1 Rab27a−/− tumors with reduced TEV production (Figures 1A and S1F). Given the growth of Rab27a−/− tumors is relatively slow, and to exclude the αPD-1 antitumor effect stemming from small tumor size, we administrated Rab27a−/− tumor-bearing mice postponed αPD-1 treatment time and found that αPD-1 treatment still inhibited Rab27a−/− tumor growth. Moreover, the difference in tumor size between immunoglobulin G (IgG) and αPD-1 treatment groups was more considerable (Figure 1B). Next, we inhibited TEV secretion by Coro1a knockout (Figures S1G–S1J) according to our previous publication18 to rule out the effects of Rab27a knockout itself and obtained similar results (Figure 1C). Then, we transferred EVs from B16F10 cells (B16F10-EVs) or EVs from Lewis lung carcinoma (LLC) cells (LLC-EVs) into LLC lung tumor mice and found that B16F10-EVs rather than LLC-EVs conferred resistance of αPD-1 therapy to LLC tumor. However, EVs from B16F10 Pdl1−/− (B16F10 Pdl1−/−-EVs) without PD-L1 did not (Figures 1D and S1K). Besides, TEV treatment alone at our current dose did not affect tumor growth (Figure S1L). Given that LLC-EVs carried lower membrane-associated PD-L1 than B16F10-EVs, we isolated LLC-EVs from PD-L1-overexpressed LLC cells (LLC-PD-L1-EVs) with increased surface PD-L1 (Figure S1M) and found that LLC-PD-L1-EVs conferred resistance of αPD-1 therapy to LLC tumor (Figure 1E). Also, LLC-PD-L1-EVs alone did not affect tumor growth (Figure S1L). Therefore, TEVs mediate αPD-1 therapy resistance via PD-L1.
Figure 1.
TEV PD-L1 mediates αPD-1 therapy resistance
(A–C) Tumor volume of B16F10 or 4T1 tumor-bearing mice (A–C) or 4T1 Rab27a−/−, B16F10 Rab27a−/− (A and B), 4T1 Coro1a−/−, or B16F10 Coro1a−/− (C) tumor-bearing mice received αPD-1 treatment after 7 (A and C) or 14 (B) days’ tumor inoculation.
(D and E) Tumor volume of αPD-1-treated LLC tumor-bearing mice with LLC-EVs, B16F10-EVs, or B16F10 Pdl1−/−-EVs (D) or LLC-EVs or LLC-PD-L1-EVs (E) treatment.
(F) Circ-EV PD-L1 concentration of lung and gastric tumor patients with αPD-1 therapy. SD, stable disease; PR, partial response; CR, complete response.
(G) PFS (lung cancer) or OS (gastric cancer) of Circ-EV PD-L1lo and Circ-EV PD-L1hi patients in (F). Representative results from three (A, C, and D) or two (B and E) independent experiments are shown.
Data are presented as mean ± SD and analyzed using the unpaired Student’s t test in (A)–(C), one-way ANOVA followed by Tukey test in (D)–(F), and log rank test in (G). ns, not significant; ∗p < 0.05; ∗∗p < 0.01; and ∗∗∗p < 0.001. See also Figure S1.
Then, we explored the role of TEV PD-L1 in αPD-1 therapy resistance of tumor patients and evaluated PD-L1 levels on circulating EVs (Circ-EVs) of lung and gastric cancer patients who received αPD-1 therapy (Table S1). We found that the Circ-EV PD-L1 concentration of αPD-1 therapy-sensitive patients was significantly lower than that of αPD-1 therapy-insensitive patients (Figure 1F). Moreover, patients with low Circ-EV PD-L1 (Circ-EV PD-L1lo) exhibited favorable progress-free survival (PFS) or overall survival (OS) relative to high Circ-EV PD-L1 (Circ-EV PD-L1hi) (Figure 1G). Thus, TEV PD-L1 mediates αPD-1 therapy resistance.
PD-L1 is sorted onto EVs in a ubiquitination-dependent manner
Since TEV PD-L1 mediated αPD-1 therapy resistance, reducing PD-L1 on TEVs is probably promising to overcome this resistance. We first determined the PD-L1 levels on TEVs during tumor progression. After the manual ground and enzyme digestion, TT-EVs from mice with 2-, 3- and 4-week 4T1 tumors (2-, 3- and 4-WK EVs) were isolated and identified. 3- and 4-WK EVs were slightly larger than 2-WK EVs, and all EVs contained comparable exosome-like markers (Figures S2A–S2C). To confirm the minimal introduction of intracellular vesicles into the isolated EVs, we performed mechanical homogenization and obtained ∼2.9 times more vesicles from 4-WK TTs, probably due to increased intracellular vesicle release (Figure S2D). Moreover, when comparing the 4-WK TT-EVs isolated in two ways, SEC13, an endoplasmic reticulum-derived COPII vesicle component, was absent in TT-EVs separated by manual ground but abundant in TT-EVs isolated by mechanical homogenization. A similar pattern occurred in the endoplasmic reticulum-resident GRP94 and the Golgi apparatus-associated protein GM130. Moreover, a stronger signal of early endosome marker EEA1 was detected in TT-EVs separated by mechanical homogenization (Figure S2E). So, if the isolated TT-EVs are mixed with intracellular vesicles, the amount is minimal. Then, we detected and found that PD-L1 on 2-, 3- and 4-WK EVs sequentially increased (Figure 2A). Correspondingly, their immunosuppressive capacities on CD8+ T cell proliferation were also successively enhanced, and αPD-L1 ablated that of 4-WK EVs (Figures 2B and 2C). Furthermore, CD63+, CD81+, and CD47+ EV subsets in 2-, 3- and 4-WK EVs were indistinguishable (Figure S2F), suggesting that the overall surface proteins on 2-, 3- and 4-WK EVs may not change significantly.
Figure 2.
PD-L1 is sorted onto EVs in a ubiquitination-dependent manner
(A) Nano-flow cytometry detected PD-L1 on the indicated EVs.
(B and C) Flow cytometry assessed carboxifluorescein diacetate succinimidyl ester (CFSE)-labeled CD8+ T cell proliferation treated with the indicated EVs (B) or 4-WK TEVs with or without αPD-L1 pretreatment (C).
(D) Nano-flow cytometry evaluated CD45+, CD144+, CD41+, CD235a+, or FAP+ TT-EVs among PD-L1+ TT-EVs.
(E and F) Nano-flow cytometry evaluated PD-L1 on FLAG+ EVs from 4T1-CD63-FLAG tumor-bearing mice (E). CFSE dilution determined these EVs’ effect on CD8+ T cell proliferation (F).
(G–I) PD-L1 on Circ-EVs of breast cancer patients was measured by nano-flow cytometry (G); these EVs’ inhibitory effect on human CD8+ T cell proliferation was measured by CFSE dilution (H); and their correlation analysis (I).
(J–L) CD63 and PD-L1 (J) or PD-L1 sorting into Rab5Q79L-GFP endosomes (L) in 4-WK SN-treated 4T1 cells were detected by confocal microscopy (J and L) and statistically analyzed (K).
(M) Nano-flow cytometry measured PD-L1 on EVs from 4-WK SN-treated 4T1 cells transfected with negative control (NC) or Hrs small interfering RNA (siRNA).
(N–P) PD-L1 ubiquitination in 4-WK SN-treated 4T1 cells (N), PD-L1 and HRS colocalization in these cells (O), and statistical analysis (P).
(Q) PD-L1 on EVs from Ub-overexpressing 4T1 and MCF7 cells.
(R) Total internal reflection fluorescence microscopy determined PD-L1 sorting into Rab5Q79L-GFP endosomes of Ub-overexpressing MCF7 cells.
(S) PD-L1 on EVs from Ub-overexpressing 4T1 cells with or without CSN5 overexpression. Scale bar, 10 μm. Representative results from three independent experiments are shown.
Data are presented as mean ± SD and analyzed using the one-way ANOVA followed by Tukey test in (A)–(C), (F), (M), and (S), Mann-Whitney rank-sum test in (H) and (L), Spearman rank-order correlation test in (I), and unpaired Student’s t test in others. ns, not significant; ∗p < 0.05; ∗∗p < 0.01; and ∗∗∗p < 0.001. See also Figure S2.
Human B lymphoma Raji cells present antigens to human T cell leukemia Jurkat T cells and activate them. However, if Jurkat T cells express PD-1 and nuclear factor of activated T cells (NFAT)-GFP (Jurkat T-PD-1-NFAT-GFP), in the presence of PD-L1, T cell activation will be inhibited, which can be determined by GFP fluorescence intensity. By this system, we also observed a stronger immunosuppressive ability of 4-WK EVs than 2-WK EVs from TTs of human MCF7 breast tumor-bearing mice, along with the higher PD-L1 on 4-WK EVs (Figures S2G and S2H).
The TT-EVs contained EVs from tumor and non-tumor cells. To identify whether tumor cells are the primary source of PD-L1+ TT-EVs, we analyzed the percentage of non-tumor cell-derived EVs in PD-L1+ TT-EVs. When labeled with CD45, CD31, CD41, or CD235a, markers corresponding to immune cells, endothelial cells, platelets, or red blood cells, respectively, as previously described,19 and fibroblast activation protein (FAP) for carcinoma-associated fibroblasts, only EVs from immune cells contained PD-L1+ EVs accounting for 26.8% of total PD-L1+ TT-EVs. Excluding this part, the remaining EVs were about 73.2%, likely close to the TEV proportion (Figure 2D), thus indicating that tumor cells are the primary cellular origin of PD-L1+ TT-EVs. To exclude the influence of non-TEVs in TT-EVs, we constructed a plasmid with a flag tag inserted into the second loop of CD63.20 This plasmid was transfected into 4T1 cells to enable the presentation of the flag on EVs (Figure S2I). 27.7% FLAG+ EVs were detected (Figure S2J). As expected, 4-WK FLAG+ TEVs possessed higher membrane-associated PD-L1 than 2-WK FLAG+ TEVs (Figure 2E). After isolating FLAG+ TEVs from TTs (2-WK and 4-WK FLAG+ TEVs), we found that 4-WK FLAG+ TEVs also more strongly inhibited CD8+ T cell proliferation (Figures 2F and S2K). Next, we found that PD-L1 on Circ-EVs significantly increased in breast cancer patients with advanced stages, along with increased ability of the corresponding EVs to inhibit CD8+ T cell proliferation (Figures 2G and 2H). Furthermore, PD-L1+ Circ-EVs and CD8+ T cell proliferation were inversely correlated (Figure 2I). These results suggest that, as tumors progress, increased PD-L1 is sorted to TEVs, mediating their enhanced immunosuppressive capacity.
Then, we investigated how PD-L1 is sorted onto TEVs. First, we confirmed that the digestion supernatant of 4-WK 4T1 TTs (4-WK SN) induced more PD-L1 on 4T1-EVs than on 2-WK SN (Figure S2L). However, neither supernatant affected the membrane and total PD-L1 of 4T1 cells (Figures S2M and S2N). Furthermore, 4-WK SN promoted PD-L1 localization in CD63+ multivesicular bodies (CD63+ MVBs) (Figures 2J and 2K). When Rab5Q79L enlarged endosomes, we also detected increased PD-L1 on intraluminal vesicles (ILVs) of 4-WK SN-treated 4T1 cells (Figure 2L). Altogether, these results suggest that factor(s) from tumor cells or cells in the tumor microenvironment (TME) participate in the MVBs sorting of PD-L1.
Proteins are sorted into MVBs via ESCRT-dependent and ESCRT-independent manners. Neither silencing CD63, HSP70, and syntenin-1 nor GW4869-mediated inhibition of neutral sphingomyelinase activity in 4T1 cells reduced PD-L1 on 4T1-EVs induced by 4-WK SN, whereas silencing of ESCRT-0 subunit HRS did (Figures 2M, S2O, and S2P). Ubiquitinated proteins are recognized by HRS and then sorted into MVBs. As expected, ubiquitinated PD-L1 in 2-WK 4T1 TTs was lower than that in 4-WK TTs (Figure S2Q). That was also increased in 4-WK SN-treated 4T1 cells, along with the colocalization of PD-L1 and HRS (Figures 2N–2P). Furthermore, on overexpression of Ub to enhance ubiquitinated PD-L1 (Figure S2R), increased PD-L1 was detected on 4T1-EVs and EVs from human breast adenocarcinoma cell line MCF7 cells (MCF7-EVs) (Figure 2Q). We also observed increased budding of PD-L1+ ILVs into Rab5Q79L endosomes induced by Ub overexpression in MCF7 cells (Figure 2R). In contrast, decreased PD-L1 on 4T1-EVs was observed when ubiquitinated PD-L1 was reduced by COP9 signalosome 5 (CSN5), which has been reported to mediate PD-L1 deubiquitination (Figures 2S and S2S). Furthermore, Ub overexpression also increased PD-L1 on EVs from B16F10 cells (B16F10-EVs) (Figure S2T). However, Ub overexpression did not affect the EV numbers secreted by these cells (Figure S2U).
The isolated EVs will inevitably be mixed with a small number of microvesicles. Annexin A1 is a specific marker of microvesicles.21 We found that ubiquitination did not affect PD-L1 on Annexin A1+ EVs from 4T1 cells (Figure S2V), excluding ubiquitination-mediated microvesicle sorting of PD-L1. Altogether, TME-induced PD-L1 ubiquitination ESCRT-dependently facilitates TEV sorting of PD-L1.
UBE4A-mediated ubiquitination is responsible for the EV sorting of PD-L1
E3 ligases Cullin3-speckle-type pox virus and zinc finger (POZ) protein complex and beta-transducin repeat-containing protein (β-TrCP) can induce PD-L1 ubiquitination.22,23 Cullin3 and β-TrCP overexpression promoted PD-L1 ubiquitination and decreased PD-L1 in 4T1 cells, but neither increased PD-L1 on 4T1-EVs (Figures S3A and S3B). To identify the E3 ligase(s) responsible for PD-L1 ubiquitination and subsequent EV sorting, proteins interacting with PD-L1 were analyzed by mass spectrometry (MS) from PD-L1-overexpressing HEK293 cells and PD-L1 pulled down 3 E3 ligases including UBE3C, UBE4A, and MID-1 (Figure S3C). Because the number of peptides detected and coverage for each protein are meager, we validated that PD-L1 did interact with UBE3C, UBE4A, and MID-1 (Figure S3D). UBE3C and UBE4A, rather than MID-1 overexpression, markedly induced PD-L1 ubiquitination, but only UBE4A increased PD-L1 on EVs (Figures S3E and S3F). Echoing ubiquitinated PD-L1 levels in 2- and 4-WK 4T1 TTs (Figure S2Q), UBE4A mRNA and protein levels also sequentially increased (Figures 3A and 3B). UBE4A overexpression also enhanced PD-L1 ubiquitination in 4T1 cells (Figure 3C). In contrast, UBE4A silencing reduced PD-L1 ubiquitination in 4T1, B16F10, and MCF7 cells (Figure S3G). Neither UBE4A overexpression nor silencing affected total and membrane-associated PD-L1 (Figures 3C, S3G, and S3H). UBE4A overexpression also did not affect Pdl1 mRNA levels in these cells (Figure S3I). Furthermore, UBE4A and PD-L1 pulled down each other (Figure 3D). These results indicate that UBE4A is PD-L1’s E3 ligase, mediating PD-L1 sorting onto EVs.
Figure 3.
UBE4A-mediated ubiquitination is responsible for the EV sorting of PD-L1
(A and B) Ube4a mRNA (A) and UBE4A protein (B) levels in 2-, 3-, 4-WK 4T1 TTs.
(C) Ubiquitinated PD-L1 in UBE4A-overexpressing 4T1 cells.
(D) PD-L1 or UBE4A was pulled down in 4T1 cells, and then UBE4A or PD-L1 was detected by western blotting.
(E) HRS and PD-L1 colocalization in UBE4A-overexpressing 4T1 cells.
(F) PLA assay detected the colocalization of PD-L1 and HRS complexes and CD63+ MVBs in CD63-GFP-overexpressing 4T1 cells with or without UBE4A overexpression.
(G) PD-L1 sorting into Rab5Q79L-GFP endosomes of 4T1 cells with or without HRS silencing. Arrows indicate inward budding of PD-L1.
(H) PD-L1 on EVs from the indicated UBE4A-overexpressing cells.
(I) CD8+ T cell proliferation in the presence of EVs from 4T1 cells with or without UBE4A overexpression.
(J and K) The ubiquitinated PD-L1 in HEK293 cells expressing Ub, PD-L1, the serial mutants (J), or PD-L1K75R mutant (K), with (J and K) or without (K) UBE4A overexpression.
(L and M) PD-L1K75R on EVs from cells in (K) (L) and these EVs’ effect on CD8+ T cell proliferation (M). Scale bar, 10 μm. Representative results from three independent experiments are shown.
Data are presented as mean ± SD and analyzed using the one-way ANOVA followed by Tukey test except for unpaired Student’s t test in (E), (F), (H), and (L). ns, not significant; ∗p < 0.05; ∗∗p < 0.01; and ∗∗∗p < 0.001. See also Figure S3.
UBE4B, a UBE4A paralog, ubiquitinates the epidermal growth factor receptor and is subsequently recognized by HRS.15 To verify whether UBE4A also induces EV sorting of ubiquitinated PD-L1 through HRS, we first confirmed that UBE4A overexpression notably enhanced the colocalization of PD-L1 and HRS (Figure 3E). Moreover, the proximity ligation assay (PLA) results also showed increased PD-L1 and HRS complexes and the complexes in CD63+ MVBs of UBE4A-overexpressing HEK293 cells (Figure 3F). Consistently, UBE4A overexpression markedly increased the budding of PD-L1+ ILVs into Rab5Q79L endosomes, which was repealed by HRS silencing (Figure 3G). Correspondingly, UBE4A overexpression increased PD-L1 on 4T1-EVs, MCF7-EVs, and B16F10-EVs and opposite results were obtained in UBE4A-silenced cells (Figures 3H, S3J, and S3K). However, UBE4A overexpression did not affect the EV secretion by these cells (Figure S3L). Functionally, EVs from UBE4A-overexpressing 4T1 cells (4T1-UBE4A-EVs) more powerfully inhibited CD8+ T cell proliferation (Figure 3I). Furthermore, 4T1-UBE4A-EVs led to fewer interferon (IFN)-γ+CD8+ and Gzm B+CD8+ T cells (Figure S3M). OT-I CD8+ T cells transgenically express a T cell receptor recognizing an epitope of OVA257–264 in the context of H-2b. When detecting OT-I CD8+ T cell cytotoxicity to OVA-overexpressing B16F10 cells (B16F10-OVA), EVs from B16F10 cells with UBE4A overexpression also more strongly inhibited OT-I CD8+ T cell killing ability than B16F10-EVs (Figure S3N). These results indicate that UBE4A-mediated PD-L1 ubiquitination promotes EV sorting of PD-L1.
To determine the site(s) of UBE4A-mediated PD-L1 ubiquitination, we equally divided the PD-L1 sequence containing lysines into three segments and constructed different PD-L1 truncations with individual deletion of amino acids (aa)19–132 (PD-L1Δ19-132), 133–225 (PD-L1Δ133-225), and 226–290 (PD-L1Δ226-290) (Figure S3O). PD-L1Δ19-132, but not the other truncations, abolished PD-L1 ubiquitination (Figure S3P). Besides, PD-L1Δ19-132 had a comparable affinity to UBE4A compared with full-length PD-L1 (Figure S3Q). Then, we constructed mutants by replacing 9 lysines in aa 19 and 132 with arginines individually. K75R mutation almost eliminated PD-L1 ubiquitination, and UBE4A overexpression no longer induced PD-L1K75R mutant ubiquitination (Figures 3J and 3K). These results indicate that K75 is the ubiquitination site of UBE4A in PD-L1. Subsequently, we characterized the functional relevance of K75R mutation in PD-L1 and found that UBE4A overexpression did not increase PD-L1K75R on HEK293-EVs, and both EVs showed similarly inhibited T cell proliferation (Figures 3L and 3M). Therefore, UBE4A-mediated K75 site ubiquitination of PD-L1 is responsible for its EV sorting.
UBE4A suppresses antitumor immunity by increasing TT-EV PD-L1
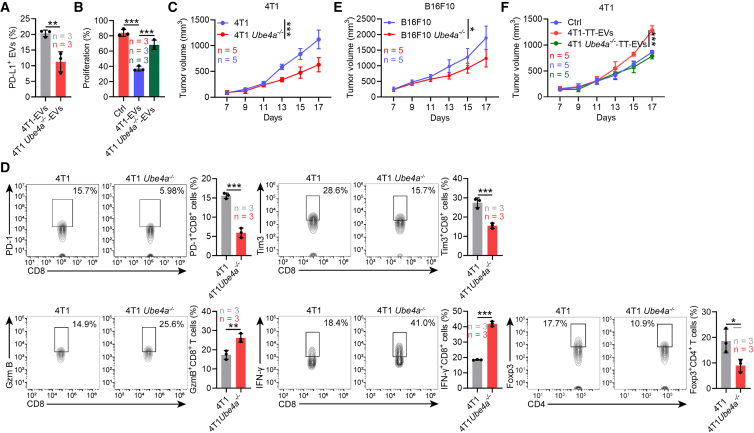
Then, we investigated UBE4A’s effects on antitumor immunity. We constructed UBE4A knockout 4T1 (4T1 Ube4a−/−) cells and found that UBE4A deficiency altered neither cell PD-L1 levels nor viability (Figures S4A and S4B). Furthermore, UBE4A did not affect the morphology, general markers, and size distribution of 4T1-EVs (Figures S4C–S4E). UBE4A also did not alter CD9+CD63+ EV production by 4T1 cells (Figure S4F). However, compared with 4T1-EVs, EVs from 4T1 Ube4a−/− cells (4T1 Ube4a−/−-EVs) had reduced membrane-associated PD-L1 and dampened ability to suppress CD8+ T cell proliferation (Figures 4A and 4B). We also observed notable growth inferiority of the 4T1 Ube4a−/− tumor relative to the 4T1 tumor (Figure 4C). Similar results could not be obtained in thymus-deficient nude mice (Figure S4G), indicating the involvement of antitumor T cell responses. Therefore, we examined the effects of tumor cell UBE4A deficiency on T cell subsets in the draining lymph nodes (dLNs). Exhausted CD8+ T cells (PD-1+ or Tim-3+) were decreased, and activated CD8+ T cells (IFN-γ+ or Granzyme B+, Gzm B) were increased in the dLNs of 4T1 Ube4a−/− tumor-bearing mice. Besides, the frequency of Foxp3+CD4+ Treg cells was greatly descending (Figure 4D). Similar changes in CD8+ T cell subsets and Treg cells of tumor-infiltrating and Circ-T cells were also observed (Figure S4H). However, we found comparable major histocompatibility complex (MHC)-II+CD11c+ DCs and Ki67+ DC subsets in the dLNs of both groups of mice (Figure S4I). Subsequently, we found that the tumor progression was also slightly inhibited in the mice bearing B16F10 melanoma with UBE4A knockout (B16F10 Ube4a−/−) compared with that bearing wild-type (WT) B16F10 melanoma (Figures 4E and S4J). These results suggest that antitumor responses are effectively induced in UBE4A deficiency tumor-bearing mice.
Figure 4.
UBE4A suppresses antitumor immunity by increasing TT-EV PD-L1
(A) Nano-flow cytometry detected PD-L1 on 4T1-EVs and 4T1 Ube4a−/−-EVs.
(B) CFSE dilution measured CD8+ T cell proliferation in the presence of 4T1-EVs and 4T1 Ube4a−/−-EVs.
(C–E) Tumor volume of 4T1 and 4T1 Ube4a−/− (C) or B16F10 and B16F10 Ube4a−/− (E) tumor-bearing WT mice (C and E). The indicated CD8+ T cell subsets and Treg cells in dLNs from tumor mice in (C) (D).
(F) Tumor volume of 4T1 tumor-bearing mice treated with 4T1-TT-EVs or 4T1 Ube4a−/−-TT-EVs. Representative results from three (A–D) or two (E and F) independent experiments are shown.
Data are presented as mean ± SD and analyzed using the unpaired Student’s t test except for one-way ANOVA followed by Tukey test in (B). ns, not significant; ∗p < 0.05 and ∗∗∗p < 0.001. See also Figure S4.
To address the role of EV PD-L1 in this process, we isolated TT-EVs and found lower PD-L1 on EVs from 4T1 Ube4a−/− TTs (4T1 Ube4a−/−-TT-EVs) than on EVs from 4T1 TTs (4T1-TT-EVs) (Figure S4K). Reduced PD-L1 on Circ-EVs from 4T1 Ube4a−/− tumor-bearing mice was also detected, and 4T1 Ube4a−/−-TT-EVs hardly suppressed CD8+ T cell proliferation in vitro (Figures S4L and S4M). Furthermore, 4T1-TT-EVs accelerated 4T1 tumor progression relative to 4T1 Ube4a−/−-TT-EVs (Figure 4F). Altogether, UBE4A suppresses antitumor CD8+ T cell responses by increasing TT-EV PD-L1.
Increased tumor cell-derived MFGE8 promotes UBE4A upregulation during tumor progression
Next, we found that 4-WK SN induced higher mRNA and protein levels of UBE4A than 2-WK SN (Figures 5A and 5B). Therefore, we surmised that specific secreted factor(s) in TTs responsible for UBE4A upregulation increased as the tumor progressed. To find out this/these factor(s), we first determined the molecular weight (MW) range of this/these factor(s). We sequentially filtered 4-WK SN with 100, 50, and 30 kDa filters and found that filtrate of 100 kDa but not 50 and 30 kDa upregulated UBE4A in 4T1 cells (Figure S5A), indicating that the MW of this/these factor(s) ranged from 50 to 100 kDa. Meanwhile, we analyzed 2- and 4-WK TTs by MS and found 77 differently expressed proteins between 2- and 4-WK TTs, of which 18 were upregulated (Figure S5B). Vitronectin (VTN), MFGE8, and Coagulation factor X stood out after screening by criteria of 50–100 kDa MW and secreted proteins among these 18 proteins (Figure S5C). VTN and MFGE8 activate integrin signaling, which is critical to tumor development. We then verified that VTN and MFGE8 in 4-WK SN were higher than those in 2-WK SN (Figure 5C). However, only murine MFGE8 (mMFGE8) but not VTN (mVTN) recombinant proteins induced UBE4A mRNA and protein expression in 4T1 cells and increased 4T1-EV PD-L1 (Figures 5D–5F). Similar results were obtained in human MFGE8 (hMFGE8)-treated MCF7 and mMFGE8-treated B16F10 cells (Figures S5D and S5F). Correspondingly, EVs from MFGE8-treated 4T1 cells more potently inhibited CD8+ T cell proliferation and activation and the OT-I CD8+ T cell-mediated killing of B16F10-OVA cells was more significantly impaired by EVs from MFGE8-treated B16F10 cells (Figures 5G, S5G, and S5H). Next, we excluded that MFGE8 affected EV production of 4T1, B16F10, and MCF7 cells (Figure S5I).
Figure 5.
Increased tumor cell-derived MFGE8 promotes UBE4A upregulation during tumor progression
(A–C) UBE4A mRNA (A), protein (B), and MFGE8 and VTN protein (C) levels in 4T1 cells stimulated with 2-WK or 4-WK SN for 24 h (A) or 48 h (B and C) were measured by real-time PCR (A) or detected by western blotting (B and C).
(D and E) UBE4A mRNA (D) and protein (E) levels in 4T1 cells stimulated with mMFGE8 or mVTN for 24 h (D) or 48 h (E).
(F) Nano-flow cytometry determined PD-L1 on EVs from mMFGE8- or mVTN-stimulated 4T1 cells.
(G) CFSE dilution measured CD8+ T cell proliferation with EVs from mMFGE8-stimulated 4T1 cells.
(H) Western blotting on UBE4A proteins in 4-WK SN-stimulated 4T1 cells with TR-14035 or Cilengitide treatment.
(I–K) Tumor volume of 4T1 tumor-bearing mice with Cilengitide treatment (I), UBE4A in TTs (J), and PD-L1 on TT-EVs (K).
(L–O) 4T1 (L–N) or 4T1-CD63-FLAG (O) tumor-bearing mice were treated with mMFGE8 and Cilengitide. Tumor volume (L), UBE4A in TTs (M), and PD-L1 on TT-EVs (N and O).
(P) Tumor volume of 4T1 and 4T1 Itgav−/− tumor-bearing mice. Arrows indicate the day mMFGE8 treatment started.
(Q and R) UBE4A in TTs (Q) and PD-L1 on TT-EVs (R) from mice in (P).
(S) Western blotting on UBE4A in 4-WK SN- and RM026-treated 4T1 and B16F10 cells.
(T and U) PD-L1 on EVs from 4T1 and B16F10 cells stimulated with 4-WK SN and RM026 (T) and the effect of these EVs on CD8+ T cell proliferation (U).
(V–Z) RM026-treated 4T1 (V–T) or 4T1-CD63-FLAG (Z) tumor-bearing WT mice. Tumor volume (V), the indicated CD8+ T cell subsets and Treg cells in dLNs from these tumor mice (W), UBE4A proteins in 2- and 4-WK TTs of these mice (X), and PD-L1 on EVs from 2- and 4-WK TTs of these mice (Y and Z). Representative results from two (I–K and L–N) or three (others) independent experiments are shown.
Data are presented as mean ± SD and analyzed using the one-way ANOVA followed by Tukey test in (A)–(H), (L), and (S)–(U) and unpaired Student’s t test in (I)–(K), (M)–(R), and (V)–(Z). ns, not significant; ∗p < 0.05; ∗∗p < 0.01 and ∗∗∗p < 0.001. See also Figures S5 and S6.
αvβ3 and αvβ5 integrins are known receptors of MFGE8.24 Cilengitide (a selective αvβ3 and αvβ5 integrin inhibitor) rather than TR-14035 (a selective α4β1 and α4β7 integrin inhibitor) abolished 4-WK SN-induced UBE4A upregulation (Figure 5H). Cilengitide also significantly inhibited 4T1 tumor progression in immune-complete mice but not nude mice (Figures 5I and S5J). However, Cilengitide treatment significantly reduced UBE4A in TTs and PD-L1 on TT-EVs from both strains of mice (Figures 5J, 5K, S5K, and S5L); thus, αvβ3 and αvβ5 integrin signalings are responsible for increased UBE4A and TT-EV PD-L1 during tumor progression. To dissect MFGE8’s role in this process, we treated 4T1 tumor-bearing mice with mMFGE8 and found that mMFGE8 significantly promoted 4T1 tumor growth, along with increased UBE4A in TTs and PD-L1 on TT-EVs. However, these results were negated in tumor-bearing mice simultaneously treated with mMFGE8 and Cilengitide (Figures 5L–5N), indicating that MFGE8 upregulates UBE4A and EV PD-L1 depending on αvβ3 and αvβ5 integrins.
Since immune cell-derived EVs accounted for a considerable PD-L1+ EVs in TT-EVs, we wondered whether MFGE8 could increase PD-L1 on both immune cell-derived EVs and TEVs. First, we found that mMFGE8 notably increased UBE4A in Gr1+CD11b+ myeloid- MDSCs rather than CD4+ and CD8+ T cells, F4/80+ macrophages, CD11c+MHC-II+ DCs, and CD19+ B cells (Figure S5M). Correspondingly, mMFGE8 enhanced PD-L1 percentage in Gr1+ EVs (Figure S5N). αvβ3 and αvβ5 integrins are also enriched in DCs and macrophages.25,26 However, we detected faint UBE4A signals in macrophages and DCs regardless of mMFGE8 treatment when obvious UBE4A signals were found in 4T1 cells and MDSCs (Figure S5O), which probably resulted in the inability of MFGE8 to increase PD-L1 on EVs from macrophages and DCs. Subsequently, we confirmed that mMFGE8 also increased TEV PD-L1, evidenced by enhanced PD-L1 on FLAG+ EVs of TTs from 4T1-CD63-FLAG tumor-bearing mice with mMFGE8 treatment (Figure 5O). Then, to investigate whether MFGE8-mediated activation of αvβ3 and αvβ5 integrin signalings in tumor cells, MDSCs, or both dictated tumor development, we established αv integrin-deficient 4T1 (4T1 Itgav−/−) cells (Figure S5P). Consistent with a previous publication,27 αv deficiency caused sharply retarded tumor growth (Figure 5P). Therefore, we treated 4T1 Itgav−/− tumor-bearing mice with mMFGE8 when the tumor size was comparable with 4T1 tumors at the beginning of treatment. Unlike 4T1 tumors whose growth was significantly accelerated by mMFGE8, mMFGE8 hardly affected 4T1 Itgav−/− tumor growth, accompanied by unaltered UBE4A in TTs and TT-EV PD-L1 of 4T1 Itgav−/− tumor-bearing mice (Figures 5P–5R). Altogether, these results indicate that MFGE8 mainly upregulates tumor cell UBE4A and PD-L1 on TEVs in αvβ3 and αvβ5 integrin-dependent manners to suppress antitumor immunity.
Next, we explored the source of increased MFGE8 during tumor progression. First, we confirmed that 4T1, B16F10, and MCF7 cells secreted high levels of MFGE8, and concentrated supernatant from tumor cells started to induce UBE4A in their parental cells (Figures S5Q and S5R). Then, we established MFGE8-deficient 4T1 (4T1 Mfge8−/−) cells and found that tumor growth was notably inhibited by MFGE8 knockout (Figures S5S and S5T). Besides, circulation MFGE8 levels in 4T1 Mfge8−/− tumor-bearing mice were markedly lower than those in 4T1 tumor-bearing mice, and increased MFGE8 of 4-WK 4T1 Mfge8−/− tumor-bearing mice was no longer detected (Figure S5U). Correspondingly, increases in UBE4A and PD-L1 on TT-EVs of 4-WK TTs were also eliminated in 4T1 Mfge8−/− tumor-bearing mice (Figures S5V and S5W). Consistent with these results, MFGE8 levels in the circulation of 4-WK B16F10 tumor-bearing Mfge8−/− mice were higher than those in 2-WK B16F10 tumor-bearing Mfge8−/− mice, and both were comparable to tumor-bearing WT mice (Figure S5X). Correspondingly, tumor growth in Mfge8−/− mice resembled that in WT mice (Figure S5Y). Altogether, tumor cells are the primary source of elevated MFGE8 during tumor progression.
To evaluate whether enhanced antitumor immunity could be achieved by MFGE8 blockade, we generated a rabbit mAb against mMFGE8 in which the constant region was replaced with mouse IgG (clone RM026). RM026 dissociation constant (KD) was 0.10 nM (Figure S6A). When used as detection Abs in ELISA assay, mMFGE8 was only detected in the serum of WT but not Mfge8−/− mice by RM026, supporting its specific binding to mMFGE8 (Figure S6B). With RM026, 4-WK SN no longer increased UBE4A in 4T1 and B16F10 cells (Figure 5S). Meanwhile, RM026 eliminated the increase in PD-L1 on both EVs and abolished their immunosuppressive priority induced by 4-WK SN (Figures 5T, 5U, S6C, and S6D). When RM026 neutralized MFGE8 in 4T1 tumor-bearing mice, the tumor development was significantly suppressed, accompanied by the markedly decreased exhausted CD8+ T cells, Treg cells, and increased frequency of activated CD8+ T cells in the dLNs (Figures 5V and 5W). Besides, similar MHC-II+CD11c+ DCs and Ki67+ DC subsets in dLNs of these mice were detected, and similar changes in CD8+ T cell subsets and Treg cells of tumor-infiltrating and Circ-T cells between these mice were also obtained (Figures S6E and S6F). Furthermore, 2- and 4-WK TTs from RM026-treated tumor-bearing mice had comparable levels of UBE4A proteins and the comparable PD-L1 on 2- and 4-WK 4T1-TT-EVs (Figures 5X and 5Y). Similar results were obtained in TEVs from RM026-treated 4T1-CD63-FLAG tumor-bearing mice (Figure 5Z). However, RM026 treatment did not affect the progression of 4T1 Ube4a−/− tumors in WT mice or 4T1 tumors in Pd1−/− mice (Figures S6G and S6H). Besides, RM026 did not inhibit 4T1 Mfge8−/− tumor growth, excluding the off-target effects of RM026 (Figure S6I).
Unlike B16F10 Ube4a−/− tumor, the growth of which is significantly inhibited (Figure 4E), an inhibitory tendency of B16F10 tumor growth was observed in RM026-treated tumor mice, which is not statistically significant (Figure S6J). Correspondingly, PD-L1 on TT-EVs from RM026-treated B16F10 tumor-bearing mice was more than that on TT-EVs from B16F10 Ube4a−/− tumor-bearing mice (Figure S6K). Thus, RM026 inhibits tumor development by decreasing TEV PD-L1.
MFGE8-induced activation of c-Fos and c-Jun jointly dictates Ube4a transcription
Activation of the Src family kinases and their downstream Syk is the main signal-transduction pathway of integrin outside-in signaling.28 Pretreatment of 4T1 cells with Lck and Fyn inhibitor PP1 abolished mMFGE8-induced UBE4A mRNA and protein expression (Figures 6A and 6B). Lck’s role was excluded because Fyn inhibitor SU6656 completely repealed mMFGE8-induced UBE4A (Figure 6C). Furthermore, 4T1 cells expressing a constitutively active form of Syk (CA-Syk) had notably upregulated UBE4A and mMFGE8 no longer induced UBE4A in these cells (Figure 6D). Syk involvement was further confirmed due to the failure induction of UBE4A in 4T1 cells treated with Syk inhibitor R406 (Figure 6E). Syk mediates the activation of ERK, JNK, p38, NFAT, and nuclear factor κB (NF-κB) signaling pathways.29,30 To pinpoint which signaling cascade is involved in mMFGE8-mediated UBE4A upregulation, we applied serial inhibitors and found that, when NF-κB (PDTC), p38 (SB203580), or NFAT inhibitor (11R-VIVIT) did not inhibit mMFGE8-induced increase in UBE4A proteins, ERK (U0126) or JNK(SP600125) inhibitor repealed that (Figure 6F), suggesting ERK and JNK pathways are essential for this process.
Figure 6.
MFGE8-induced c-Jun and c-Fos jointly dictate Ube4a transcription
(A–C) Ube4a mRNA (A) and protein (B and C) levels in mMFGE8-stimulated 4T1 cells with PP1 (A and B) or SU6656 (C).
(D) UBE4A protein in CA-Syk-overexpressing 4T1 cells with or without mMFGE8 treatment.
(E–H) Western blotting analyzed UBE4A proteins in mMFGE8-stimulated 4T1 cells with the indicated concentration of R406 (E) and the indicated inhibitors (F and G) or accompanied by c-Fos or c-Jun silencing (H).
(I) c-Fos and c-Jun silencing effect in 4T1 cells.
(J) ChIP-seq analyzed the c-Fos binding peak in the Ube4a promoter of 4T1 cells.
(K) ChIP-PCR analyzed the binding of c-Fos or c-Jun to the Ube4a promoter in c-Jun or c-Fos silenced 4T1 cells with or without mMFGE8 stimulation.
(L) Dual-luciferase analyzed the potential regulatory effect of c-Fos, c-Jun, or both on Ube4a promoter activity in 293T cells following cotransfection with plasmids expressing luciferase reporter containing Ube4a promotor sequence (Ube4a-luc) or NC-luc, Fos, Jun or empty vector, and the Renilla luciferase reporter. Representative results from three independent experiments are shown.
Data are presented as mean ± SD and analyzed using the one-way ANOVA followed by Tukey test in (D) and unpaired Student’s t test in others. ns, not significant; ∗∗p < 0.01 and ∗∗∗p < 0.001.
c-Fos and c-Jun, which constitute the AP-1 transcription complex, are mainly activated by ERK and JNK, respectively.31,32 Since ERK and JNK inhibitors eliminated mMFGE8-induced UBE4A upregulation in 4T1 cells, we assumed whether c-Fos and c-Jun jointly induce Ube4a transcription. First, AP-1 inhibitor T-5224 eliminated the mMFGE8-induced increase in UBE4A proteins (Figure 6G). Then, we found that either c-Fos or c-Jun silencing notably inhibited mMFGE8-mediated UBE4A upregulation (Figures 6H and 6I). Chromatin immunoprecipitation sequencing (ChIP-seq) revealed a c-Fos binding peak in the Ube4a promotor (−3,405 to −3,673) (Figure 6J). Furthermore, ChIP-PCR experiments verified the binding of c-Fos and c-Jun to this sequence, which was inhibited by c-Fos or c-Jun silencing, respectively. Additionally, both bindings were enhanced by mMFGE8 stimulation (Figure 6K). These data suggest the reciprocal roles of c-Fos and c-Jun in regulating Ube4a transcription. To confirm this, we inserted this region in luciferase reporter vectors and found that c-Fos and c-Jun joint rather than alone overexpression significantly increased luciferase activity (Figure 6L). In summary, these results indicate that MFGE8-activated c-Fos and c-Jun corporately transactivate Ube4a transcription.
MFGE8 blockade overcomes tumor resistance to αPD-1 therapy
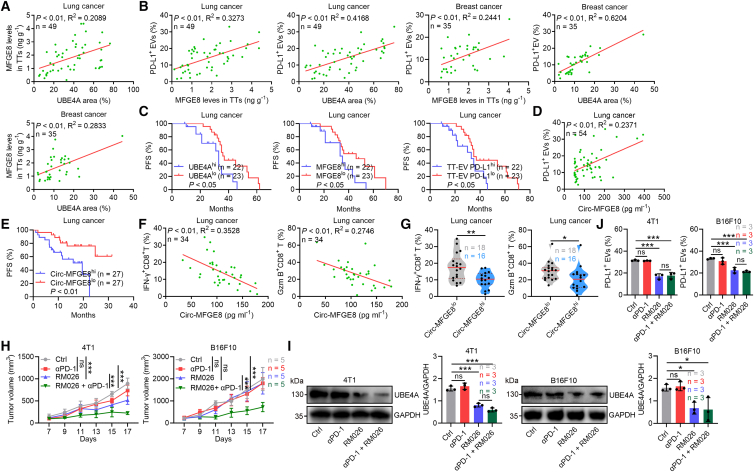
To extend our findings to humans, we detected UBE4A and MFGE8 protein levels in TTs of lung and breast cancer patients (Table S2) and found that their levels were increased with tumor progression (Figures S7A and S7B). Moreover, UBE4A and MFGE8 were positively correlated (Figure 7A). We also isolated and measured PD-L1 levels on TT-EVs and found increased TT-EV PD-L1 with tumor progression (Figure S7C). As expected, UBE4A and MFGE8 positively correlated with PD-L1 levels on TT-EVs (Figure 7B). Furthermore, lung patients with low levels of UBE4A, MFGE8, or PD-L1 on TT-EVs (UBE4Alo, MFGE8lo, or TT-EV PD-L1lo) had better PFS than those with high levels of UBE4A, MFGE8, and PD-L1 on TT-EVs (UBE4Ahi, MFGE8hi, or TT-EV PD-L1hi) (Figure 7C). Low PD-L1 levels on prostate cancer cells are due to high PD-L1 sorting on TEVs.12 Echoing this study, PD-L1 levels on cells from TTs of breast cancer patients were inversely correlated with PD-L1 levels on TT-EVs (Figure S7D). Subsequently, we analyzed the Cancer Cell Line Encyclopedia (CCLE) database and found that most types of human cancers expressed higher UBE4A, MFGE8, ITGB3, ITGB5, and ITGAV than non-small cell lung cancer (Figure S7E), suggesting that the MFGE8-UBE4A axis-mediated regulation of EV sorting of PD-L1 is probably widespread across cancers.
Figure 7.
MFGE8 blockade overcomes tumor resistance to αPD-1 therapy
(A–C) ELISA measured MGFE8 protein levels in TT SN, nano-flow cytometry analyzed PD-L1 on EVs from lung and breast cancer patients’ TTs, and immunohistochemistry detected UBE4A proteins in TTs of these patients. Correlation between the corresponding indicators (A and B). PFS of the grouped patients (C).
(D–G) MFGE8 proteins and PD-L1 on EVs in sera of another cohort of lung cancer patients before αPD-1 therapy were detected by ELISA and nano-flow cytometry, respectively, and the correlation was analyzed (D). PFS of the grouped patients after αPD-1 therapy (E). Circulating IFN-γ+CD8+ and Gzm B+CD8+ T cells of these patients after αPD-1 therapy and their correlations with MFGE8 protein levels were analyzed (F). Circulating IFN-γ+CD8+ and Gzm B+CD8+ T cells were compared in the grouped patients (G).
(H–J) αPD-1-treated 4T1 or B16F10 tumor-bearing mice with or without RM026 treatment. Tumor volume (H), UBE4A proteins in TTs (I), and PD-L1 on EVs from TTs (J) of these mice. Representative results from three independent experiments are shown. Data are presented as mean ± SD and analyzed using the Spearman rank-order correlation test in (A), (B), (D), and (F), log rank test in (C) and (E), unpaired Student’s t test in (G), and one-way ANOVA followed by Tukey test in (H)–(J). ns, not significant; ∗p < 0.05; ∗∗p < 0.01; and ∗∗∗p < 0.001. See also Figure S7.
We also investigated the relationship between Circ-MFGE8 and Circ-EV PD-L1 levels. Like TTs, Circ-MFGE8 and Circ-EV PD-L1 levels of lung tumor patients were also positively correlated (Figure 7D). Besides, Circ-MFGE8 levels of lung tumor patients were higher than those of healthy control (Figure S7F). Notably, compared with patients with high levels of Circ-MFGE8 (Circ-MFGE8hi) before αPD-1 therapy, patients with low levels of Circ-MFGE8 (Circ-MFGE8lo) had a better outcome after αPD-1 treatment (Figure 7E). We also found that Circ-MFGE8 levels before αPD-1 therapy were inversely correlated with IFN-γ+CD8+ and Gzm B+CD8+ T cells in circulation after αPD-1 therapy (Figure 7F). Furthermore, patients with Circ-MFGE8lo had higher circulation IFN-γ+CD8+ and Gzm B+CD8+ T cells (Figure 7G). These results suggest that MFGE8 is responsible for αPD-1 therapy resistance and predicts antitumor responses of αPD-1 therapy.
Next, we tested the effect of MFGE8 blockade on αPD-1 therapy resistance. First, we found that exogenous supplementation of mMFGE8 conferred αPD-1 therapy resistance to LLC tumors and increased UBE4A in LLC TTs and PD-L1 on LLC TT-EVs (Figures S7G–S7I). Then, in mice with αPD-1 therapy-resistant 4T1 and B16F10 tumors, combination with RM026 rather than αPD-1 alone greatly inhibited 4T1 tumor progression. Although neither αPD-1 nor RM026 prevented B16F10 tumor progression, their combination notably did (Figure 7H). When detecting UBE4A levels in TTs, we found that both RM026 alone or with αPD-1 reduced that and UBE4A was comparable between both groups of TTs (Figure 7I). PD-L1 on TT-EVs and Circ-EVs was also similarly reduced in both groups (Figures 7J and S7J). To confirm that RM026 sensitizes αPD-1 to B16F10 tumors by reducing TEV PD-L1, we supplemented B16F10-EVs and B16F10 Pdl1−/−-EVs into B16F10 tumor-bearing mice with combination therapy and found that B16F10-EVs rather than B16F10 Pdl1−/−-EVs repealed antitumor effects of combination therapy (Figure S7K). Therefore, MFGE8 blocking overcomes αPD-1 therapy resistance by reducing TEV PD-L1.
Finally, we tested the toxicity of RM026. RM026 treatment alone or in combination with αPD-1 caused neither impairment of liver and kidney functions nor apparent pathological damage to major organs in 4T1 tumor-bearing mice (Figures S7L and S7M), indicating that MFGE8 is a safe target.
Discussion
PD-L1+ TEVs’ role in αPD-1 therapy resistance is still elusive. We found that inhibiting EV secretion of 4T1 and B16F10 tumor cells repealed their resistance to αPD-1. Furthermore, transferring B16F10-EVs rather than LLC-EVs conferred resistance of LLC tumor to αPD-1 therapy via PD-L1, and less PD-L1 was on LLC-EVs. After LLC-EVs with increased membrane-associated PD-L1 were obtained from PD-L1-overexpressing LLC cells, these EVs started to negate αPD-1 therapeutic effects on LLC tumors. Thus, PD-L1+ TEVs mediate αPD-1 therapy resistance.
Inhibition of TEV release by Rab27a, smpd3, or Coro1a knockout10,18 notably suppresses tumor progression by activating antitumor immunity. However, this approach cannot be replicated in tumor patients due to the lack of safe and effective gene editing techniques in vivo. Instead, unselective suppression of EV secretion may cause unpredictable consequences because cell secretion of EVs is a critical physiological phenomenon. Moreover, no ideal drug is currently available to inhibit EV production. Therefore, reducing PD-L1 on TEVs is likely a promising strategy to overcome αPD-1 therapy resistance, which requires identifying specific mechanisms responsible for TEV sorting of PD-L1. We found that PD-L1 was sorted onto TEVs in an HRS-dependent fashion by UBE4A-mediated ubiquitination. Protein cargos are primarily sorted into MVBs in an ESCRT-dependent manner, and HRS is the subunit of ESCRT-0.13 Unsurprisingly, PD-L1 is sorted to EVs via HRS. However, HRS is physiologically essential because Hrs−/− mice are embryonic lethality. Therefore, HRS is likely an undruggable target, emphasizing the importance of deciphering the upstream events of HRS-mediated EV sorting of PD-L1, such as UBE4A.
Many E3 ligases can ubiquitinate PD-L1. Here, we did not reveal why UBE4A-ubiquitinated PD-L1 is specifically sorted onto EVs. HRS-mediated endosome recruitment of E3 ligase precedes substrate ubiquitination on microdomains of the endosomal membrane and subsequent HRS recognition. UBE4B, a homolog to UBE4A, is recruited to endosomes by binding HRS.15 Resembling UBE4B, UBE4A is likely endosomal localization and ubiquitinates PD-L1 on endosomal membrane, leading to the EV-sorting fate of UBE4A-ubiquitinated PD-L1. However, our results do not exclude other E3 ligases responsible for EV sorting of PD-L1 via the ESCRT pathway. Alternatively, protein cargoes are sorted to EVs independent of ESCRT. Although HRS silencing abolished the 4-WK SN-induced increase in TEV PD-L1, it did not wholly eliminate PD-L1 on TEVs. Thus, ESCRT-independent mechanisms also mediate EV sorting of PD-L1.
In phase 3 clinical trials, PD-L1 on the tumor or immune cells was considered a marker of αPD-1/αPD-L1 therapy sensitivity.33 PC3 prostate cancer cells highly express Pdl1 mRNA, but PC3 cells packaged greater amounts of PD-L1 onto EVs, and EV PD-L1 resists αPD-1/αPD-L1 blockade.10 We found that PD-L1 levels on TT-EVs of lung and breast cancer patients were inversely correlated with PD-L1 on tumor or immune cells. Therefore, tumor or immune cells with high membrane-associated PD-L1 probably pack less PD-L1 onto EVs and produce EVs with lower PD-L1, resulting in sensitivity to αPD-1/αPD-L1 therapy, which explains why surface PD-L1 on the tumor or immune cells is a marker of αPD-1/αPD-L1 therapy sensitivity. UBE4A increased PD-L1 on TEVs but did not alter PD-L1 levels on tumor cells, indicating that UBE4A is not responsible for PD-L1 exchange between cells and EVs. As mentioned earlier, other mechanisms contribute to EV sorting of PD-L1, thus responsible for PD-L1 exchange between tumor cells and TEVs. UBE4A increased TEV PD-L1 but did not affect the total PD-L1 protein in tumor cells. Reasonably, the total cellular PD-L1 should be reduced because UBE4A does not enhance PD-L1 synthesis. Increased UBE4A-mediated PD-L1 ubiquitination probably reduced PD-L1 ubiquitination by other E3 ligases, such as Cullin3 and β-TrCP, thereby decreasing PD-L1 degradation via proteasomes. Besides, PD-L1 is degraded via lysosomes.34 UBE4A-mediated PD-L1 ubiquitination may also disturb the PD-L1 fate of lysosomal degradation, thereby maintaining cellular PD-L1 unchanged.
We demonstrated that MFGE8 activates integrin signaling in tumor cells and promotes tumor cells to secret EVs with increased PD-L1 and immunosuppressive abilities by upregulating UBE4A as the tumor progresses. Increased MFGE8 was also detected during tumor progression, which stemmed from enhanced MFGE8 of tumor source because enhanced MFGE8 was no longer detected in advanced 4T1 tumors with MFGE8 deficiency. Besides MFGE8, other molecules abundant in the TME also bind to αvβ3 and αvβ5 integrins, such as fibronectin and periostin.35,36 However, increases in TT UBE4A and TT-EV PD-L1 were eliminated by MFGE8 deficiency in tumors. Therefore, the possibility that other molecules upregulate UBE4A via αvβ3 and αvβ5 integrins is excluded. As a secreted protein, MFGE8’s functions can be easily blocked by the neutralizing Ab, such as RM026. However, unlike UBE4A knockout, which inhibited 4T1 and B16F10 tumor progression, RM026 only inhibited 4T1 tumor progression. In tumor cells, UBE4A consists of the basic and MFGE8-induced UBE4A. RM026 eliminated MFGE8-induced UBE4A rather than basic UBE4A. PD-L1 levels on TT-EVs from RM026-treated B16F10 tumor-bearing mice were higher than those on TT-EVs from B16F10 Ube4a−/− tumor-bearing mice. Therefore, relatively high PD-L1 levels on TEVs induced by the basic level of UBE4A probably restrict the therapeutic effects of RM026 on B16F10 tumor-bearing mice. Although RM026 alone could not inhibit B16F10 tumor growth, the RM026-mediated decrease in TT-EVs was sufficient to sensitize the B16F10 tumor to αPD-1 therapy. So, both 4T1 and B16F10 tumors were markedly inhibited by αPD-1 and RM026 combination therapy. Furthermore, we found that patients with low Circ-MFGE8 had a better outcome after αPD-1 treatment. Thus, MFGE8 is a potential target for overcoming αPD-1 therapy resistance and a promising biomarker to predict αPD-1 therapy responsiveness.
Although we only validated MFGE8-UBE4A axis-mediated regulation of EV sorting of PD-L1 in lung and breast cancer patients, UBE4A, MFGE8, ITGB3, ITGB5, and ITGAV are extensively expressed in various human tumors. Therefore, MFGE8-UBE4A axis-mediated regulation of EV sorting of PD-L1 is probably widespread across cancers. Besides TEVs, MFGE8 induced MDSC UBE4A in TTs and upregulated PD-L1 on Gr1+ EVs. However, MFGE8 could not affect 4T1 Itgav−/− tumor growth, suggesting that MFGE8 mainly enhances TEV PD-L1 to suppress antitumor immunity. Relative to TEV PD-L1, MFGE8-mediated increase in Gr1+ EV PD-L1 is probably insufficient to antagonize antitumor immunity, likely due to low Gr1+ EV proportion. Given that MDSC proportion and their secreted EVs greatly vary among different individuals, MFGE8-induced Gr1+ EV PD-L1 may affect the antitumor immunity of some cancer patients. Anyway, reducing EV PD-L1 and enhancing antitumor immunity can be achieved by targeting the MFGE8-UBE4A axis. Thus, our findings probably direct broad-spectrum antitumor drug development.
Limitations of the study
We systematically elucidated how tumor cells manipulate PD-L1 sorting onto EVs to induce αPD-1 therapy resistance. However, there are some limitations to this study. Due to current technology limitations, intracellular particles are inevitably introduced when isolating EVs from TT. Furthermore, though reducing PD-L1+ EVs, the RM026 is ineffective in antagonizing the B16F10 tumor. In tumor cells, UBE4A consists of the basic and MFGE8-induced UBE4A. RM026 can eliminate MFGE8-induced UBE4A, but tumor cells still express the basic level of UBE4A, which can sort PD-L1 onto EVs. We did not elucidate basic and MFGE8-induced UBE4A weights in TEV sorting of PD-L1. Additionally, we did not evaluate the effects of sex and gender on the results, which may limit the generalizability of our findings.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Zhijian Cai (caizj@zju.edu.cn).
Materials availability
Plasmids and cell lines generated in this study are available from the lead contact without restriction.
Data and code availability
-
•
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via PRIDE and are publicly available as of date of publication. The accession number is listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (U24A201955, 82130053, 82270086, 82071812, and 82071812), the Natural Science Foundation of Fujian Province, China (2024J08340), the Natural Science Foundation of Xiamen, China (3502Z202471062), and the Natural Science Foundation of Zhejiang Province (LY19H100003). We thank Yingying Huang in the Core Facilities, Zhejiang University School of Medicine, for her technical support. We thank Chenyu Yang in the Center of Cryo-Electron Microscopy (CCEM), Zhejiang University, for her technical assistance with transmission electron microscopy.
Author contributions
Z.C., Y.H., Jianli Wang, and H.F. designed and supervised the research. W.W., J.C., S.W., X.S., and J.Y. performed the experiments. J.C. performed the MS analysis. W.W., J.C., Jiang Wang, and Z.C. analyzed the data and wrote the paper.
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-mouse CD9 APC (1:500) | BioLegend | Cat#124812; RRID:AB_2783071 |
| anti-human CD63 APC (1:500) | BioLegend | Cat#353007; RRID: AB_10916393 |
| anti-mouse CD81 APC (1:500) | BioLegend | Cat#104909; RRID: AB_2562993 |
| anti-human CD81 APC (1:500) | BioLegend | Cat#349510; RRID: AB_2564021 |
| anti-mouse CD274 PE (1:500) | BioLegend | Cat#124308; RRID: AB_2073556 |
| anti-human CD274 PE (1:500) | BioLegend | Cat#329706; RRID: AB_940368 |
| anti-mouse UBE4A (1:1000, cross-reaction with human) | ABclonal | Cat#A3354; RRID:AB_2765070 |
| anti-mouse c-Jun (1:1000) | CST | Cat#9165T; RRID:AB_2130165 |
| anti-mouse c-Fos (1:1000) | CST | Cat#2250T |
| anti-mouse Ubiquitin (1:1000, cross-reaction with human) | CST | Cat#3936T |
| anti-mouse HA (1:1000) | CST | Cat#51064-2-AP |
| anti-mouse MFGE8 (1:1000, cross-reaction with human) | ABclonal | Cat#A12322; RRID: AB_2759173 |
| anti-mouse VTN (1:1000) | ABclonal | Cat#7448; RRID: AB_2783071 |
| anti-mouse HGS (1:1000) | Abcam | Cat#ab155539; RRID: AB_3293616 |
| anti-mouse syntenin (1:1000) | Proteintech | Cat#22399-1-AP; RRID: AB_2879100 |
| anti-mouse HSP70 (1:1000) | BD bioscience | Cat#610608; RRID: AB_397942 |
| eBioscienceTM Fixable Viability Dye eFluorTM 520 (1:500) | Invitrogen | Cat#65-0867-14 |
| anti-mouse CD3e (2 μg mL-1) | Bio X Cell | Cat#BE0001-1;RRID:AB_1107634 |
| anti-mouse CD28 (2 μg mL-1) | Bio X Cell | Cat#BE0015-5;RRID:AB_1107628 |
| Purified anti-mouse CD274 Antibody (1:500) | BioLegend | Cat#124302; RRID: AB_961228 |
| Purified anti-human CD274 Antibody (1:500) | BioLegend | Cat#329702; RRID: AB_940372 |
| anti-mouse CD45 PB (1:500) | BioLegend | Cat#103126; RRID: AB_493535 |
| anti-mouse CD4 APC/Cyanine7 (1:500) | BioLegend | Cat#100526; RRID: AB_312727 |
| anti-mouse CD8α APC/Cyanine7 (1:500) | BioLegend | Cat#100714;RRID:AB_312753 |
| anti-mouse CD8α APC (1:500) | BioLegend | Cat#100712;RRID:AB_312751 |
| anti-mouse PD-1 PE (1:500) | BioLegend | Cat#135205; RRID: AB_1877232 |
| anti-mouse IFN-γ PE (1:500) | BioLegend | Cat#505808; RRID: AB_315402 |
| anti-mouse Ki-67 PE (1:500) | BioLegend | Cat#151209; RRID: AB_2716014 |
| anti-mouse FOXP3 APC (1:500) | Invitrogen | Cat#17-5773-82;RRID:AB_469457 |
| Mouse anti-CD63 (IF) (1:200) | Invitrogen | Cat#MA1-19281 |
| Rabbit anti-CD63 (WB) (1:1000) | ABclonal | Cat#A5271; RRID: AB_2766092 |
| Rabbit anti-GRP94 (1:1000) | ABclonal | Cat#A0989 |
| Rabbit anti-Tsg101 (1:1000) | ABclonal | Cat#A1692; RRID: AB_2763744 |
| Rabbit anti-Alix (1:1000) | Proteintech | Cat#12422-1-AP;RRID:AB_2162467 |
| Rabbit anti-PD-L1 (IF) (1:200) | Proteintech | Cat#28076-1-AP; RRID:AB_2881052 |
| Rabbit anti-PD-L1 (WB) (1:1000, cross-reaction with human) | ABclonal | Cat#A1645; |
| Mouse anti-GAPDH (1:1000, cross-reaction with human) | ABclonal | Cat#AC002; RRID: AB_2736879 |
| Rabbit anti-Rab27a (1:1000) | ABclonal | Cat#A1934; RRID: AB_2862644 |
| Rabbit anti-Phospho-Syk (Tyr525, Tyr526) (1:1000) | Invitrogen | Cat#MA5-14918 |
| Goat anti-mouse IgG HRP (1:200) | MultiSciences | Cat#70-GAM007;RRID:AB_2927718 |
| Goat anti-rabbit IgG HRP (1:200) | MultiSciences | Cat#70-GAR007;RRID:AB_2827833 |
| Purified anti-mouse CD63 Antibody (1:500) | BioLegend | Cat#143901; RRID: AB_11203908 |
| Mouse Control IgG | Abclonal | Cat#AC011; RRID: AB_2770414 |
| Rabbit Control IgG | Abclonal | Cat#AC005; RRID: AB_2771930 |
| Goat anti-rabbit IgG DyLight 488 | MultiSciences | Cat#70-GAR4882 |
| Goat anti-mouse IgG DyLight 488 | MultiSciences | Cat#70-GAM4882 |
| Goat anti-mouse IgG DyLight 594 | MultiSciences | Cat#70-GAM5942 |
| Goat anti-rabbit IgG DyLight 594 | MultiSciences | Cat#70-GAR5942 |
| Purified anti-mouse CD31 Antibody (1:500) | BioLegend | Cat#109502; RRID: AB_2904286 |
| Purified anti-mouse CD41 Antibody (1:500) | BioLegend | Cat#133901; RRID: AB_1626143 |
| Purified anti-mouse CD235a Antibody (1:500) | BioLegend | Cat#116702; RRID: AB_2924454 |
| anti-mouse Gr1 APC/Cyanine7 (1:500) | BioLegend | Cat#108423; RRID: AB_2137486 |
| anti-mouse CD11b APC (1:500) | BioLegend | Cat#101211; RRID: AB_312794 |
| anti-mouse CD11c APC/Cyanine7 (1:500) | BioLegend | Cat#117323; RRID: AB_830646 |
| anti-mouse CD19 APC (1:500) | BioLegend | Cat#152409; RRID: AB_2629838 |
| Anti-mouse Fibroblast activation protein- α (FAP) (1:500) | Invitrogen | Cat#PA5-99313;RRID:AB_2818246 |
| Purified anti-mouse CD45 Antibody (1:500) | BioLegend | Cat#160302; RRID: AB_2876568 |
| Bacterial and virus strains | ||
| E. coli | ATCC | ATCC 11775 |
| Biological samples | ||
| Human lung cancer tissues | Zhejiang University School of Medicine Second Affiliated Hospital | N/A |
| Human breast cancer tissues | Wenzhou Medical University affiliated Dongyang Hospital | N/A |
| Human blood of breast cancer patients | Zhejiang University School of Medicine Second Affiliated Hospital | N/A |
| Human blood of gastric cancer patients | Zhejiang University School of Medicine Second Affiliated Hospital | N/A |
| Human blood of healthy volunteers | Zhejiang University School of Medicine Second Affiliated Hospital | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Anti-mMFGE8 IgG1 chimeric mAb | Sino Biological | N/A |
| Antifade mounting medium with DAPI | VECTASHIELD | Cat#H-1200 |
| JetPEI | Polyplus | Cat#101-10N |
| PEI | Polysciences | Cat#23966-2 |
| TransIT-TKO | Mirus | Cat#MIR 2150 |
| SYBR | Vazyme | Cat# Q121-02 |
| MG132 | Medchemexpress | Cat#HY-13259 |
| FBS | HyClone | Cat: SV30160.03 |
| Duolink® In Situ PLA® Probe anti-Rabbit PLUS | Sigma | Cat#DUO92002 |
| Duolink® In Situ PLA® Probe anti-Mouse MINUS | Sigma | Cat#DUO92004 |
| Duolink® In Situ Detection Reagents Red | Sigma | Cat#DUO92008 |
| Duolink® In Situ Wash Buffers | Sigma | Cat#DUO82049 |
| OptiPrepTM (Iodixanol) | StemCell | Cat#07820 |
| penicillin/streptomycin | Keyi | Cat#CP011 |
| puromycin | MedChemExpress | Cat#HY-B1743A |
| TRIzol | Takara | Cat#9109 |
| DNAse I | Thermo | Cat#18047019 |
| collagenase IV | Thermo | Cat#17104019 |
| PMA | Sigma | Cat#P1585 |
| ionomycin | Sigma | Cat#407951 |
| Recombinant Mouse MFGE8 protein | ABclonal | Cat#RP01490 |
| Recombinant Human MFGE8 protein | ABclonal | Cat#RP03233 |
| Recombinant Mouse VTN protein | ABclonal | Cat#RP02818 |
| brefeldin A solution | MedChemExpress (Shanghai, China) | Cat#HY-16592 |
| Critical commercial assays | ||
| Simple ChIP Plus Sonication Chromatin IP Kit | CST | Cat#56383S |
| cDNA Synthesis Kit | Vazyme | Cat#R323-01 |
| BCA Protein Assay Kit | Thermo | Cat#23225 |
| Mouse biotin positive selection Kit | StemCell | Cat#17665 |
| Human MFGE8 ELISA kits | JONLNBIO | Cat#JL15107-96T |
| Mouse MFGE8 ELISA kits | JONLNBIO | Cat#JL21981-96T |
| Deposited data | ||
| Original MS data | This paper | PXD057691 |
| Original MS data | This paper | PXD058079 |
| Experimental models: Cell lines | ||
| 4T1 | the Cell Bank of the Chinese Academy of Sciences | Cat#TCM32 |
| B16F10 | the Cell Bank of the Chinese Academy of Sciences | Cat#TCM36 |
| LLC | the Cell Bank of the Chinese Academy of Sciences | Cat#TCM7 |
| B16F10-OVA | Dr. Qibin Leng(Guangzhou Medical University, Guangzhou, China) | N/A |
| MCF7 | the Cell Bank of the Chinese Academy of Sciences | Cat#TCHu74 |
| HEK293T | the Cell Bank of the Chinese Academy of Sciences | Cat#GNHu43 |
| Experimental models: Organisms/strains | ||
| Mice: C57BL/6J | Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China) | N/A |
| Mice: BALB/c | Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China) | N/A |
| Mice: Nude mice | Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China) | N/A |
| Mice: C57BL/6, Mfge8−/− | Cyagen Biosciences (Suzhou, Jiangsu, China) | N/A |
| Oligonucleotides | ||
| siRNA for gene knockdown, see Table S3 | This paper | N/A |
| Primers for RT-PCR, see Table S3 | This paper | N/A |
| Primers for ChIP-qPCR, see Table S3 | This paper | N/A |
| Recombinant DNA | ||
| HA-mouse-Ube4a | Miao Ling Plasmid Sharing Platform | N/A |
| HA-mouse-Ube3c | Miao Ling Plasmid Sharing Platform | N/A |
| HA-mouse-Mid1 | Miao Ling Plasmid Sharing Platform | N/A |
| GFP-mouse-Jun | Miao Ling Plasmid Sharing Platform | N/A |
| Flag-mouse-Fos | Miao Ling Plasmid Sharing Platform | N/A |
| Flag-mouse-Pdl1 | Miao Ling Plasmid Sharing Platform | N/A |
| Rab5Q79L-GFP | Prof. Yuehai Ke (Zhejiang University) | N/A |
| psPAX2 | Addgene | Cat#12260 |
| pMD2.G | Addgene | Cat#12259 |
| PCDNA3.1 | Invitrogen | Cat#V79520 |
| pGL3-Basic | Miaolingbio | Cat#P0193 |
| lentiCRISPRv2 puro | Addgene | Cat#52961 |
| Software and algorithms | ||
| ImageJ | ImageJ | https://imagej.net/Welcome |
| Graphpad 8.0 | Graphpad 8.0 | https://www.graphpad.com/scientificsoftware/prism/ |
| FlowJo (V10) | TreeStar | https://www.flowjo.com/solutions/flowjo/downloads |
| Other | ||
| Anti-FLAG®M2 Magnettic Beads | Sigma | Cat#M8823 |
| Protein A/G PLUS-Agarose | Santa Cruz | Cat#sc2003 |
| 38.5 mL ultracentrifuge tubes | Beckman Coulter | Cat#326823 |
Experimental model and subject details
Mice
Female mice of the C57BL/6J and BALB/c strain and nude mice, aged between 6 and 8 weeks, were acquired from Joint Ventures Sipper BK Experimental Animal Co. in Shanghai, China. Specifically, the Mfge8−/− C57BL/6 mice were genetically engineered by Cyagen Biosciences (Suzhou, Jiangsu, China). Pd1−/− BALB/c strain mice were acquired from Dr. Tasuku Honjo (Kyoto University, Japan). All the mice in a controlled facility that maintained specific pathogen-free conditions. The experimental procedures on the mice received the necessary approval from the Animal Care and Use Committee of the Zhejiang University School of Medicine.
Human samples
Blood samples were collected from 32 healthy donors and 54 lung cancer patients before αPD-1 therapy. Blood samples were also collected from 54 lung cancer patients and 40 gastric cancer patients who had undergone αPD-1 therapy. Tumor tissues (TTs) were collected from 49 lung cancer patients and 35 breast cancer patients. Samples of TTs derived from lung cancer patients and the blood of breast and gastric tumor patients or healthy volunteers were procured from the Second Affiliated Hospital, Zhejiang University School of Medicine. TTs derived from breast cancer patients were obtained from Affiliated Dongyang Hospital of Wenzhou Medical University. We allocated the samples based on disease stage and response to αPD-1 therapy. In some experiments, the samples were separated into high and low groups based on the median levels of MFGE8, UBE4A and TT-EV PD-L1. These sample acquisitions were conducted with the necessary approval from the Ethics Committee. Before obtaining the samples, all the patients diagnosed with cancer and the healthy volunteers were duly informed about the purpose and utilization of their samples. In compliance with ethical guidelines, consent forms were provided to and signed by all participants in this study.
Cell lines
4T1 (TCM32), B16F10 (TCM36), LLC (TCM7), MCF7 (TCHu74) and HEK293 (GNHu43) cells were purchased from the Chinese Academy of Sciences Institute (Shanghai, China). B16F10-OVA cells were provided by Dr. Qibin Leng (Guangzhou Medical University, Guangzhou, China). Fetal bovine serum (F101, Vazyme, Nanjing, Jiangsu, China) was ultracentrifugated at 120,000 × g for 1 h to remove EVs and then added into DMEM (C0006, Keyi, Hangzhou, Zhejiang, China) or RPMI-1640 (C0003, Keyi) at final concentration of 10% (v v−1). 4T1, B16F10, B16F10-OVA, MCF7 and HEK293 cells were cultured with 10% EV-depleted fetal bovine serum and 1% penicillin/streptomycin (CP011, Keyi). LLC cells were cultured in RPMI-1640 with 10% EV-depleted fetal bovine serum and 1% penicillin/streptomycin. All cells were cultured at 37°C in a humidified atmosphere with 5% CO2. All cell lines were routinely examined for mycoplasma infection.
Method details
CRISPR-Cas9 mediated construction of gene knockout cells
The gRNAs, with sequences for their target genes listed in Table S3, were annealed and cloned into the Lenti-CRISPR-v2 vector. To deplete target genes, lentiviruses were produced by transfecting HEK293T cells with a combination of target plasmid and package plasmids and the lentiviruses were collected 72 h later. B16F10 and 4T1 cells were transiently transfected with the lentiviruses for 48 h and then selected with 2.5 μg mL−1 puromycin for 3 days. Approximately 300 cells were then transferred into a fresh medium without selection antibiotics and seeded in a 6-cm dish to allow colony formation from single cells. Colonies were then picked and expanded for knockout validation by immunoblot or ELISA.
Separation of EVs
4T1, B16F10, and LLC cells were seeded onto 15-cm plates at a density of 3 million cells per plate. These cells were cultured for 48 h, and then the media from 10 plates were collected, followed by differential centrifugation. Firstly, the supernatants were centrifuged at 300 × g for 10 min, followed by a centrifugation step at 2,000 × g for 20 min, both carried out at 4°C. Subsequently, another centrifugation was performed at 10,000 × g for 30 min at 4°C. For TT-EV isolation, TTs were manually detached and gently ground in 3 mL DMEM with a syringe plunger to minimize the release of intracellular vesicles due to cell disruption. Then type IV collagenase (Sigma–Aldrich, St. Louis, MO, USA) was added into this buffer at a final concentration of 1 mg mL−1 and enzymatically digested for 1 h at 37°C. In some experiments, TTs were detached and subjected to mechanical homogenization. The program was run for 45 s, paused for 15 s, repeated 12 times, and set at 60 Hz to disrupt the cell membrane. The TT fragments containing supernatants were centrifuged at 300 × g for 10 min. Then, all the resulting supernatants were filtered through 0.22 μm syringe filters (Millipore, Billerica, MA, USA) and collected in 38.5 mL ultracentrifuge tubes (326823, Beckman Coulter, Brea, CA, USA). EVs were concentrated by ultracentrifugation using an SW32Ti rotor (L-90K with SW32Ti rotor, Beckman Coulter) at 100,000 × g for 70 min at 4°C. Subsequently, the EV pellets were resuspended in sterile PBS. The protein content of the isolated EVs was quantified using BCA protein assay without adding any detergent (Thermo Fisher Scientific, Waltham, CA, USA).
To isolate Flag+ TT-EVs, 100 μg of TT-EVs from 4T1-CD63-Flag tumor-bearing mice was incubated with biotinylated anti-Flag (final concentration 1 mg mL−1) for 4 h at room temperature (RT) with gentle shaking. Then, the biotin-coated Flag+ EVs were captured by Dynabeads and isolated using a magnet, followed by a detachment from the beads using the CELLectionTM Biotin Binder Kit according to the manufacturer’s instructions. Subsequently, the detached EVs were washed by centrifugation at 100,000 × g for 1 h.
Establishment of plasmid stable expression cell lines
The lentiviruses carrying targeted plasmids were prepared by co-transfecting HEK293T cells with packaging plasmid psPAX2 (Addgene, 12260) and envelope plasmid pMD2.G (Addgene, 12259), followed by viral supernatant collection after 72 h. To generate stable expression cells, parental cells were transiently transfected with the lentiviruses for 24 h, and then the cells were selected with 2.5 μg mL−1 puromycin (MedChemExpress, HY-B1743A) for 2 days. The positive cells were sorted into a 96-well plate on a MoFlo Astrios EQs Cell Sorter (Beckman Coulter).
Cell treatments in vitro
2 × 105 mL−1 tumor cells in a 24-well plate were stimulated with TT (0.5 g) supernatant, acquired from the tumor tissue after the enzyme digestion and centrifugation in RPMI-1640 complete culture medium. In some experiments, after tumor cells were attached, the medium was replaced with fresh medium mixed with inhibitors, including Cilengitide (HY-16141), TR-14035 (HY-15770), R406 (HY-12067), U0126(HY-12031A), SP600125 (HY-12041), PDTC (HY-18738), SB203580 (HY-10256), 11R-VIVIT (HY-P1430A), T-5224 (HY-12270), PP1 (HY-13804), SU6656 (HY-B0789), all from MedChemExpress (Shanghai, China). Sometimes, tumor cells were stimulated with supernatant from the corresponding cells in 1 × 106 mL−1 culture density with 1× and 100× concentration for 24 h.
Electron microscopy and NTA
EV suspension was placed on 200-mesh carbon-coated copper grids at RT for 2 min. Any excess suspension was carefully removed from the grids using filter paper. Subsequently, the EVs were subjected to negative staining by uranyl acetate, performed at RT for 5 min. After the staining step, the EVs were washed twice with PBS and left to dry. The dried EVs were then observed using an FEI Tecnai T10 electron microscope operating at 100 kV (Thermo FEI, Hillsboro, OR, USA).
To measure particle sizes and concentrations, NTA analyzed EVs using a NanoSight NS300 system (Malvern PANalytical, Malvern, UK) configured with a 488 nm laser and high-sensitivity sCMOS camera.
Immunoprecipitation and western blotting
EVs and cells were lysed in the SDS buffer. The lysates were then boiled at 100°C for 10 min. Following the lysis and boiling step, the samples were resolved using SDS-polyacrylamide gel and transferred onto PVDF membranes (Millipore). These membranes were blocked with 5% milk for 2 h. Next, the membranes were incubated overnight with the appropriate primary Abs at 4°C, followed by incubation with the corresponding secondary Abs at RT for 2 h. An Enhanced Chemiluminescence Kit (MultiSciences, Hangzhou, Zhejiang, China) was employed to detect the protein bands.
For immunoprecipitation, the cells were lysed in co-immunoprecipitation lysis buffer consisting of 50 mM Tris-HCl, 5 mM EDTA, 150 mM NaCl, 0.5% (v v−1) NP-40, and 10% (v v−1) glycerol at pH 7.4. The lysis buffer was supplemented with 1 mM PMSF, 1 mM Na3VO,4 and 10 mM NaF. The lysate was then incubated with M2-Flag beads (Sigma–Aldrich) overnight at 4°C for immunoprecipitation. The immunoprecipitates were washed at least three times with lysis buffer and subsequently analyzed using the indicated Abs by western blotting.
Tumor model and treatment
On day 0, mice were subcutaneously injected with 2 × 106 tumor cells as follows: B16F10, B16F10 Rab27a−/−, B16F10 Coro1a−/−, B16F10 Ube4a−/−, 4T1, 4T1 Rab27a−/−, 4T1 Coro1a−/−, 4T1 Ube4a−/−, 4T1 Itgav−/−, 4T1 Mfge8−/− and LLC cells. On day 7, the tumor-bearing mice were randomized into groups and received treatments as described below. The mice with LLC tumors were intravenously injected with 20 μg LLC-EVs, B16F10-EVs or B16F10 Pdl1−/−-EVs every 2 days to supply TEVs. The mice were intravenously injected with 10 μg mMFGE8 every 3 days or 100 μg mMFGE8-neutralizing Ab RM026 every 3 days to block mMFGE8. In some experiments, the EV dose of 100 μg was used to observe the tumor-promoting effect of TT-EVs alone. For αPD-1 treatment, the mice were intraperitoneally injected with 100 μg of αPD-1 every 3 days. In some experiments, B16F10 Rab27a−/− or 4T1 Rab27a−/− tumor-bearing mice received αPD-1 14 days after tumor inoculation. The mice were intraperitoneally injected with 100 μg Cilengitide to block αvβ3 and αvβ5 functions every 3 days. On day 20, the mice were euthanized, and the dLNs, tumor-infiltrating immune cells and blood were collected for flow cytometry analysis. Tumor growth was monitored every 2 days by measuring the length and width of the tumors. Tumor volume was calculated using the formula: 0.5 × length × width.2
Flow cytometry analysis
For intracellular staining, cells were stimulated with PMA (50 ng mL−1, Sigma–Aldrich), ionomycin (1 μg mL−1, Sigma–Aldrich), and brefeldin A solution (eBioscience, San Diego, CA, USA) at 37 °C for 4 h before intracellular staining. The flow cytometry data were analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
Nano-flow cytometry analysis
The nano-flow cytometry was performed as previously described with minor modifications.37 EVs isolated from cell culture media, sera or TTs were divided into 50 μL PBS with 1 × 1010 particle ml−1 concentration. For each 50 μL sample, 0.25 μL specific primary antibodies were added into each sample overnight, and then 0.25 μL secondary antibodies were incubated at 37°C for 1 h. For FAP staining, EVs were pre-treated with 1× permeabilization buffer (eBioscience) for 1 h before incubating with a primary antibody. After incubation, the mixture was washed twice with 1 mL PBS by centrifugation at 100,000 g for 20 min at 4°C. The pellets were resuspended in 100 μL PBS, diluted to the approximate concentration, and analyzed by Flow NanoAnalyzer (N30E, NanoFCM Inc., Xiamen, Fujiang, China).
CD8+ T cell proliferation and activation assay
Mouse and human CD8+ T cells were isolated from splenocytes and lymph nodes using the Botin CD8+ T cells Isolation Kit (StemCell, Vancouver, BC, CNA), following the manufacturer’s instructions. The isolated CD8+ T cells were seeded into 96-well plates at a density of 2 × 106 cells ml−1. The cells were then stimulated with 2 μg mL−1 mouse or human anti-CD3 and anti-CD28-coated Dynabeads (Thermo Fisher Scientific) in the presence of 20 μg ml−1 EVs. Some CD8+ T cells were labeled with CFSE before stimulation. To block PD-L1 on EVs, 20 μg ml−1 EVs were incubated with 10 μg mL−1 αPD-L1 or IgG isotype antibodies. Then, the excessive antibodies were removed through ultracentrifugation. After three days of incubation, the cells were harvested and analyzed using flow cytometry to evaluate their proliferation according to CFSE dilution and IFN-γ+CD8+ and Gzm B+CD8+ T cells by intracellular staining.
Detection of CD8+ T cell cytotoxicity
CD8+ T cells were isolated from OT-I mice splenocytes, mixed with 10 μg B16F10-EVs and activated using anti-CD3 (2 μg mL−1) and CD28 (2 μg mL−1) for 2 days. B16F10 cells were labeled with CFSE and seeded onto 48-well plates at a density of 1 × 104 cells per well. The activated CD8+ T cells were cultured with adherent B16F10 cells overnight at an effector-to-target ratio of 10:1. The cell mixture was collected, and cell killing was measured using flow cytometry. The killing efficiency (%) was calculated as follows: [(Ctrl cell number - CFSE+ cell number)/Ctrl cell number] × 100.
Immunofluorescence
The cells were fixed with precooled methyl alcohol for 10 min at −20 °C and then permeabilized by 0.1% Triton X-100 for 10 min at RT. Non-specific binding sites were blocked by incubating the cells with a blocking buffer containing 5% bovine serum albumin and 3% goat serum in PBS. Then, the cells were incubated with the corresponding primary Abs overnight at 4 °C in the blocking buffer. The following day, after three washes in PBS, the cells were incubated with DyLight 488-labeled secondary Abs for 1 h at RT and washed in PBS. Finally, nuclei were stained with DAPI (Thermo Fisher Scientific). The stained sections were imaged using an Olympus IX83-FV3000 confocal microscope (Olympus Corp, Tokyo, Japan). Images were analyzed using ImageJ software (NIH, Bethesda, MD, USA).
Plasmid and DNA, siRNA transfection
HA-mouse-Ube4a, HA-mouse-Ube3c, HA-mouse-Mid1, GFP-mouse-Jun, Flag-mouse-Fos, Flag-mouse-Pdl1 and various mutants were constructed and acquired from the Miao Ling Plasmid Sharing Platform (Wuhan, Hubei, China). Rab5Q79L-GFP was provided by Prof. Yuehai Ke (Zhejiang University). According to the manufacturer’s protocol, B16F10 and HEK293 cells were transfected with plasmids using JetPEI Transfection Reagent (Polyplus, Beijing, China) or PEI (Polysciences, Warrington, PA, USA) or were transfected with scramble NC or target siRNA using INTERFERin (Polyplus) or TransIT-TKO Transfection Reagent (Mirus Bio, Madison, WI, USA).
MS
MS protein content analysis was performed using the Q Exactive system (Thermo Fisher Scientific). The results were presented in the affiliated Source Data file 1.
Real-time PCR
The process of extracting total RNA involved using TRIzol reagent, following the standard protocol. Once the RNA was obtained, it was reverse transcribed into complementary DNA (cDNA) using the cDNA Synthesis Kit (TaKaRa, Dalian, Liaoning, China), following the instructions provided by the manufacturer. Real-time PCR analysis was performed with Power SYBR Green (TaKaRa) using the following reaction conditions: 95°C for 30 s, followed by 40 cycles of 5 s at 95°C and 34 s at 60°C. These conditions ensured proper amplification of the target genes. The obtained PCR data were subsequently analyzed using the 2-ΔΔCt.
PLA
PLA was carried out using Duolink In Situ PLA reagents according to the manufacturer’s instructions (Sigma–Aldrich) by the corresponding primary Abs. Samples were imaged using an Olympus IX83-FV3000 confocal microscope (Olympus Corp), and the images were analyzed with ImageJ software (NIH).
ELISA
Sandwich ELISA measured serum EVs in the individual samples. Briefly, 96-well ELISA plates were coated with 4 μg mL−1 purified anti-human CD63 Abs in coating buffer and incubated overnight at 37°C. After blocking with assay diluent, serum samples in triplicate were added to individual wells and incubated overnight at 37°C. The plates were washed, and the bound EVs were detected by incubation with biotin anti-human PD-L1 and avidin-HRP for 2 h at 37°C. Then, the signal was developed with tetramethylbenzidine, and the samples were blocked with 2 M H2SO4, after which absorbance at 450 nm was measured with a SpetraMax M5 microplate reader (Molecular Devices, San Jose, CA, USA). The concentration of αPD-L1 on the EVs was calculated based on the linear range of the ELISA data. Serial dilutions of biotinylated αPD-L1 were used to make a standard curve. The standard curve results demonstrated that the established ELISA exhibited a reliable linear detection range from 0 to 2 ng μL−1. The MFGE8 level in mouse and human serum was detected by ELISA kits (JiangLaibio, Shanghai, China) according to the manufacturer’s instructions.
Generation of mMFGE8-neutralizing mAb
Anti-mMFGE8 IgG1 chimeric mAb (Sino Biological, Beijing, China) was generated by cloning the variable region of Abs from immunized rabbits and the constant region of mouse IgG1 Abs into the pcDNA3.1 vector, and the clone RM026 was selected as the most effective one to inhibit mMFGE8-induced upregulation of UBE4A in 4T1 cells.
ChIP-seq
The ChIP-seq was conducted using a ChIP Assay Kit (Cell Signaling Technology, MA, USA), following the instructions provided by the manufacturer. Briefly, cells from 10-cm dishes were collected for the ChIP-seq assay. The proteins and DNA within the cells were crosslinked using 1% formaldehyde for 10 min at 37°C. Then, the chromatin was sheared into fragments using the Bioruptor Pico (Diagenode, Liege, NJ, USA) instrument. The shearing process typically involved 25 cycles to obtain chromatin fragments ranging in size from 200 to 500 base pairs. The lysates were incubated and immunoprecipitated with specific Abs. The immunoprecipitated DNA fragments associated with the target proteins were isolated and purified for real-time PCR. According to the manufacturer’s protocol, the immunoprecipitated DNA was purified and subjected to sequencing library preparation using the RK2055 (Abclonal, Wuhan, Hubei, China). The DNA libraries were sequenced with Illumina Novaseq 6000 at HaploX Biotechnology (Shenzhen, Guangdong, China). Sequenced reads of 50 bp were obtained using the sequenced reads PE150. All reads were mapped to the mouse genome mm10.
Luciferase reporter assay
The Ube4a gene binding site of c-Fos TF is acquired from ChIP-seq. The genomic DNA from 4T1 cells was used as the starting material. The specific Ube4a gene binding site was amplified from the genomic DNA by PCR and cloned into the pGL3 luciferase reporter vector. Then, HEK293 cells were transfected with the Ube4a-luc reporter vector, plasmids for expressing c-Fos proteins, and the pRL-cytomegalovirus plasmid as an internal control. The transfection was performed using the JetPEI Transfection Reagent (Polyplus) for 24 h. The luciferase activity in cell lysates was detected using a Dual-Luciferase Reporter Assay System, and the data were normalized to Renilla luciferase activity. An empty pCR-cytomegalovirus vector was used as the control.
Quantification and statistical analysis
Data are expressed as the mean ± SD. For normally distributed data, Unpaired Student’s t test was used for comparisons between two groups, one-way or two-way ANOVA for comparisons among multiple groups, the log rank test for survival rate analysis, and Spearman rank-order correlation test for Pearson correlation. The Mann-Whitney rank-sum test was used to compare the two groups for data that were not normally distributed. All statistical analyses were performed using Graph Prism 8.0 software (GraphPad Software Inc., San Diego, CA, USA). Statistical significance was considered if a p value was <0.05.
Published: January 21, 2025
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2024.101922.
Contributor Information
Yuzhou He, Email: hyzxy1995@126.com.
Huajun Feng, Email: fenghuajun@zafu.edu.cn.
Zhijian Cai, Email: caizj@zju.edu.cn.
Supplemental information
References
- 1.Page D.B., Postow M.A., Callahan M.K., Allison J.P., Wolchok J.D. Immune modulation in cancer with antibodies. Annu. Rev. Med. 2014;65:185–202. doi: 10.1146/annurev-med-092012-112807. [DOI] [PubMed] [Google Scholar]
- 2.Hamanishi J., Mandai M., Matsumura N., Abiko K., Baba T., Konishi I. PD-1/PD-L1 blockade in cancer treatment: perspectives and issues. Int. J. Clin. Oncol. 2016;21:462–473. doi: 10.1007/s10147-016-0959-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin A.M., Nirschl T.R., Nirschl C.J., Francica B.J., Kochel C.M., Van Bokhoven A., Meeker A.K., Lucia M.S., Anders R.A., DeMarzo A.M., Drake C.G. Paucity of PD-L1 expression in prostate cancer: innate and adaptive immune resistance. Prostate Cancer Prostatic Dis. 2015;18:325–332. doi: 10.1038/pcan.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng M., Xiong G., Cao Z., Yang G., Zheng S., Song X., You L., Zheng L., Zhang T., Zhao Y. PD-1/PD-L1 and immunotherapy for pancreatic cancer. Cancer Lett. 2017;407:57–65. doi: 10.1016/j.canlet.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Ribovski L., Joshi B., Gao J., Zuhorn I. Breaking free: endocytosis and endosomal escape of extracellular vesicles. Extracell. Vesicles Circ. Nucl. Acids. 2023;4:283–305. doi: 10.20517/evcna.2023.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7 doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreola G., Rivoltini L., Castelli C., Huber V., Perego P., Deho P., Squarcina P., Accornero P., Lozupone F., Lugini L., et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J. Exp. Med. 2002;195:1303–1316. doi: 10.1084/jem.20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y., Gu Y., Cao X. The exosomes in tumor immunity. OncoImmunology. 2015;4 doi: 10.1080/2162402X.2015.1027472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S.T., Cristescu R., Bass A.J., Kim K.M., Odegaard J.I., Kim K., Liu X.Q., Sher X., Jung H., Lee M., et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat. Med. 2018;24:1449–1458. doi: 10.1038/s41591-018-0101-z. [DOI] [PubMed] [Google Scholar]
- 10.Poggio M., Hu T., Pai C.C., Chu B., Belair C.D., Chang A., Montabana E., Lang U.E., Fu Q., Fong L., Blelloch R. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177:414–427.e13. doi: 10.1016/j.cell.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J., Yang J., Wang W., Guo D., Zhang C., Wang S., Lu X., Huang X., Wang P., Zhang G., et al. Tumor extracellular vesicles mediate anti-PD-L1 therapy resistance by decoying anti-PD-L1. Cell. Mol. Immunol. 2022;19:1290–1301. doi: 10.1038/s41423-022-00926-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanson P.I., Cashikar A. Multivesicular body morphogenesis. Annu. Rev. Cell Dev. Biol. 2012;28:337–362. doi: 10.1146/annurev-cellbio-092910-154152. [DOI] [PubMed] [Google Scholar]
- 13.Juan T., Fürthauer M. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin. Cell Dev. Biol. 2018;74:66–77. doi: 10.1016/j.semcdb.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 14.Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brügger B., Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 15.Sirisaengtaksin N., Gireud M., Yan Q., Kubota Y., Meza D., Waymire J.C., Zage P.E., Bean A.J. UBE4B protein couples ubiquitination and sorting machineries to enable epidermal growth factor receptor (EGFR) degradation. J. Biol. Chem. 2014;289:3026–3039. doi: 10.1074/jbc.M113.495671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peinado H., Alečković M., Lavotshkin S., Matei I., Costa-Silva B., Moreno-Bueno G., Hergueta-Redondo M., Williams C., García-Santos G., Ghajar C., et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bobrie A., Krumeich S., Reyal F., Recchi C., Moita L.F., Seabra M.C., Ostrowski M., Théry C. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72:4920–4930. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- 18.Fei X., Li Z., Yang D., Kong X., Lu X., Shen Y., Li X., Xie S., Wang J., Zhao Y., et al. Neddylation of Coro1a determines the fate of multivesicular bodies and biogenesis of extracellular vesicles. J. Extracell. Vesicles. 2021;10 doi: 10.1002/jev2.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu Z.L., Liu X.C., Wu M., Shi S., Fu Q.Y., Jia J., Chen G. Untouched isolation enables targeted functional analysis of tumour-cell-derived extracellular vesicles from tumour tissues. J. Extracell. Vesicles. 2022;11 doi: 10.1002/jev2.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ran N., Gao X., Dong X., Li J., Lin C., Geng M., Yin H. Effects of exosome-mediated delivery of myostatin propeptide on functional recovery of mice. Biomaterials. 2020;236 doi: 10.1016/j.biomaterials.2020.119826. [DOI] [PubMed] [Google Scholar]
- 21.Jeppesen D.K., Fenix A.M., Franklin J.L., Higginbotham J.N., Zhang Q., Zimmerman L.J., Liebler D.C., Ping J., Liu Q., Evans R., et al. Reassessment of exosome composition. Cell. 2019;177:428–445.e18. doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J., Bu X., Wang H., Zhu Y., Geng Y., Nihira N.T., Tan Y., Ci Y., Wu F., Dai X., et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature. 2018;553:91–95. doi: 10.1038/nature25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C.W., Lim S.O., Xia W., Lee H.H., Chan L.C., Kuo C.W., Khoo K.H., Chang S.S., Cha J.H., Kim T., et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat. Commun. 2016;7 doi: 10.1038/Ncomms12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aziz M., Jacob A., Matsuda A., Wang P. Review: milk fat globule-EGF factor 8 expression, function and plausible signal transduction in resolving inflammation. Apoptosis. 2011;16:1077–1086. doi: 10.1007/s10495-011-0630-0. [DOI] [PubMed] [Google Scholar]
- 25.Rubartelli A., Poggi A., Zocchi M.R. The selective engulfment of apoptotic bodies by dendritic cells is mediated by the alpha(v)beta 3 integrin and requires intracellular and extracellular calcium. Eur. J. Immunol. 1997;27:1893–1900. doi: 10.1002/eji.1830270812. [DOI] [PubMed] [Google Scholar]
- 26.Finnemann S.C., Rodriguez-Boulan E. Macrophage and retinal pigment epithelium phagocytosis: apoptotic cells and photoreceptors compete for alphavbeta3 and alphavbeta5 integrins, and protein kinase C regulates alphavbeta5 binding and cytoskeletal linkage. J. Exp. Med. 1999;190:861–874. doi: 10.1084/jem.190.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morandi E.M., Verstappen R., Zwierzina M.E., Geley S., Pierer G., Ploner C. ITGAV and ITGA5 diversely regulate proliferation and adipogenic differentiation of human adipose derived stem cells. Sci. Rep. 2016;6 doi: 10.1038/Srep28889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abram C.L., Lowell C.A. The ins and outs of leukocyte integrin signaling. Annu. Rev. Immunol. 2009;27:339–362. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurosaki T., Shinohara H., Baba Y. B cell signaling and fate decision. Annu. Rev. Immunol. 2010;28:21–55. doi: 10.1146/annurev.immunol.021908.132541. [DOI] [PubMed] [Google Scholar]
- 30.Kingeter L.M., Lin X. C-type lectin receptor-induced NF-κB activation in innate immune and inflammatory responses. Cell. Mol. Immunol. 2012;9:105–112. doi: 10.1038/cmi.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis R.J. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 32.Roskoski R. ERK1/2 MAP kinases: Structure, function, and regulation. Pharmacol. Res. 2012;66:105–143. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016;375:1823–1833. doi: 10.1056/Nejmoa1606774. [DOI] [PubMed] [Google Scholar]
- 34.Liu X., Yin M., Dong J., Mao G., Min W., Kuang Z., Yang P., Liu L., Zhang N., Deng H. Tubeimoside-1 induces TFEB-dependent lysosomal degradation of PD-L1 and promotes antitumor immunity by targeting mTOR. Acta Pharm. Sin. B. 2021;11:3134–3149. doi: 10.1016/j.apsb.2021.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy P.A., Begum S., Hynes R.O. Tumor angiogenesis in the absence of fibronectin or its cognate integrin receptors. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wasik A., Ratajczak-Wielgomas K., Badzinski A., Dziegiel P., Podhorska-Okolow M. The role of periostin in angiogenesis and lymphangiogenesis in tumors. Cancers. 2022;14:4225. doi: 10.3390/Cancers14174225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian Y., Gong M., Hu Y., Liu H., Zhang W., Zhang M., Hu X., Aubert D., Zhu S., Wu L., Yan X. Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J. Extracell. Vesicles. 2020;9 doi: 10.1080/20013078.2019.1697028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via PRIDE and are publicly available as of date of publication. The accession number is listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.