Summary
Tumor-associated neutrophils (TANs) play a critical role in the progression and prognosis of triple-negative breast cancer (TNBC), with N2-type TANs known for their pro-tumor characteristics. This study introduces CT-1, a derivative of cryptotanshinone that effectively suppresses TNBC growth while selectively reducing the proportion of N2-type TANs within tumor tissue. Notably, CT-1 induces simultaneous ferroptosis in both N2-type TANs and TNBC cells, a dual mechanism that enhances its therapeutic efficacy. The study identifies ferritin heavy chain 1 (FTH1), a key protein in iron metabolism, as the direct target of CT-1. By targeting FTH1, CT-1 facilitates the interaction between NCOA4 and ferritin, triggering ferritinophagy-mediated ferroptosis. These findings position CT-1 as a promising therapeutic agent, offering a strategy to combat TNBC by inducing ferroptosis in both N2-type TANs and cancer cells. This approach underscores the potential of FTH1 as a therapeutic target for treating TNBC.
Keywords: N2-type tumor-associated neutrophils, FTH1, ferroptosis, cryptotanshinone derivative, TNBC
Graphical abstract

Highlights
-
•
CT-1 dual-targets N2-type TANs and TNBC cells to combat TNBC
-
•
CT-1 selectively eliminates N2-type TANs without affecting other immune cells
-
•
CT-1 induces ferritinophagy-mediated ferroptosis by targeting FTH1
-
•
CT-1 promotes lysosomal autophagic degradation of FTH1
Liu et al. explored the efficacy and underlying mechanism of CT-1 in combating TNBC. CT-1 effectively inhibits TNBC by targeting FTH1 and facilitating the interaction between NCOA4 and ferritin, thereby triggering ferritinophagy-mediated ferroptosis in both N2-type TANs and TNBC cells.
Introduction
Breast cancer accounts for 11.7% of all new cancer cases worldwide, making it the most prevalent cancer worldwide.1 Among these, triple-negative breast cancer (TNBC) stands out due to its aggressive nature, poor prognosis, and high metastatic potential, resulting in a dismal 5-year survival rate of just 11%.2 The efficacy of traditional chemotherapy and targeted therapies is notably limited in TNBC because of the absence of specific molecular targets that are present in other breast cancer subtypes. Consequently, there is an urgent need to develop effective therapeutic strategies and drugs to improve survival outcomes and reduce mortality in TNBC patients.3
Neutrophils, key immune cells with anti-inflammatory functions, play critical roles in the development, prognosis, metastasis, and prognosis of TNBC.4 Tumor-associated neutrophils (TANs) are strongly correlated with poor outcomes in TNBC and are categorized into two subtypes: anti-tumor N1-type and pro-tumor N2-type.5,6 In the early stages of TNBC, N1-type TANs predominantly inhibit tumor growth by secreting type I interferon (IFN-α) and activating natural killer (NK) cells via interleukin (IL)-18.7 However, as TNBC progresses, the proportion of N2-type TANs increases. These N2-type TANs promote TNBC cell proliferation by releasing neutrophil elastase (NE) and upregulating a proliferation-inducing ligand (APRIL).8,9 Additionally, they contribute to tumor angiogenesis by secreting chemokines, vascular endothelial growth factor (VEGF), and matrix metalloproteinase-9 (MMP-9).10 Their interactions with circulating tumor cells (CTCs) also lead to the release of neutrophil extracellular traps (NETs), which facilitate blood-borne metastasis and increase the risk of pulmonary metastasis.11,12,13,14 While blocking or eliminating neutrophil infiltration into tumors can inhibit TNBC growth and metastasis to some extent, neutrophils are the most abundant immune cells in the body, and completely obstructing their migration could compromise the overall immune defense. Therefore, the precise targeting and selective elimination of N2-type TANs have become a significant focus in the development of TNBC therapies.15,16,17
Ferroptosis, a regulated form of non-apoptotic cell death characterized by excessive peroxidation of polyunsaturated phospholipids,18 is intricately linked to various biological processes, including iron metabolism, amino acid metabolism, and lipid metabolism, as well as the biosynthesis of glutathione, nicotinamide adenine dinucleotide phosphate (NADPH), phospholipids, and coenzyme Q10.19 Among these processes, the content of polyunsaturated lipids is a key determinant of ferroptosis susceptibility.18,20,21 Recent single-cell transcriptome and whole-transcriptomic analyses have revealed an enrichment and upregulation of genes involved in ferroptosis regulation within TANs.19,22,23 Lipid accumulation is more pronounced in N2-type TANs within breast cancer tumor tissues, with these stored lipids being transferred to tumor cells to fuel their growth and metastasis.24,25 This suggests that N2-type TANs may be especially susceptible to ferroptosis.
TNBC also exhibits aberrant gene expression patterns associated with ferroptosis, including upregulation of iron metabolism-related transferrin receptor and lipid metabolism-related long-chain acyl-CoA synthetase (ACSL4), along with the downregulation of iron-export transport proteins.19,26 These dysregulated gene expressions, particularly in iron and lipid metabolism, provide TNBC with an abundance of iron and lipids, making the cancer cells particularly vulnerable to ferroptosis inducers. Thus, identifying a precise target capable of inducing simultaneous ferroptosis in both N2-type TANs and TNBC cells is of significant therapeutic interest.
In this study, we discovered a small molecule, CT-1, a derivative of cryptotanshinone (CTS), which effectively suppresses TNBC growth while significantly reducing the proportion of N2-type TANs within tumor tissue. Additionally, CT-1 induces simultaneous ferritinophagy-induced ferroptosis in both N2-type TANs and TNBC cells in vitro and in vivo, by targeting FTH1 and facilitating the interaction between NCOA4 and ferritin. These findings suggest that CT-1, as a promising therapeutic agent, can induce ferroptosis in both N2-type TANs and TNBC cells, offering a strategy for TNBC treatment by targeting FTH1.
Results
N2-type TANs and TNBC cells are more susceptible to ferroptosis
Consistent with the previous report,27 there is a large amount of neutrophil infiltration in breast cancer tissues, with a higher prevalence of N2-type TANs than N1-type TANs (Figure 1A). The infiltration of neutrophils is negatively correlated with the prognosis of breast cancer (Figure 1B). Furthermore, the abundance of neutrophils in TNBC is significantly elevated compared to other subtype breast cancers, with higher expression of N2-type TANs’ markers relative to N1-type TANs (Figures 1C and 1D). The immunofluorescence analysis of TNBC tissues corroborates these findings (Figures 1E and 1H). To investigate the role of N1- and N2-type TANs in TNBC, the N1- and N2-type TANs were established using the model cell line-human promyelocytic leukemia cells (HL-60) (Figure S1A), following the reported method.28 The morphological changes (Figure S1B) and N1/N2-related markers (N1: CD95, NOS2, CCL3, N2: CD206, CCL2, and ARG2) were detected to assess the polarization of HL-60 cells (Figure S1D). After co-culturing with N1-type TANs, the survival and migration of human breast cancer cells (MDA-MB-231) were inhibited, while N2-type TANs promoted survival and migration (Figures 1F and 1G). Further analysis finds that the ferroptosis-related genes are elevated in TNBC cancer epithelial cells and neutrophils (Figure 1I). The oil red O staining also demonstrates that N2-type TANs possess higher lipid content than N1-type TANs (Figure 1J and S1C), suggesting a greater susceptibility to ferroptosis in N2-type TANs. Recent literature has reported that TANs could promote the metastasis of breast cancer by resisting ferroptosis.29 Therefore, simultaneously promoting ferroptosis in N2-type TANs and tumor cells may be an effective strategy for treating TNBC. In addition, identifying a precise target capable of inducing simultaneous ferroptosis in N2-type TANs and TNBC cells is important.
Figure 1.
N2-type TANs and TNBC cells are more susceptible to ferroptosis
(A) The cell types and the distribution of N1/N2-type TANs in breast cancer tissues were displayed using uniform manifold approximation and projection (UMAP) (GSE176078; 100,000 cells; 26 patients).
(B) Kaplan-Meier survival curves were generated for breast cancer patients categorized into high and low neutrophil infiltration groups by TIMER2.0 (http://timer.cistrome.org/).
(C) The expression of FCGR3B in different subtypes of breast cancers. FCGR3B is a prototypical marker gene expressed in neutrophils, with its expression levels serving as an indicator of neutrophil proportions.
(D) The expression of N1-type TANs and N2-type TANs markers in different subtype breast cancers.
(E and H) The immunofluorescence analysis of N1-type (CD66+CD54+) and N2-type (CD66+PD-L1+) TANs in TNBC tissues (n = 3).
(F and G) The cell survival and migration of MDA-MB-231 co-cultured with HL-60, N1-type TANs, and N2-type TANs, respectively (n = 3).
(I) The expression of ferroptosis-related genes in different cells.
(J) Oil red staining of N1 and N2 (n = 5).
Statistics: p values are from the two-tailed unpaired t test. The data are represented as mean ± SD.
CT-1 induced simultaneous ferroptosis in N2-type TANs and TNBC cells
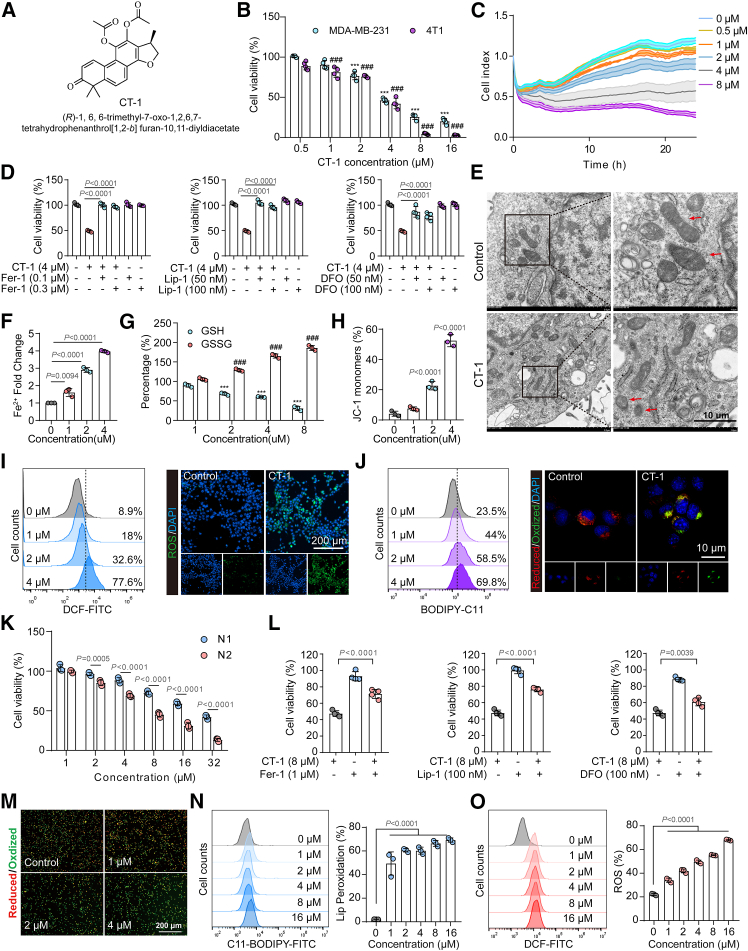
CT-1 is a derivative of CTS, an active component of the traditional Chinese medicine Danshen, whose structure, 1H-NMR, 13C-NMR, and mass spectrometry (MS) spectra are shown in Figures 2A and S2. CCK-8 and RTCA results showed that CT-1 could inhibit the proliferation of human and murine TNBC cell lines (MDA-MB-231, 4T1, and experimental mammary tumor-6 [EMT6]) in a dose-dependent manner (Figures 2B, 2C, and S4A). The efficacy of CT-1 is significantly better than that of CTS (Figure S1H). To investigate the effect of CT-1 on neutrophils, the N1- and N2-type TANs were established using the model cell line HL-60. The cell counting kit-8 (CCK-8) results showed that N2-type TANs were more sensitive to CT-1 (Figure 2K). A synergistic cytotoxic effect was observed when N2-type TANs were co-cultured with MDA-MB-231 cells and treated with CT-1, as depicted in Figures S1G and S1K.
Figure 2.
CT-1 induced simultaneous ferroptosis in N2-type TANs and TNBC cells
(A) The chemical structure of CT-1.
(B) 4T1 and MDA-MB-231 cells viability were assessed using CCK-8 assays after treatment with CT-1 for 24 h (n = 4).
(C) Real-time cellular analysis (RTCA) assays in 4T1 cells (n = 3).
(D) Cell viability of 4T1 cells treated with CT-1 in the presence of ferrostatin-1 (Fer-1, 0.1 μM, 0.3 μM), liproxstatin-1 (Lip-1, 50 nM, 100 nM), or deferoxamine (DFO, 50 nM, 100 nM) for 24 h (n = 4).
(E) Representative TEM images of 4T1 cells following CT-1 (4 μM) treatment for 24 h. Scale bars, 10 μm.
(F and G) Fe2+ fold change (n = 3) with oxidized glutathione (GSSG) and GSH levels in 4T1 cells following CT-1 treatment for 24 h (n = 3).
(H) JC-1 monomer production in 4T1 cells after CT-1 treatment for 24 h. Scale bars, 200 μm (n = 3).
(I and J) Flow cytometry assay and representative fluorescence images for (I) 2',7'-Dichlorofluorescein (DCF) and (J) C11-BODIPY labeling of 4T1 cells following CT-1 treatment for 24 h, indicating the ROS and lipid peroxidation.
(K) The cell viability of N1 and N2 cells was evaluated through CCK-8 assays following CT-1 treatment for 24 h (n = 3).
(L) Cell viability of N1 and N2 cells treated with CT-1 in the presence of ferrostatin-1 (Fer-1, 1 μM), liproxstatin-1 (Lip-1, 100 nM), or deferoxamine (DFO, 100 nM) for 24 h (n = 4).
(M) Representative fluorescence images for C11-BODIPY labeling of N2 cells following CT-1 treatment for 24 h; scale bars, 200 μm
(N and O) Flow cytometry assay for DCF and C11-BODIPY labeling of 4T1 cells following CT-1 treatment for 24 h, indicating ROS and lipid peroxidation, respectively (n = 3).
Statistics: p values are from one-way ANOVA with Tukey’s multiple-comparison test. ∗∗∗p < 0.001 and ###p < 0.001 compared with the control groups. The data are represented as mean ± SD.
Transcriptomic RNA sequencing (RNA-seq) was utilized for further mechanism investigation. The gene set enrichment analysis (GSEA) indicated that the top pathways regulated by CT-1 were related to intracellular iron homeostasis and ferroptosis (Figures S3A and S3B). There was a significant change in the expression of ferroptosis-related genes (Figure S3C). Then, the ferroptosis inhibitors (ferrostatin-1 [Fer-1], liproxstatin-1 [Lip-1], and deferoxamine [DFO]) were used to explore whether CT-1 could induce ferroptosis. All three ferroptosis inhibitors could reverse the inhibitory effect of CT-1 on N2-type TANs and TNBC cells (Figures 2D, 2L, and S3D), suggesting that CT-1 exerted its anti-tumor action via ferroptosis. To further ensure the generalizability of our findings, the primary murine neutrophils were used to validate the results (Figures S1E and S1F). As shown in Figure S3K, CT-1 inhibited the proliferation of primary murine N2-like neutrophils with an half maximal inhibitory concentration (IC50) of 7.16 μM. And the inhibitory effects of CT-1 were significantly reversed by all three ferroptosis inhibitors (Figures S3L–S3N). Additionally, lipid peroxidation, a key feature of ferroptosis, was promoted in a dose-dependent manner (Figure S3O). These results suggest that CT-1 exerts its inhibitory action through the induction of ferroptosis.
Transmission electron microscopy (TEM) showed that CT-1 increased ferroptotic morphological characteristics in 4T1 cells, such as reduced mitochondrial size, disappearance of mitochondrial cristae, and increased mitochondrial membrane density (Figure 2E). Labile iron (Fe2+), a key marker of ferroptosis, directly or indirectly increases intracellular iron levels, elevating oxidative stress and ferroptosis. After administration of CT-1, the level of Fe2+ was significantly increased (Figure 2F). Glutathione (GSH), overexpressed in tumor cells, is a critical antioxidant that maintains redox metabolism balance and protects tumor cells from reactive oxygen species (ROS)-mediated damage. Therefore, the oxidized GSH (GSSG) level is often considered a vital marker for assessing ferroptosis intensity. The GSSG/GSH ratio was increased after treatment with CT-1, indicating the oxidation of GSH (Figure 2G). CT-1 could also inhibit the form of 5,5',6,6'-tetrachloro-1,1',3,3'-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) polymers, demonstrating the decrease of cellular mitochondrial membrane potential (Figures 2H, S3I, and S3J). Flow cytometry assay and fluorescence photography results found that CT-1 could promote the generation of ROS and lipid peroxidation in TNBC cells in a dose-dependent manner (Figures 2I, 2J, and S3E–S3H). The same effects were observed in N2-type TANs (Figures 2M–2O). These findings demonstrated that CT-1 could induce ferroptosis in N2-type TANs and TNBC cells. We also assessed the impact of CTS and CT-1 on human mammary epithelial cells (michigan cancer foundation-10A [MCF-10A]). The results suggested a heightened sensitivity of TNBC cells to CT-1 compared to the mammary epithelial cells (Figures S1I and S1J).
CT-1 inhibited the growth of TNBC tumors
To evaluate the anti-tumor effect of CT-1 in vivo, 4T1 and EMT6 cells were inoculated into the fourth mammary fat pads of BALB/c mice to establish orthotopic breast cancer models. The tumor-bearing mice were injected daily with CT-1 via the tail vein, as shown in Figures 3A and 3G. Compared to the control group, CT-1 significantly inhibited the growth of 4T1 and EMT6 tumors (Figures 3B, 3C, 3H, and 3I), reduced the tumor weight (Figures 3D and 3J), and extended the survival time (Figure 3K). H&E staining, TUNEL, and Ki67 assays indicated that CT-1 promoted TNBC tumor cell necrosis and apoptosis and inhibited their proliferation (Figures S4B–S4D). And the ferroptosis-related markers (4-hydroxynonenal [4-HNE], ROS, GPX4, and FTH1) in tumor tissues were analyzed by immunohistochemistry staining to confirm the role of CT-1 in inducing ferroptosis in vivo. The results showed that the ROS (dihydroethidium [DHE]-positive) and lipid peroxidation (4-HNE positive) were significantly elevated, while the expressions of GPX4 and FTH1 were notably reduced following CT-1 treatment (Figures 3F, 3L, and S4E). These findings suggest that CT-1 effectively induces ferroptosis in vivo. Additionally, the administration of CT-1 demonstrated no toxicological impacts on the subjects, as indicated by the stable body weight of the mice (Figures 3E and S5B). This observation was further corroborated by consistent results in histological evaluations, as shown in Figure S5A. These findings suggested that CT-1 markedly suppressed TNBC tumor growth by inducing ferroptosis.
Figure 3.
CT-1 inhibited the growth of TNBC tumors
(A) The treatment regimen diagrams (n = 5).
(B) Tumor volume was monitored every day.
(C and D) Post-sacrificed tumor specimens were photographed, and tumor weight was recorded.
(E) Body weight was monitored daily.
(F) Statistical analysis of immunohistochemical staining of ferroptosis-related markers (4-HNE, DHE, GPX4, and FTH1) in EMT6 tumor tissues (n = 3).
(G) The treatment regimen diagrams (n = 6).
(H) Tumor volume was monitored every day.
(I and J) Post-sacrificed tumor specimens were photographed, and their weights were meticulously recorded.
(K) Survival rates of the animals were tracked and statistically analyzed using a log rank test, with MS indicating median survival (t test, n = 6).
(L) Statistical analysis of immunohistochemical staining of ferroptosis-related markers (4-HNE, DHE, GPX4, and FTH1) in 4T1 tumor tissues (n = 3).
Statistics: p values are from one-way ANOVA with Tukey’s multiple-comparison test. The data are represented as mean ± SD.
To further investigate the pharmaceutical properties of CT-1, pharmacokinetic experiments and acute toxicity test were conducted. The results are shown in Figures S5C and S5D. The t1/2 of CT-1 in rats was 1.82 ± 0.31 h, administered via tail vein injection at a dose of 10 mg/kg. The Cmax was 4.52 ± 0.82 mg/mL. The area under curve (AUC)0-∞ and AUC0-t were 15.91 ± 3.1 and 15.1 ± 3.07 h mg/L, respectively. Additionally, the acute toxicity test showed that CT-1, at doses of 50 mg/kg and 25 mg/kg, had no significant impact on body weight, organ indices, and blood parameters in mice (Figure S6). No clinical signs were observed in any of the animals up to 14 days post-exposure, and no deaths occurred, indicating that CT-1 possesses a favorable safety profile.
CT-1 reduced the infiltration of pro-tumor TANs
Recognizing the critical role of TANs in TNBC development, the effects of CT-1 on the TANs were further explored. An orthotopic TNBC mice model was established and treated with CT-1 or a control solvent for 14 days. Then, the tumors were collected for cytometry by time-of-flight (CyTOF) analysis (Figure 4A). Through visualization of high-dimensional data using t-distributed stochastic neighbor embedding (viSNE) analysis, 36 cell subpopulations were identified, with a minimum of ten distinct cell populations observed in both treatment groups, based on the established gating strategy (Figures S7A and S7B). A comparative analysis of these cell populations revealed notable shifts in the tumor immune microenvironment (TIME), including a reduction in neutrophils (CD11b+Ly6G+), an increase in myeloid cells (CD45+CD11b+), and a significant rise in macrophages in the CT-1-treated mice (Figures 4B–4D). These findings suggested that CT-1 may enhance the infiltration of various leukocytes into the tumor, including CD8+ T cells, B cells, dendritic cells, and monocytes. In relation to principal-component analysis (PCA), Figure 4E illustrates the seven tumor populations primarily responsible for group differentiation. To assess the influence of CT-1 on neutrophil differentiation, a trajectory analysis was conducted to investigate the dynamic evolution of neutrophils and their interactions with other immune cells. These findings revealed a temporal transition of naive neutrophils into CCR2+ and MMP12+ neutrophils subtypes (Figures 4F and 4G). Additionally, CT-1 inhibited the differentiation of neutrophils into CCR2+ and MMP12+ (Figure 4H). After testing the expression of pro-tumor TANs marker genes, the results indicated a significant increase in the expression levels of CD54, IL-2, inducible nitric oxide synthase (iNOS), and Arg1 in N1-type TANs, while MMP1 exhibited notable expression in pro-tumor TANs (Figures S7C and S7D).
Figure 4.
CT-1 reduced the infiltration of pro-tumor TANs
(A) Analysis of tumor-derived single-cell suspensions via CyTOF (control group, n = 4; CT-1 group, n = 3).
(B) Immune landscapes of peripheral CD45+ cells with an equal number (1 × 106 cells) from each group. Representative cell distribution by t-distributed stochastic neighbor embedding (tSNE) plot of tumor-infiltrating leukocytes overlaid with color-coded clusters in 4T1 tumors from saline-treated versus CT-1-treated BALB/c mice. Elliptical dotted lines accentuate the clusters where notable differences were observed between the two groups.
(C and D) Frequency comparison of major immune cell types and neutrophils in between groups.
(E) PCA analysis depicting individual mice; CT-1-treated mice are marked in red (with a surrounding red ellipse), and saline-treated mice are marked in blue (with a surrounding blue ellipse).
(F–H) Pseudotime-ordered analysis of cancer cells from saline and CT-1 samples. Pseudotime (F), neutrophil subgroups (G), and group (H) are labeled by colors.
(I) Quantitative results for N2-type TANs.
(J and K) Quantitative results for anti-tumor and pro-tumor TANs. NOS2 and CD206 are the markers for anti-tumor and pro-tumor TANs, respectively (n = 3).
(L) Representative multiplex immunohistochemistry images of the spatial distribution and abundance for Ly6G, CD11b, NOS2, CD206, and DAPI in tumor sections. Scale bar, 100 μm.
Statistics: p values are from the two-tailed unpaired t test. The data are represented as mean ± SD.
CT-1 could reduce the proportion of pro-tumor TANs within 4T1 tumor tissues (Figure 4I). Notably, CD206 was identified as an inhibitor of anti-tumor immunity.22 Supporting these findings, flow cytometry analysis demonstrated that CT-1 decreased the expression of immunosuppressive cytokines CD206 and increased NOS2 in TANs within 4T1 tumors (Figures 4J and 4K). Consistent with these observations, immunofluorescence analysis of tumor sections showed that CT-1 reduced the presence of CD206 N2-type TANs and increased the population of NOS2 N1-type TANs (Figure 4L). These data suggested that CT-1 could eliminate pro-tumor TANs and reverse neutrophil-mediated tumor immunosuppression.
FTH1 was a direct target of CT-1
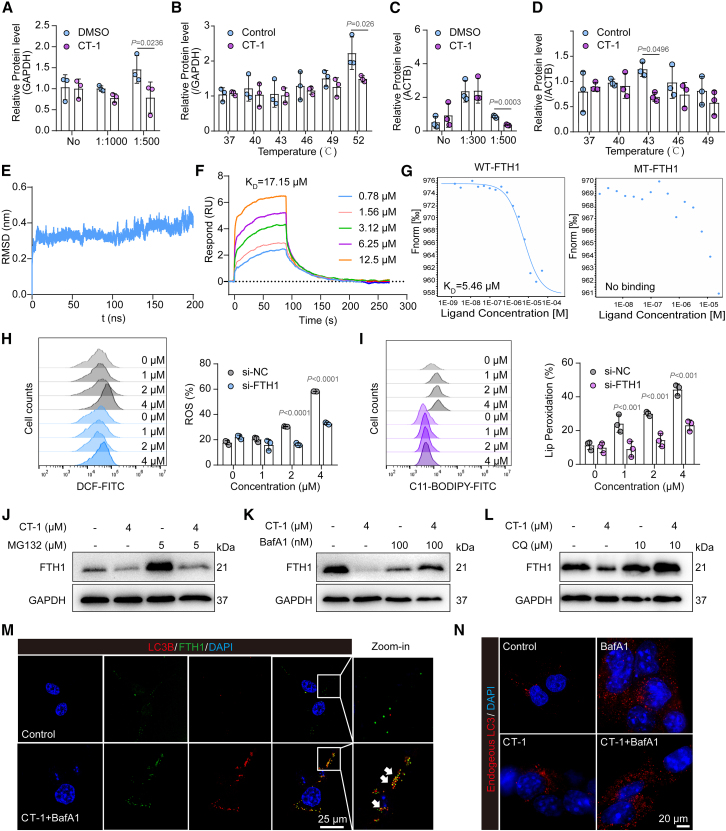
CT-1 could induce ferroptosis in TNBC cells, but the molecular target of CT-1 in TNBC cells remains unclear. The ferroptosis-related proteins were prioritized for follow-up. Using the online target predict tool omics and text based target enrichment and ranking (OTTER) to analyze the transcriptome and proteome, the top-ranked target FTH1 was identified (Figure S8A). FTH1 is a subunit of ferritin that catalyzes the oxidation of Fe2+ to Fe3+, which plays a pivotal role in ferroptosis. To identify that FTH1 is the molecular target of CT-1 in ferroptosis, a drug affinity responsive target stability (DARTS) assay was performed. The immunoblot analysis of the DARTS sample revealed the decreased stabilization of FTH1 during the proteolysis process when treated with CT-1 at 100 μM in 4T1 cells (Figures 5A and S9A). Then, the cellular thermal shift assay (CETSA) also proved that CT-1 could decrease the thermal stability of FTH1 in the intact living 4T1 (Figures 5B and S9B) at 49°C and 52°C. Subsequent FTH1 and CT-1 interaction validation was conducted using CETSA and DARTS assays in N2-type TANs. These results indicated that CT-1 could decrease the enzyme and thermal stability of FTH1 (Figures 5C, 5D, S9C, and S9D), suggesting that CT-1 promoted ferroptosis in N2-type TANs in an FTH1-dependent manner.
Figure 5.
CT-1 targeted FTH1 and promoted its NCOA4-mediated ferritinophagy
(A and B) Immunoblot analysis of DARTS and CETSA samples revealed the decreased stabilization of FTH1 during the proteolysis and heating processes in 4T1 cells (n = 3).
(C) Immunoblot analysis of the DARTS sample further revealed the increased stabilization of FTH1 during the proteolysis process in N2-type TANs (n = 3).
(D) CT-1 significantly decreased the thermal stability of FTH1 in CETSA assays at 43°C in N2-type TANs (n = 3).
(E) Root-mean-square deviation (RMSD) showcases the conformational change of FTH1 upon binding with CT-1.
(F) SPR analysis of CT-1 binding to FTH1 (KD = 17.15 μM).
(G) MST analysis of CT-1 binding to WT-FTH1 (KD = 5.46 μM) and MT-FTH1.
(H and I) FTH1 knockdown through siRNA significantly alleviated the ROS production and lipid peroxidation of CT-1 on 4T1 cells (n = 3).
(J–L) BafA1 and CQ but not MG132 reversed CT-1-mediated FTH1 degradation.
(M) Immunofluorescence analysis was conducted to observe the colocalization in 4T1 cells post-CT-1 treatment (4 μM), with or without BafA1 (100 nM) for 24 h. Endogenous FTH1 was marked with a green fluorescent signal, while LC3 was highlighted in red. The cell nuclei were distinctly stained with DAPI, emitting a blue fluorescence. The images show the potential colocalization of FTH1 and LC3, as the white arrows indicate. Scale bars, 25 μm.
(N) Immunofluorescence analysis of the content of endogenous LC3 in cells treated with or without BafA1 and CT-1. Scale bars, 20 μm.
Statistics: p values are from one-way ANOVA with Tukey’s multiple-comparison test. The data are represented as mean ± SD.
Molecular dynamics simulation showed the binding of FTH1 with CT-1 (Figures 5E and S9E). The results of surface plasmon resonance (SPR) displayed that CT-1 directly bonded to FTH1 with a KD value of 17.15 μM (Figure 5F). Next, the molecular docking predicted that the binding sites of FTH1 were Pro128 and Thr123 (Figure S9G). To verify the critical role of Pro128 and Thr123 in the complex interaction, these two sites were mutated, and microscale thermophoresis (MST) was used to test affinity. The results showed that the KD value between CT-1 and the wild-type FTH1 was 5.46 μM, while there was no binding with the mutant FTH1 (Figure 5G). This result further confirmed the critical role of Pro128 and Thr123 in CT-1 binding with FTH1. Additionally, the expression level of FTH1 was notably higher in N2-type TANs, as illustrated in Figure S9F. These findings suggested a potential rationale for the preferential targeting of N2-type TANs by CT-1. Subsequently, the FTH1 was knocked down using small interfering RNA (siRNA), and the inhibitory effect of CT-1 on TNBC cells was reversed (Figure S9H). Additionally, the markers of ferroptosis, ROS, and lipid peroxidation were significantly reversed in a dose-dependent manner (Figures 5H, 5I, and S9I–S9K). To further validate the role of FTH1 in the anti-tumor effect of CT-1, the lentivirus-mediated FTH1 knockdown stable cell line was constructed. Firstly, the impact of FTH1 on TNBC tumor growth was assessed (Figure S10A). Upon inoculation of 4T1 cells with FTH1 knockdown into the mouse mammary fat pad, a significant deceleration in growth rate was observed compared to the control group. Additionally, the inhibitory efficacy in the shFTH1 group was not significant compared to the control group, indicating that FTH1 knockdown attenuated the suppressive effect of CT-1 on the growth of 4T1 tumors in vivo (Figures S10B–S10E).
NCOA4-mediated ferritinophagy was crucial for CT-1-induced ferroptosis
To explore the specific mechanism of CT-1 action on FTH1, qRT-PCR was used to assess the mRNA expression levels of FTH1 after CT-1 treatment, and no significant alterations were observed (Figure S11A). Subsequent addition of the protein synthesis inhibitor cycloheximide (CHX) revealed that CT-1 accelerated the degradation of FTH1 protein (Figures S11B and S11C). Protein degradation primarily occurs via two pathways: the proteasomal pathway and the autophagy-lysosomal pathway. Initially, the proteasome inhibitor MG132 was introduced, revealing its inability to antagonize the degradative effect of CT-1 on FTH1 protein (Figures 5J, S11D, S11N, and S11O). Then, the addition of lysosomal inhibitors Bafilomycin A1 (BafA1) or chloroquine (CQ) resulted in significant inhibition of FTH1 protein degradation induced by CT-1 (Figures 5K, 5L, S11J, and S11K). Both BafA1 and CQ were able to reverse the effects of CT-1 (Figures S11E, S11F, and S11V), indicating that CT-1 promoted lysosomal autophagic degradation of FTH1 rather than via the proteasomal pathway.
NCOA4 is a key regulator of lysosomal autophagic degradation of FTH1, which aligns with our observation that CT-1 promotes the autophagic degradation of FTH1—a process significantly inhibited by lysosomal inhibitors like BafA1 or CQ. Then the relationship between NCOA4-mediated FTH1 degradation and CT-1-induced ferroptosis in TNBC cells was further explored. Western blot analysis showed that CT-1 led to a dose-dependent decrease in FTH1 and NCOA4 levels in TNBC cells (Figures S11H, S11L, S11P, and S11S). Knockdown of NCOA4 or FTH1 attenuated the effects of CT-1 in MDA-MB-231 cells (Figures S11Q, S11R, S11T, and S11U). The results of co-immunoprecipitation (coIP) experiments demonstrated that CT-1 enhances the interaction between NCOA4 and FTH1 (Figures S11G and S11W). Confocal microscopy analysis revealed that treatment with CT-1 and BafA1 increased the colocalization of FTH1 and LC3B, indicating that CT-1 promoted the lysosomal autophagy of FTH1 (Figure 5M). And the autophagy marker LC3B-II was significantly increased after treating with CT-1 in a time-dependent manner (Figures S11I and S11M). Pre-treatment with autophagy inhibitor BafA1 further increased the content of endogenous LC3 in cells treated with CT-1 (Figure 5N). These findings suggest that CT-1-triggered NCOA4-mediated autophagy played a crucial role in the degradation of FTH1 protein, ultimately contributing to ferroptosis induced by CT-1.
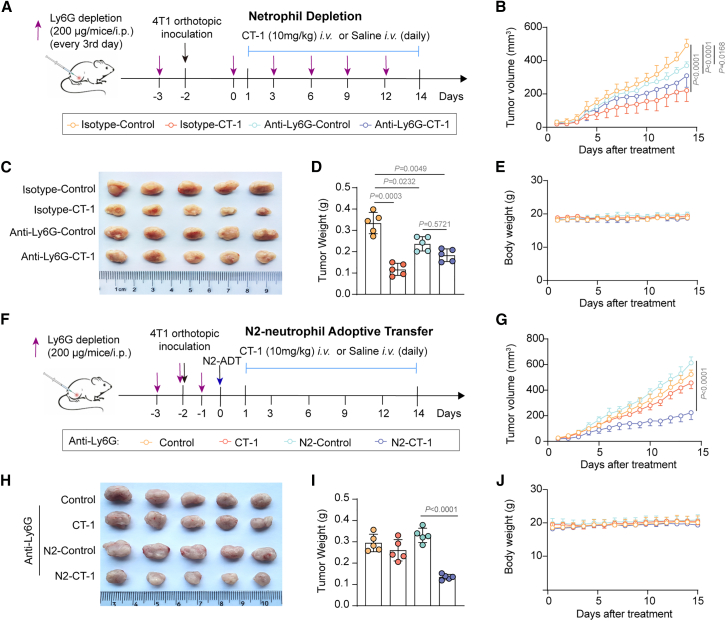
Blocking N2-type TANs reversed the anti-tumor effects of CT-1
Further, confirming the role of TANs as the primary immune cells mediating the inhibitory effects of CT-1 on tumor growth, 4T1 tumor-bearing mice were treated with anti-Ly6G antibody to deplete neutrophils (Figure S12A). The anti-Ly6G antibody was injected every three days, with the control group receiving an immunoglobulin G (IgG) isotype control, and CT-1 was administered daily. The experimental setup is illustrated in Figure 6A. Compared to the isotype control group in wild-type tumor-bearing mice, blocking neutrophils resulted in a deceleration of tumor growth, and the efficacy of CT-1 was diminished (Figures 6B–6E). To further confirm that CT-1 reduces TNBC tumor growth by inhibiting N2-type TANs, the in vivo adoptive transfer experiments were performed (Figure 6F). As shown in Figures 6G–6J, adoptive transfer of N2-type TANs restored the anti-tumor efficacy of CT-1. The flow cytometry analysis demonstrated that CT-1 decreased the proportion of N2-type TANs and the expression of immunosuppressive cytokines CD206 within 4T1 tumors (Figure S12B). These results suggest that CT-1 exerted its effects based on inhibiting N2-type TANs.
Figure 6.
Blocking N2-type TANs reversed the anti-tumor effects of CT-1
(A) The diagrams illustrate the neutrophil depletion experiment. 4T1 cells (6 × 105) were administered intravenously via the tail vein of BALB/c mice either with or without the intravenous (i.v.) administration of CT-1 (10 mg/kg) every day plus injections of either an isotype or an anti-Ly6G antibody every three days (n = 5).
(B) Tumor volume was monitored daily.
(C and D) Following the sacrifice of mice, the tumors were photographed and tumor weight (D) was recorded.
(E) Body weight was monitored daily.
(F) The diagrams illustrate the N2-TANs adoptive transfer experiments. The primary murine neutrophils were isolated from bone marrow and activated by TGF-β1. The activated N2-like neutrophils were suspended in 100 μL PBS before adoptively transferred into the recipient mice with neutrophils depletion by tail vein injection after subcutaneous tumor inoculation.
(G) Tumor volume was monitored daily.
(H and I) Following the sacrifice of mice, the tumors were photographed and tumor weight (I) was recorded.
(J) Body weight was monitored daily.
Statistics: p values are from one-way ANOVA with Tukey’s multiple-comparison test. Data are represented as mean ± SD.
CT-1 suppressed the growth of TNBC PDOs
Patient-derived organoids (PDOs), as a clinically relevant and practical model, have shown promise in drug response prediction, mirroring the morphology and properties of original human tumors. Particularly in TNBC PDOs, previous research has highlighted their applicability in preclinical drug trials and forecasting treatment outcomes. In this study, a prospective, observational investigation was conducted to evaluate the efficacy of CT-1 using the TNBC PDO model. CT-1 was administered to PDOs at different concentrations over six days, as illustrated in Figure 7A. CT-1 impeded the formation and viability of the organoids. Live/Dead staining was utilized to investigate further the effects of CT-1 on the viability of the organoids, distinguishing between viable (green) and non-viable (red) organoids. The results showed a significant reduction in the quantity and viability of TNBC organoids following CT-1 treatment, with IC50 values of 2.35 μM, 3.69 μM, and 6.74 μM on three different PDOs (Figures 7B and 7C).
Figure 7.
CT-1 inhibited the growth of TNBC PDOs
(A) Schematic representation of the experimental design.
(B) The influence of CT-1 on organoids was assessed in a concentration-dependent manner, evaluating organoid sizes (%) (n = 3).
(C) Visualization of PDO post-CT-1 treatment for 6 days, stained with Live/Dead fluorescent dye, conducted in triplicate. Scale bars, 200 μm.
The data are represented as mean ± SD.
Discussion
TNBC remains one of the most challenging subtypes of breast cancer to treat, largely due to its lack of specific therapeutic targets and the presence of a highly immunosuppressive tumor microenvironment. TANs are closely linked to TNBC progression, metastasis, staging, grading, and poor patient outcome.4,10,30 Current strategies aiming to block TANs infiltrating or eliminating TANs within tumor tissues have shown limited success or remain under investigation.15,16,17 Given that neutrophils are the most abundant immune cells in the body, their widespread removal or blockade could compromise overall immune function. Therefore, the selective targeting of pro-tumor N2-type TANs represents a promising approach in TNBC therapy.
N2-type TANs are particularly problematic as they contribute significantly to TNBC progression by promoting tumor growth, angiogenesis, immune suppression, and metastasis through mechanisms such as the release of NETs.12,16,31,32 Therefore, developing innovative strategy and drugs that can selectively eliminate N2-type TANs without affecting the beneficial N1-type TANs is crucial for advancing TNBC treatment. Our study introduces CT-1, a small molecule capable of effectively inhibiting N2-type TANs at low doses (e.g., 4 μM in vitro and 5 mg/kg in vivo), while sparing N1-type TANs. These findings suggest that CT-1 could be a promising targeted agent for enhancing cancer immunotherapy by specifically depleting pro-tumor N2-type TANs. Through CyTOF analysis, we observed a significant reduction in TANs within the tumor microenvironment following CT-1 treatment. Moreover, the TANs affected by CT-1 exhibited decreased immunosuppressive functions, as evidenced by reduced levels of the N2 marker Arg1. CT-1 also induced ferroptosis specifically in TNBC cells and N2-type TANs, sparing other immune cells and thereby mitigating the immunosuppressive side effects commonly associated with traditional ferroptosis inducers.23,33,34,35
Ferroptosis, a regulated form of cell death driven by lipid peroxidation, is increasingly recognized as a key mechanism in tumor suppression.21 Our study reveals that N2-type TANs have elevated lipid content compared to N1-type TANs, making them more vulnerable to ferroptosis. CT-1 exploits this vulnerability by targeting FTH1 in both TNBC cells and N2-type TANs, inducing ferroptosis through the promotion of NCOA4-mediated ferritinophagy. This dual targeting of FTH1 in both cell types underpins the synergistic anti-tumor effect of CT-1.
Ferritin, composed of FTH1 and ferritin light chain (FTL) subunits, plays a crucial role in maintaining iron homeostasis by sequestering excess cellular iron, thus preventing oxidative damage from the Fenton reaction.36 FTH1-mediated ferroptosis is triggered by the release of free Fe2+ through NCOA4-dependent ferritinophagy, leading to increased ROS production and lipid peroxidation.37,38,39 Our study demonstrates that CT-1 enhances the interaction between FTH1 and NCOA4, promoting lysosomal autophagy of FTH1 and consequently inducing ferroptosis. The elevated expression levels of FTH1 and NCOA4 in neutrophils, compared to other immune cells, may explain the specific impact of CT-1 on these cells (Figure S8B). Blocking TANs or knocking down FTH1 significantly diminishes the therapeutic efficacy of CT-1 in TNBC tumors, suggesting that the effect of CT-1 is not solely attributed to a singular mechanism but rather to synergistic collaboration. We observed that CT-1 achieved a tumor inhibition rate of 61.55% when administered without neutrophil blockade. However, when neutrophils were blocked, the tumor inhibition rate was reduced to 19.58%. This suggests that the contribution of neutrophil ferroptosis to the overall anti-TNBC effect is approximately 40%. These findings indicate that the ferroptosis induced by CT-1 in both TANs and TNBC cells plays important roles in its anti-tumor efficacy.
CT-1 is a derivative of CTS. CTS is a quinone diterpenoid compound isolated from the roots of Danshen,40,41 known for its anti-cancer,42,43,44 antioxidant,45 and anti-inflammatory properties.45,46 Previous studies have indicated the anti-TNBC potential of high-dose CTS, yet its efficacy is hindered by inadequate water solubility and in vivo stability. To address these issues, we optimized the drug properties of CTS by synthesizing five structural derivatives and investigating their structure-activity relationships. The derivatives containing an α, β-unsaturated ketone structure in the A ring demonstrate the most promising anti-TNBC effects. Among them, CT-1 could induce ferroptosis in both N2-type TANs and tumor cells to inhibit TNBC in vitro and in vivo, which deserves further investigation.
Our study reveals the high expression of FTH1 in both pro-tumor TANs and TNBC cells, highlighting its potential as a therapeutic target for neutrophil-specific cancer therapies. CT-1, the small-molecule compound targeting FTH1, represents a therapeutic approach for TNBC by inducing ferroptosis in both TANs and TNBC cells.
In summary, our study introduces CT-1 as a small molecule with significant potential substantial therapeutic potential against TNBC. CT-1 effectively inhibits TNBC by targeting FTH1 and promoting the interaction between NCOA4 and ferritin, thereby inducing ferritinophagy-mediated ferroptosis in both N2-type TANs and TNBC cells. These findings underscore CT-1’s dual-action mechanism as a promising strategy for treating TNBC by inducing simultaneous ferroptosis in both pro-tumor neutrophils and cancer cells. Targeting FTH1 to trigger ferroptosis in N2-type TANs and TNBC cells emerges as a compelling approach for TNBC therapy, offering avenues for future clinical applications.
Limitations of the study
Given the limitations of our study, which was conducted on mouse models of TNBC, future research should involve xenograft models derived from immunized human patients to validate these findings and explore the clinical potential of CT-1. Additionally, the impact of CT-1 on other myeloid cells, such as dendritic cells and myeloid-derived suppressor cells, warrants further investigation to fully understand its effects on the immune system.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Xin Luan (luanxin@shutcm.edu.cn).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
The RNA-seq data generated in this study have been deposited in Genome Sequence Archive for Human (GSA-Human, https://ngdc.cncb.ac.cn/gsa-human) in the National Genomics Data Center (NGDC) with accession number HRA009103. Proteomics data are available at PRIDE (https://proteomecentral.proteomexchange.org; Accession # PXD057337). All other data are available in the main text or the supplementary materials.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (82322073, 82274153, and 82173846), Oriental Scholars of Shanghai University (TP2022081), Shuguang Program of Shanghai Education Commission, Youth Project of Shanghai Oriental Talents Program, Jiangxi Province Thousand Talents Program (jxsq2023102168), Young Talent Lifting Project of China Association of Chinese Medicine [NCACM-(2021-QNRC2-A08)], Shanghai Rising-Star Program (22QA1409100), High-level Key Discipline of National Administration of Traditional Chinese Medicine (zyyzdxk-2023071), Three-year Action Plan for Shanghai TCM Development and Inheritance Program (ZY(2025-2027)-2-2-1), Innovation team of high-level local universities in Shanghai: Strategic Innovation Team of TCM Chemical Biology, CAMS Innovation Fund for Medical Sciences (CIFMS, 2023-I2M-3-009), the Organizational Key Research and Development Program of Shanghai University of Traditional Chinese Medicine (2023YZZ02), Open Project of Guangxi Key Laboratory of Bioactive Molecules Research and Evaluation (BMRE2024-KF04), China Postdoctoral Science Fund - General Fund (2024M762110), and National Key Laboratory of Lead Druggability Research (NKLYT2023010). The figures were created with BioRender (https://app.biorender.com/).
Author contributions
Conceptualization and supervision, X.L., W.Z., L.Z., Q.S., and H.C.; methodology and investigation, Y.L., J.G., and L.Y.; resources and software, Y.Y., Y.G., J.L., J.J., H.Y., and B.W.; writing – original draft and writing – review and editing, Y.L. and L.Z.
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| β-Actin antibody | EpiZyme | Cat#LF201 |
| GAPDH antibody | EpiZyme | Cat#LF205 |
| anti-Ly6G | BioXCell | Cat#BE0075-1 |
| FTH1 antibody | Abcam | Cat#ab65080; RRID: AB_10564857 |
| SQSTM1 antibody | Abcam | Cat#ab109012; RRID: AB_2810880 |
| FTH1 antibody | Cell Signaling Technology | Cat#4393S; RRID: AB_11217441 |
| LC3B antibody | Cell Signaling Technology | Cat#83506S; RRID: AB_2800018 |
| NCOA4 antibody | Cell Signaling Technology | Cat#66849S; RRID: AB_3064842 |
| NCOA4 antibody | Santa Cruz Biotechnology Inc | Cat#sc-373739 |
| Alexa Fluor 647 conjugate | Cell Signaling Technology | Cat#4418S; RRID: AB_1904017 |
| HRP-linked anti-mouse IgG | Cell Signaling Technology | Cat#7076P2; RRID: AB_330924 |
| HRP-linked anti-rabbit IgG | Cell Signaling Technology | Cat#7074S; RRID: AB_2099233 |
| Normal mouse IgG | Santa Cruz Biotechnology Inc | Cat#sc-2025 |
| Chemicals, peptides, and recombinant proteins | ||
| RPMI-1640 medium | Thermo Fisher Scientific | Cat#11875093 |
| Leibovitz’s L-15 medium | Thermo Fisher Scientific | Cat#11415064 |
| DMEM medium | Meilunbio | Cat#MA0212 |
| IMDM medium | Thermo Fisher Scientific | Cat#31980030 |
| FBS | Thermo Fisher Scientific | Cat#10091155 |
| penicillin/streptomycin | Meilunbio | Cat#PWL062 |
| erythrocyte lysis buffer | Biosharp | Cat#BL503B |
| staining buffer | BD Pharmingen | Cat#554656 |
| phosphate-buffered saline (PBS) | Meilunbio | Cat#MA0015 |
| 2′, 7′-dichlorodihydrofluorescein (DCFH) | Beyotime | Cat#S0033S |
| BSA | Meilunbio | Cat#MA0100 |
| pronase | Roche | Cat#10165921001 |
| phosphatase | Roche | Cat#04906837001 |
| complete protease inhibitors | Roche | Cat#4693116001 |
| IgG2A | BioXcell | Cat#BE0085 |
| MG132 | Meilunbio | Cat#MB5137 |
| CQ | Topscience | Cat#T8689 |
| baf A1 | Topscience | Cat#T6740 |
| Recombinant human FTH1 protein | Novoprotein | Cat#P02794 |
| Neofect™ DNA transfection reagent | Beijing SBS Genetech Co. Ltd | Cat#TF20121201 |
| Critical commercial assays | ||
| MACS-based neutrophil isolation kit | Milteyni Biotec | Cat#130-097-658 |
| CCK-8 solution | Meilunbio | Cat#MA0225-4 |
| GSH and GSSG assay kit | Beyotime | Cat#S0053 |
| Mito-FerroGreen | Dojindo | Cat#M489 |
| FastPure Cell/Tissue Total RNA Isolation Kit | Vazyme | Cat#RC101 |
| HiScript II Q RT SuperMix | Vazyme | Cat#R223 |
| 2×Universal SYBR Green Master Mix | Vazyme | Cat#Q511 |
| Deposited data | ||
| RNA-seq raw data | This paper | GSA-Human: HRA009103 |
| mass spectrometry proteomics raw data | This paper | PRIDE: PXD057337 |
| Experimental models: Cell lines | ||
| 293T cell | Cell Bank of the Shanghai Institute of Cell Biology | SCSP-502 |
| 4T1 cell | Cell Bank of the Shanghai Institute of Cell Biology | SCSP-5056 |
| MDA-MB-231 cell | Cell Bank of the Shanghai Institute of Cell Biology | SCSP-5043 |
| EMT6 cell | ATCC | CRL-2755 |
| HL-60 cell | Cell Bank of the Shanghai Institute of Cell Biology | SCSP-5210 |
| Oligonucleotides | ||
| siRNA Sequences | GenePharma | Table S1 |
| primer sequences | Sangon Biotech | Table S2 |
| Recombinant DNA | ||
| FTH1 | GenePharma | MTTASTSQVRQNYHQDSEAAINRQIN LELYASYVYLSMSYYFDRDDVALKNF AKYFLHQSHEEREHAEKLMKLQNQR GGRIFLQDIKKPDCDDWESGLNAME CALHLEKNVNQSLLELHKLATDKNDPH LCDFIETHYLNEQVKAIKELGDHVTNLR KMGAPESGLAEYLFDKHTLGDSDNES |
| Software and algorithms | ||
| Flowjo v10 | Flowjo | https://www.flowjo.com/ |
| Graphpad Prism 8.0 | Graphpad Prism | https://www.graphpad.com/ |
| ImageJ | National Institutes of Health | https://imagej.nih.gov/ij/ |
Experimental model and study participant details
Cell lines and cell culture
Authenticated 293T, 4T1, MDA-MB-231, EMT6, and HL-60 cell lines were purchased from the Cell Bank of the Shanghai Institute of Cell Biology (SIBS). 4T1 and EMT6 cells were cultured in RPMI-1640 medium, MDA-MB-231 cells were cultured in Leibovitz’s L-15 medium, and the 293T cell lines were cultured in DMEM medium. HL-60 cell lines were cultured in IMDM medium, which medium was supplemented with 20% (v/v) FBS. Each culture medium used in our experiments was enhanced with 10% (v/v) FBS and 1% penicillin/streptomycin The cell cultures were maintained at 37°C in a humidified incubator, which provided an atmosphere of 5% CO2 and 95% air.
Human samples
The human TNBC tumor tissue sections used for immunohistochemistry (IHC) staining were obtained from tumor samples (43.16 ± 59.62 cm3), which were purchased from Outdo Biotech Company (Shanghai, China). The patients, all female with an average age of 51 ± 7.55 years, were clinical diagnosed with TNBC at stage II-Ⅲ. All samples were collected by surgery after obtaining written informed consent from the patients. The study protocol was approved by the Ethics Committee of Shanghai Outdo Biotech Company (No. SHX2021YF01).
Animal experiments
The four-week-old female Balb/c mice were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. The mice were maintained in a 12 h light/dark cycle at 22°C with free access to food and water. All the animal experiments were approved by the Ethics Committee for Animal Experiments (SHUTCM) of Shanghai University of Traditional Chinese Medicine (No. PZSHUTCM2302270015).
Method details
Polarization of HL-60 cells
2×105 cells/mL HL-60 cells were cultured with 10 μM all-trans retinoic acid (ATRA) for 7 days to obtained N1-like HL-60 cells. The media was changed every 3 days for nutrient replenishment. Then the N1-like HL-60 cells were subsequently stimulated with 100 ng/mL of TGF-β1 for 3 days as N2-like HL-60 for further investigation.
Separation of primary murine neutrophils
After euthanizing the mice, the tibia and femur were removed and flushed with1640 basic medium using a 25-gauge needle. Erythrocytes were removed by centrifuging the cell suspension and treating with erythrocyte lysis buffer. Neutrophils were then washed with staining buffer and further purified with a MACS-based neutrophil isolation kit. The purified neutrophils were subsequently stimulated with 100 ng/mL of TGF-β1 as N2-like neutrophils for further investigation.
Bioinformatic analysis
The raw data of single-cell transcriptome profiling from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm. nih.gov/geo/) (GSE176078) was analyzed by the online single-cell analysis platform (Single cell portal, https://singlecell.broadinstitute.org/single_cell). The RNA-seq gene expression profile and clinical information of Breast Invasive Carcinoma (BRCA) were obtained from the TCGA database (https://gdc-portal.nci.nih.gov/). After matching with Propensity score matching (PSM), survival analysis was performed using the Kaplan–Meier method and log rank test. The R package survival (V3.40-0) was utilized for the survival analysis. The ferroptosis-related genes were obtained from the FerrDb database (http://www.zhounan.org/ferrdb/current/), and the expression levels of these genes were analyzed.
CCK-8 assay
TNBC cells were first plated in 96-well plates (Corning, model 3599) at a density of 50,000 cells per well, with each well containing 100 μL of culture. Following overnight incubation, these cells were subjected to various concentrations of CT-1 for 24 h. Subsequently, 10 μL of CCK-8 solution was added to each well, and incubation was extended for two more hours. Absorbance readings at 450 nm were obtained using a Synergy H1 microplate reader (BioTek). Data were adjusted relative to the untreated control group and expressed as '% Inhibition', calculated using the formula: (ODA – ODB)/(ODC – ODB) 100 % × 100 %. Here, ODA denotes the absorbance in wells treated with different concentrations of CT-1, ODB represents wells containing only the medium (blank group), and ODC pertains to wells with control groups.
Measurement of GSH/GSSG
The reduced glutathione (GSH) and oxidized glutathione disulfide (GSSG) in cell lysates or tissue lysates were measured with GSH and GSSG assay kit according to the manufacturer’s instruction.
Measurement of Fe2+
The cell or tissue lysates were measured with Mito-FerroGreen according to the manufacturer’s instructions.
BODIPY-C11 staining
TNBC cells (1 × 105 cells/well) were seeded in a 12-well plate (Corning, model 3513) and treated with CT-1 at a range of concentrations for 24 h. Following trypsinization, the cells were prepared for staining procedures. For the BODIPY-C11 staining process, cells were resuspended in 1 mL of phosphate-buffered saline (PBS). This suspension included 5 mM of either BODIPY 581/591 C11 or BODIPY 665/676, and the cells were incubated for 30 min at 37°C in a tissue culture incubator. Post-incubation, the cells were washed and resuspended in 200 mL of fresh PBS for immediate analysis via a flow cytometer (CytoFLEX, Beckman). For the BODIPY 581/591 C11 staining, detection was carried out for both the non-oxidized C11 (in the PE channel) and the oxidized C11 (in the FITC channel). The Mean Fluorescence Intensity (MFI) ratio of FITC to MFI of PE was calculated for each sample. The data obtained were normalized against the control samples, illustrating the relative levels of lipid ROS.
ROS measurement
TNBC cells were cultured in 12-well plates (Corning, model 3513) at a density of 1 × 105 cells/well overnight. After a 24-h incubation period with varying concentrations of CT-1, the cells were gently rinsed with PBS. This was followed by a 30-min incubation with 20 μmol/L of 2′, 7′-dichlorodihydrofluorescein (DCFH). Imaging these cells was performed using a High Content Analysis System (manufactured by PerkinElmer, USA), and the resulting fluorescence signals were quantified using flow cytometry.
siRNA transfection
siRNA, along with negative (NC) and positive controls (PC) for GAPDH, were custom-synthesized. Details of these oligonucleotide sequences are provided in Table S1. We employed TransMate for the transfection process, strictly adhering to the provided protocol. 4T1, MDA-MB-231, and N2-type HL-60 cell lines, at a density of 300,000 cells per well, were cultured in 6-well plates for this purpose. These cells were then subjected to siRNA transfection. After a 48-h post-transfection, cells were harvested to analyze protein expression levels, utilizing the Western blot technique.
Quantitative PCR analysis
RNA extraction was achieved using the FastPure Cell/Tissue Total RNA Isolation Kit. The cDNA synthesis was performed using the HiScript II Q RT SuperMix for qPCR. The quantitative PCR (qPCR) analyses on the cDNA samples were conducted using 2×Universal SYBR Green Master Mix on a StepOnePlus Real-Time PCR System (Thermo Fisher Scientific). Changes in mRNA expression levels were determined using the ΔΔCt method, with GAPDH as internal control. All results were normalized to untreated control samples for comparative analysis. The primer sequences are provided in Table S2.
Western blotting
TNBC cells (3 × 105 cells/well) were grown in 6-well plates (Corning, 3516) and exposed to CT-1 at specified concentrations. Then, the cells were lysed by pre-cooled NP-40 buffer containing a protease inhibitor. The total proteins were separated by 12.5% (EpiZyme, PG113) or 15% (EpiZyme, PG114) SDS-PAGE gel and transferred to a 0.2 μm PVDF membrane (Immobilon, ISEQ00010). Blocking of membranes was performed in 1× TBST supplemented with 5% nonfat milk, conducted at room temperature for 1 h. The detection of protein signals utilized the ChemiDoc Imaging System (Bio-Rad), and quantitative analysis was carried out using ImageJ software (NIH). For normalization, either β-Actin (1:1000) or GAPDH (1:1000) served as loading controls.
Immunofluorescence
TNBC cells (5 × 104 cells/well) were cultured in 20 mm confocal dishes and subjected to either DMSO as a solvent control or varying durations of CT-1 treatment. Cells were then fixed using 4% paraformaldehyde for 20 min and permeabilized with a 0.5% Triton X-100 solution for 5 min. Blocking was performed using 1% BSA in 1× PBS for 1 h at ambient temperature. Overnight incubation at 4°C with anti-FTH1 antibody (1:1000, CST) followed. Post-primary antibody incubation; cells were rinsed with ice-cold 1× PBS and incubated for 60 min with a fluorescently labeled secondary antibody at a dilution of 1:500. After another wash with 1× PBS, cells were stained with DAPI for 10 min. The resulting fluorescence images were captured using a GE DeltaVision OMX SR imaging system.
Co-IP
The 4T1 cells were treated with CT-1 (4 μM) for 12 h. Post-treatment, these cells were harvested, rinsed with PBS, and subsequently lysed using a cell lysis buffer (Beyotime, P0013F). 2 mg of the total protein extract was used. Then, 5 μL of anti-FTH1 antibody (concentration 1 μg/μL) or a comparable volume of normal rat IgG antibody (0.4 μg/μL) was added, and the mixture was incubated overnight at 4°C. The subsequent step involved the addition of 20 μL of protein A beads (Santa Cruz Biotechnology Inc., sc-2003) to the immune complexes, followed by a 2 h incubation at 4°C to facilitate bead conjugation. The bead conjugates were gathered and washed five times with 200 μL of precooled IP lysis buffer. The final step entailed incubating the samples with the 3×SDS loading buffer for immunoblotting analysis.
Molecular docking
The co-crystal structure of FTH1, obtained from the Protein DataBank (PDB, entry 6IHT), was utilized as a basis for modeling the interaction between CT1 and the FTH1/DNA complex. Before the simulation of FTH1’s binding affinity toward the protein, all water molecules in the crystal structure were eliminated.
Surface plasmon resonance (SPR) assay. The Biacore T200 system (Cytiva) was employed, and SPR assays were used in assessing the binding affinity of CT-1 to human FTH1. The procedure began with immobilizing proteins onto various channels of a CM5 chip using an amine-coupling method at a flow rate of 10 μL/min in a 10 mM sodium acetate buffer at pH 4.0. The sensor surface activation involved a 7-min injection of a 50 mM N-hydroxysuccinimide (NHS) and 200 mM 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) mixture. This was followed by injecting 166.5 μg/mL human FTH1 for 420 s, after which the surface was blocked using 1 M ethanolamine at pH 8.5. Different concentrations of CT-1 were then flowed through the system, with an association phase of 90 s and a dissociation phase of 120 s. Binding analysis was conducted in PBS containing 0.05% Tween 20 and 1% DMSO at pH 7.4°C and 25°C. To ensure accuracy, double reference subtractions and solvent corrections were applied to counteract bulk refractive index changes, injection noise, and data drift. The data obtained were analyzed using a Langmuir 1:1 binding model within the Biacore Evaluation software provided by Cytiva.
Microscale thermophoresis (MST) assay
Initially, the FTH1 protein was prepared in distilled water to a concentration of 20 μM. The assay was carried out in a specific buffer containing 10 mM CaCl2, 50 mM NaCl, 5 mM DTT, and 50 mM HEPES (pH 7.5) to facilitate optimal binding. Post-labeling the protein with a fluorescent dye, it was mixed with various concentrations of CT-1 and incubated in the dark for 10 min at room temperature. The solution was then loaded into capillaries for analysis with the Monolith NT.115.
CETSA
4T1 cells (3 × 105 cells/well) were grown in 6-well plates (Corning, 3516) and exposed to CT-1 at 80 μM for 3 h at 37°C. Lysis was carried out using NP-40 buffer, supplemented with phosphatase and complete protease inhibitors. The resulting proteins were divided into 7 or 8 aliquots and subjected to cooling for 3 min at varied temperatures before being prepped with 5× SDS loading buffer for immunoblotting.
DARTS
4T1 cell lysates prepared in 500 mL NP-40 with added protease and phosphatase inhibitors were divided into two tubes. One was incubated with 100 μM CT-1 and the other with an equal volume of DMSO, both at room temperature for 1 h. Following this, the mixtures were aliquoted into 100 μL portions and treated with varying doses of pronase at room temperature for 30 min. Protease inhibitors were added to halt the digestion, and the samples were then processed for immunoblotting.
Syngeneic tumor models
Four-week-old female Balb/c mice were purchased from Shanghai SLAC Laboratory Animal Co., Ltd; these mice underwent a week of acclimatization in a sterile environment before the commencement of experiments. In the syngeneic tumor models, 6 × 105 4T1 cells and 6 × 105 EMT6 cells, suspended in 100 μL of ice-cold 1× PBS, were subcutaneously (s.c.) injected into each mouse’s fourth mammary fat pad, respectively. Upon the tumors reaching approximately 50 mm3, mice were grouped (n = 6) and subjected to different treatments: vehicle control, CT-1 at 5 mg/kg and 10 mg/kg, and PTX (10 mg/kg). CT-1 was formulated in a solution of 5% DMSO, 20% PEG 400, 5% Tween 80, and 70% saline and administered via the tail vein daily. The impact of CT-1 on the survival and tumor growth in these mice was closely monitored post-therapy. Tumor volumes were measured daily until they reached 2,000 mm3, set as the experimental endpoint. Survival data were then plotted using GraphPad Prism 8.0. The mice’s tumor volumes and body weights were recorded daily, with tumor volumes calculated using the formula: (length × width2)/2. After the study, mice were sacrificed, and tumors were extracted for further analysis (including H&E staining, TUNEL assay, Ki67, and FTH1 protein detection), which was carried out by Service. Additionally, for the mice bearing 4T1 tumors, plasma was collected and centrifuged at 3,000 g for 15 min at 4°C, followed by a routine blood test using an automatic blood cell analyzer.
Mass CyTOF
Single-cell suspensions from tumors were obtained using a combination of mechanical dissociation via a gentle MACS Dissociator (Miltenyi Biotech) and enzymatic breakdown with collagenase II and dispase II. Surface epitope staining was carried out as per the guidelines provided in the Maxpar Cell Surface Staining with Fresh Fix kit (PN400276 A1). Cisplatin was utilized to identify dead cells in each sample. Cells were rinsed with cell staining buffer, then treated with a mouse FcR blocking solution for 10 min, followed by a 30-min room temperature incubation with heavy metal-conjugated cell-surface antibodies (Fluidigm), as detailed in Additional file 1: Table S1. This was succeeded by a 3-min treatment with 25 μM cisplatin at room temperature. After a double wash with cell staining buffer, the cells were fixed using a 1.6% paraformaldehyde solution. Post-fixation, cells were preserved in Fix and Perm Buffer that contained 125 nM Intercalator-Ir and stored overnight at 4°C. Before CyTOF analysis, cells underwent a double wash with staining buffer and resuspended in Cell Acquisition Solution. Following another wash and fixation with 1.6% paraformaldehyde, cells were stained with Cell-ID Intercalator-Ir (Fluidigm) for CyTOF2 mass cytometry (Fluidigm) analysis. The data obtained were subsequently processed and visualized using R software (version 4.1.2).
PDO models of TNBC
Mature organoids were collected and dissociated into single cells with TrypLE Express. 5000 cells resuspended in 4 μL Matrigel were seeded onto the center of each well in the 96-well plate, and 100μL of human breast cancer organoid culture medium (BCm) were added per well when Matrigel solidified. Plates were incubated at 37°C, 5% CO2 incubator for 3-5 days to allow tumor organoids to form. Tumor organoid viability assays were conducted by transferring the culture medium into 100 μL of BCm supplemented with serially diluted concentrations of drugs in triple replicates and kept on cultivation for about 3–5 days 10 μL of CCK-8 reagent were added to each well, followed by continued incubation for another 4 h at 37°C, 5% CO2, and then the absorbance at 450 nm was measured. Cell viabilities and inhibition rates were calculated as formula follows: Cell viability = [(As-Ab)/(Ac-Ab)] ×100%, and cell inhibition rate = [(Ac-As)/(Ac-Ab)] ×100%, whereas means the absorbances of different concentrations of drugs treated wells, Ac means the absorbances of control wells and Ab means the absorbances of blank wells. For live-dead cell detection, each well received a staining solution containing 1 μg/mL acridine orange (AO) and 1 μg/mL ethidium bromide (EB) and incubated for 5 min at ambient temperature in the dark. The wells were subjected to a double washing with PBS. Subsequently, the wells were examined under a microscope with fluorescence imaging capabilities.
In vivo neutrophil depletion
In the process of depleting neutrophils in vivo, a group of female Balb/c mice (totaling six) received intraperitoneal injections of a neutrophil-depleting antibody (anti-Ly6G at a dosage of 200 μg per mouse, clone 1A8). This was administered before injecting 4T1 tumor cells and was repeated every third day at specified intervals. A comparable concentration of mouse IgG2A (1:100) was used as an isotype control. After the experiment, the mice were sacrificed, and the effectiveness of the neutrophil depletion was confirmed using flow cytometry (CytoFLEX LX, Beckman).
In vivo adoptive transfer of N2-neutrophils
For in vivo N2-neutrophils transfer experiments, the primary murine neutrophils were isolated from bone marrow and activated by TGF-β1 as described previously. The activated N2-like neutrophils were suspended in 100 μL PBS before adoptively transferred into the recipient mice with neutrophils depletion by tail vein injection after subcutaneous tumor inoculation.
Pharmacokinetic experiments
Female SD rats (180–200 g) were administered 10 mg/kg of CT-1 intravenously via the tail vein. Blood samples were collected at various time intervals post-administration. After centrifugation and appropriate processing, the drug concentration in the serum was determined using high-performance liquid chromatography-mass spectrometry. A calibration curve was established to ensure the accuracy of the analytical method. The data were then analyzed using DAS 2.0 software to evaluate changes in drug concentration over time, calculate pharmacokinetic parameters.
Acute toxicity test
Thirty female Balb/c mice, aged 6–7 weeks, were divided into three groups, receiving CT-1 at doses of 0 mg/kg, 25 mg/kg, and 50 mg/kg, administered via tail vein injection. Clinical signs, body weight, and survival of the mice were monitored up to 14 days post-exposure. At the end of the observation period, major organs and blood samples were collected for blood parameter analysis and H&E staining.
Quantification and statistical analysis
Statistical data was evaluated using a two-tailed unpaired t-test when comparing two different groups or one-way ANOVA with Tukey’s multiple comparison tests, utilizing GraphPad Prism software version 8.0.1. A significance threshold was set at p < 0.05, ∗∗∗P < 0.001 and ###P < 0.001.
Published: January 13, 2025
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2024.101915.
Contributor Information
Lijun Zhang, Email: zhanglijun0407@shutcm.edu.cn.
Weidong Zhang, Email: wdzhangy@hotmail.com.
Xin Luan, Email: luanxin@shutcm.edu.cn.
Supplemental information
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Foulkes W.D., Smith I.E., Reis-Filho J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 3.Garrido-Castro A.C., Lin N.U., Polyak K. Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer Discov. 2019;9:176–198. doi: 10.1158/2159-8290.CD-18-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deepak K.G.K., Vempati R., Nagaraju G.P., Dasari V.R., Rao D.N., Malla R.R. Tumor microenvironment: Challenges and opportunities in targeting metastasis of triple negative breast cancer. Pharmacol. Res. 2020;153 doi: 10.1016/j.phrs.2020.104683. [DOI] [PubMed] [Google Scholar]
- 5.Quail D.F., Amulic B., Aziz M., Barnes B.J., Eruslanov E., Fridlender Z.G., Goodridge H.S., Granot Z., Hidalgo A., Huttenlocher A., et al. Neutrophil phenotypes and functions in cancer: A consensus statement. J. Exp. Med. 2022;219 doi: 10.1084/jem.20220011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coffelt S.B., Wellenstein M.D., de Visser K.E. Neutrophils in cancer: neutral no more. Nat. Rev. Cancer. 2016;16:431–446. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 7.Giese M.A., Hind L.E., Huttenlocher A. Neutrophil plasticity in the tumor microenvironment. Blood. 2019;133:2159–2167. doi: 10.1182/blood-2018-11-844548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahiddine K., Blaisdell A., Ma S., Créquer-Grandhomme A., Lowell C.A., Erlebacher A. Relief of tumor hypoxia unleashes the tumoricidal potential of neutrophils. J. Clin. Invest. 2020;130:389–403. doi: 10.1172/jci130952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bat-Erdene U., Quan E., Chan K., Lee B.M., Matook W., Lee K.Y., Rosales J.L. Neutrophil TLR4 and PKR are targets of breast cancer cell glycosaminoglycans and effectors of glycosaminoglycan-induced APRIL secretion. Oncogenesis. 2018;7:45. doi: 10.1038/s41389-018-0058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajizadeh F., Aghebati Maleki L., Alexander M., Mikhailova M.V., Masjedi A., Ahmadpour M., Hashemi V., Jadidi-Niaragh F. Tumor-associated neutrophils as new players in immunosuppressive process of the tumor microenvironment in breast cancer. Life Sci. 2021;264 doi: 10.1016/j.lfs.2020.118699. [DOI] [PubMed] [Google Scholar]
- 11.Szczerba B.M., Castro-Giner F., Vetter M., Krol I., Gkountela S., Landin J., Scheidmann M.C., Donato C., Scherrer R., Singer J., et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566:553–557. doi: 10.1038/s41586-019-0915-y. [DOI] [PubMed] [Google Scholar]
- 12.Xiao Y., Cong M., Li J., He D., Wu Q., Tian P., Wang Y., Yang S., Liang C., Liang Y., et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell. 2021;39:423–437.e7. doi: 10.1016/j.ccell.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Nolan E., Malanchi I. Neutrophil 'safety net' causes cancer cells to metastasize and proliferate. Nature. 2020;583:32–33. doi: 10.1038/d41586-020-01672-3. [DOI] [PubMed] [Google Scholar]
- 14.Xiong S., Dong L., Cheng L. Neutrophils in cancer carcinogenesis and metastasis. J. Hematol. Oncol. 2021;14:173. doi: 10.1186/s13045-021-01187-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyagi A., Sharma S., Wu K., Wu S.Y., Xing F., Liu Y., Zhao D., Deshpande R.P., D'Agostino R.B., Jr., Watabe K. Nicotine promotes breast cancer metastasis by stimulating N2 neutrophils and generating pre-metastatic niche in lung. Nat. Commun. 2021;12:474. doi: 10.1038/s41467-020-20733-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Trotter T.N., Shuptrine C.W., Tsao L.C., Marek R.D., Acharya C., Wei J.P., Yang X.Y., Lei G., Wang T., Lyerly H.K., Hartman Z.C. IL26, a Noncanonical Mediator of DNA Inflammatory Stimulation, Promotes TNBC Engraftment and Progression in Association with Neutrophils. Cancer Res. 2020;80:3088–3100. doi: 10.1158/0008-5472.CAN-18-3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nemeth T., Sperandio M., Mocsai A. Neutrophils as emerging therapeutic targets. Nat. Rev. Drug Discov. 2020;19:253–275. doi: 10.1038/s41573-019-0054-z. [DOI] [PubMed] [Google Scholar]
- 18.Jiang X., Stockwell B.R., Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021;22:266–282. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stockwell B.R., Friedmann Angeli J.P., Bayir H., Bush A.I., Conrad M., Dixon S.J., Fulda S., Gascón S., Hatzios S.K., Kagan V.E., et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pope L.E., Dixon S.J. Regulation of ferroptosis by lipid metabolism. Trends Cell Biol. 2023;33:1077–1087. doi: 10.1016/j.tcb.2023.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang F., Graham E.T., Naowarojna N., Shi Z., Wang Y., Xie G., Zhou L., Salmon W., Jia J.M., Wang X., et al. PALP: A rapid imaging technique for stratifying ferroptosis sensitivity in normal and tumor tissues in situ. Cell Chem. Biol. 2022;29:157–170.e6. doi: 10.1016/j.chembiol.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y., Song J., Zhao Z., Yang M., Chen M., Liu C., Ji J., Zhu D. Single-cell transcriptome analysis reveals tumor immune microenvironment heterogenicity and granulocytes enrichment in colorectal cancer liver metastases. Cancer Lett. 2020;470:84–94. doi: 10.1016/j.canlet.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Kim R., Hashimoto A., Markosyan N., Tyurin V.A., Tyurina Y.Y., Kar G., Fu S., Sehgal M., Garcia-Gerique L., Kossenkov A., et al. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature. 2022;612:338–346. doi: 10.1038/s41586-022-05443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veglia F., Tyurin V.A., Blasi M., De Leo A., Kossenkov A.V., Donthireddy L., To T.K.J., Schug Z., Basu S., Wang F., et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature. 2019;569:73–78. doi: 10.1038/s41586-019-1118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li P., Lu M., Shi J., Gong Z., Hua L., Li Q., Lim B., Zhang X.H.F., Chen X., Li S., et al. Lung mesenchymal cells elicit lipid storage in neutrophils that fuel breast cancer lung metastasis. Nat. Immunol. 2020;21:1444–1455. doi: 10.1038/s41590-020-0783-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang F., Xiao Y., Ding J.H., Jin X., Ma D., Li D.Q., Shi J.X., Huang W., Wang Y.P., Jiang Y.Z., Shao Z.M. Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy. Cell Metab. 2023;35:84–100.e8. doi: 10.1016/j.cmet.2022.09.021. [DOI] [PubMed] [Google Scholar]
- 27.Gentles A.J., Newman A.M., Liu C.L., Bratman S.V., Feng W., Kim D., Nair V.S., Xu Y., Khuong A., Hoang C.D., et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015;21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fridlender Z.G., Sun J., Kim S., Kapoor V., Cheng G., Ling L., Worthen G.S., Albelda S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y., Liu Z., Liu G., Zhang Y., Liu S., Gan D., Chang W., Peng X., Sung E.S., Gilbert K., et al. Neutrophils resist ferroptosis and promote breast cancer metastasis through aconitate decarboxylase 1. Cell Metab. 2023;35:1688–1703.e10. doi: 10.1016/j.cmet.2023.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.SenGupta S., Hein L.E., Xu Y., Zhang J., Konwerski J.R., Li Y., Johnson C., Cai D., Smith J.L., Parent C.A. Triple-Negative Breast Cancer Cells Recruit Neutrophils by Secreting TGF-beta and CXCR2 Ligands. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.659996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang C., Wang Z., Li L., Zhang Z., Jin X., Wu P., Sun S., Pan J., Su K., Jia F., et al. Aged neutrophils form mitochondria-dependent vital NETs to promote breast cancer lung metastasis. J. Immunother. Cancer. 2021;9 doi: 10.1136/jitc-2021-002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coffelt S.B., Kersten K., Doornebal C.W., Weiden J., Vrijland K., Hau C.S., Verstegen N.J.M., Ciampricotti M., Hawinkels L.J.A.C., Jonkers J., de Visser K.E. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma X., Xiao L., Liu L., Ye L., Su P., Bi E., Wang Q., Yang M., Qian J., Yi Q. CD36-mediated ferroptosis dampens intratumoral CD8(+) T cell effector function and impairs their antitumor ability. Cell Metab. 2021;33:1001–1012.e5. doi: 10.1016/j.cmet.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiernicki B., Maschalidi S., Pinney J., Adjemian S., Vanden Berghe T., Ravichandran K.S., Vandenabeele P. Cancer cells dying from ferroptosis impede dendritic cell-mediated anti-tumor immunity. Nat. Commun. 2022;13 doi: 10.1038/s41467-022-31218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu S., Chaudhary O., Rodríguez-Morales P., Sun X., Chen D., Zappasodi R., Xu Z., Pinto A.F.M., Williams A., Schulze I., et al. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8(+) T cells in tumors. Immunity. 2021;54:1561–1577.e1567. doi: 10.1016/j.immuni.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrison P.M., Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta. 1996;1275:161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y.C., Gong Y.T., Sun Q.Y., Wang B., Yan Y., Chen Y.X., Zhang L.J., Zhang W.D., Luan X. Ferritinophagy induced ferroptosis in the management of cancer. Cell. Oncol. 2024;47:19–35. doi: 10.1007/s13402-023-00858-x. [DOI] [PubMed] [Google Scholar]
- 38.Mancias J.D., Wang X., Gygi S.P., Harper J.W., Kimmelman A.C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509:105–109. doi: 10.1038/nature13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen X., Yu C., Kang R., Kroemer G., Tang D. Cellular degradation systems in ferroptosis. Cell Death Differ. 2021;28:1135–1148. doi: 10.1038/s41418-020-00728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y., Xie X., Hou X., Shen J., Shi J., Chen H., He Y., Wang Z., Feng N. Functional oral nanoparticles for delivering silibinin and cryptotanshinone against breast cancer lung metastasis. J. Nanobiotechnology. 2020;18:83. doi: 10.1186/s12951-020-00638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou J., Su C.M., Chen H.A., Du S., Li C.W., Wu H., Tsai S.H., Yeh Y.T. Cryptanshinone Inhibits the Glycolysis and Inhibits Cell Migration Through PKM2/β-Catenin Axis in Breast Cancer. OncoTargets Ther. 2020;13:8629–8639. doi: 10.2147/ott.S239134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen W., Liu L., Luo Y., Odaka Y., Awate S., Zhou H., Shen T., Zheng S., Lu Y., Huang S. Cryptotanshinone activates p38/JNK and inhibits Erk1/2 leading to caspase-independent cell death in tumor cells. Cancer Prev. Res. 2012;5:778–787. doi: 10.1158/1940-6207.Capr-11-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park I.J., Kim M.J., Park O.J., Park M.G., Choe W., Kang I., Kim S.S., Ha J. Cryptotanshinone sensitizes DU145 prostate cancer cells to Fas(APO1/CD95)-mediated apoptosis through Bcl-2 and MAPK regulation. Cancer Lett. 2010;298:88–98. doi: 10.1016/j.canlet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Ji Q., Qi D., Xu X., Xu Y., Goodman S.B., Kang L., Song Q., Fan Z., Maloney W.J., Wang Y. Cryptotanshinone Protects Cartilage against Developing Osteoarthritis through the miR-106a-5p/GLIS3 Axis. Mol. Ther. Nucleic Acids. 2018;11:170–179. doi: 10.1016/j.omtn.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagappan A., Kim J.H., Jung D.Y., Jung M.H. Cryptotanshinone from the Salvia miltiorrhiza Bunge Attenuates Ethanol-Induced Liver Injury by Activation of AMPK/SIRT1 and Nrf2 Signaling Pathways. Int. J. Mol. Sci. 2019;21 doi: 10.3390/ijms21010265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X., Lian L.H., Bai T., Wu Y.L., Wan Y., Xie W.X., Jin X., Nan J.X. Cryptotanshinone inhibits LPS-induced proinflammatory mediators via TLR4 and TAK1 signaling pathway. Int. Immunopharmacol. 2011;11:1871–1876. doi: 10.1016/j.intimp.2011.07.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The RNA-seq data generated in this study have been deposited in Genome Sequence Archive for Human (GSA-Human, https://ngdc.cncb.ac.cn/gsa-human) in the National Genomics Data Center (NGDC) with accession number HRA009103. Proteomics data are available at PRIDE (https://proteomecentral.proteomexchange.org; Accession # PXD057337). All other data are available in the main text or the supplementary materials.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.