Abstract
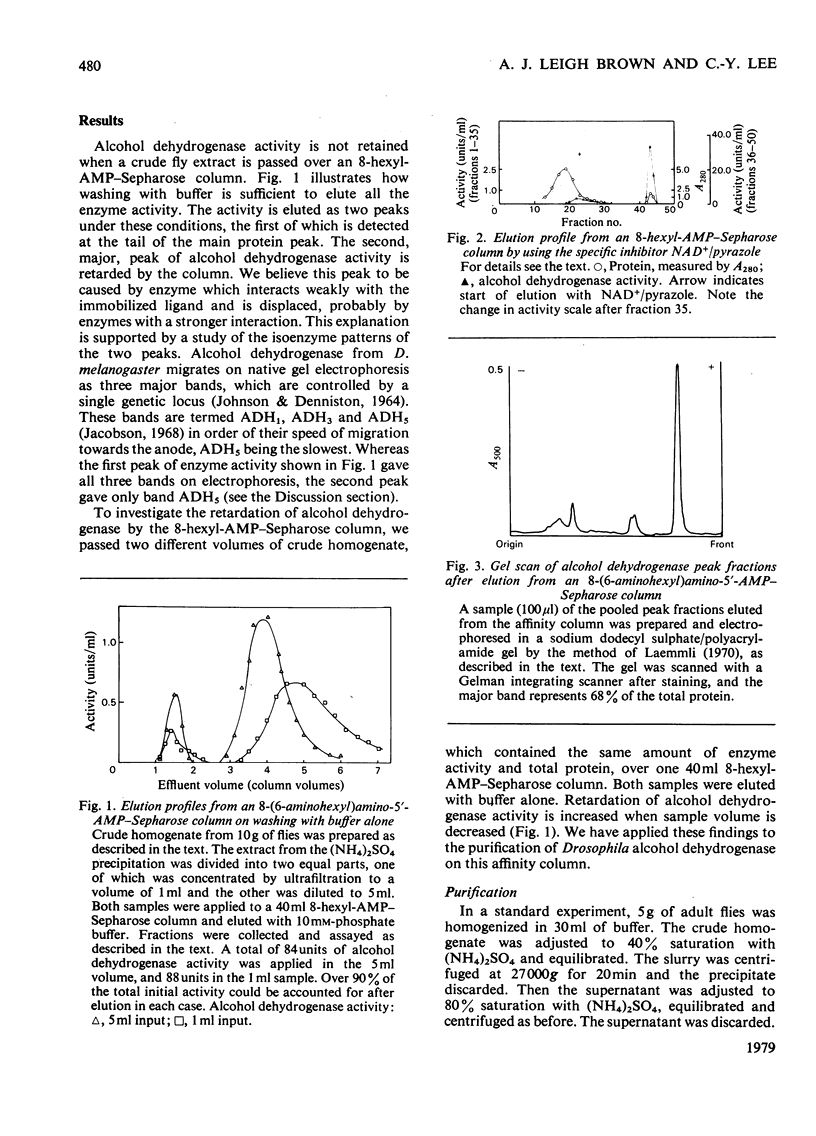
A method for the purification of alcohol dehydrogenase from Drosophila melanogaster is described. The method makes use of 8-(6-aminohexyl)amino-5'-AMP, immobilized on Sepharose 4B, as an affinity ligand. Since alcohol dehydrogenase from Drosophila shows weak affinity for this column, a novel technique was developed to separate alcohol dehydrogenase from both unbound proteins and more strongly bound enzymes. The purification procedure is simple to operate and give a homogeneous preparation in good yield after only three steps.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L., Jornvall H., Mosbach K. Preparative purification of homogenous steroid-active isozyme of horse liver alcohol dehydrogenase by affinity chromatography on an immobilized AMP-analog. Anal Biochem. 1975 Dec;69(2):401–409. doi: 10.1016/0003-2697(75)90142-6. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography of macromolecules. Adv Enzymol Relat Areas Mol Biol. 1972;36:29–89. doi: 10.1002/9780470122815.ch2. [DOI] [PubMed] [Google Scholar]
- Day T. H., Hillier P. C., Clarke B. Properties of genetically polymorphic isozymes of alcohol dehydrogenase in Drosophila melanogaster. Biochem Genet. 1974 Feb;11(2):141–153. doi: 10.1007/BF00485770. [DOI] [PubMed] [Google Scholar]
- Elliott J. I., Knopp J. A. Alcohol dehydrogenase from Drosophila melanogaster. Methods Enzymol. 1975;41:374–379. doi: 10.1016/s0076-6879(75)41083-7. [DOI] [PubMed] [Google Scholar]
- JOHNSON F. M., DENNISTON C. GENETIC VARIATION OF ALCOHOL DEHYDROGENASE IN DROSOPHILIA MELANOGASTER. Nature. 1964 Nov 28;204:906–907. doi: 10.1038/204906a0. [DOI] [PubMed] [Google Scholar]
- Jacobson K. B. Alcohol dehydrogenase of Drosophila: interconversion of isoenzymes. Science. 1968 Jan 19;159(3812):324–325. doi: 10.1126/science.159.3812.324. [DOI] [PubMed] [Google Scholar]
- Jacobson K. B., Murphy J. B., Knopp J. A., Ortiz J. R. Multiple forms of drosophila alcohol dehydrogenase. 3. Conversion of one form to another by nicotinamide adenine dinucleotide or acetone. Arch Biochem Biophys. 1972 Mar;149(1):22–35. doi: 10.1016/0003-9861(72)90295-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lange L. G., Vallee B. L. Double-ternary complex affinity chromatography: preparation of alcohol dehydrogenases. Biochemistry. 1976 Oct 19;15(21):4681–4686. doi: 10.1021/bi00666a022. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Lappi D. A., Wermuth B., Everse J., Kaplan N. O. 8-(6-Aminohexyl)-amino-adenine nucleotide derivatives for affinity chromatography. Arch Biochem Biophys. 1974 Aug;163(2):561–569. doi: 10.1016/0003-9861(74)90515-3. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Lazarus L. H., Kabakoff D. S., Russell P. J., Jr, Laver M., Kaplan N. O. Purification of kinases by general ligand affinity chromatography. Arch Biochem Biophys. 1977 Jan 15;178(1):8–18. doi: 10.1016/0003-9861(77)90165-5. [DOI] [PubMed] [Google Scholar]
- Mosbach K., Guilford H., Ohlsson R., Scott M. General ligands in affinity chromatography. Cofactor-substrate elution of enzymes bound to the immobilized nucleotides adenosine 5'-monophosphate and nicotinamide-adenine dinucleotide. Biochem J. 1972 May;127(4):625–631. doi: 10.1042/bj1270625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- THEORELL H., YONETANI T. LIVER ALCOHOL DEHYDROGENASE-DPN-PYRAZOLE COMPLEX: A MODEL OF A TERNARY INTERMEDIATE IN THE ENZYME REACTION. Biochem Z. 1963;338:537–553. [PubMed] [Google Scholar]
- Thatcher D. R. Enzyme instability and proteolysis during the purification of an alcohol dehydrogenase from Drosophila melanogaster. Biochem J. 1977 May 1;163(2):317–323. doi: 10.1042/bj1630317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigue C. L., Johnson F. M. Isozyme variability in species of the genus Drosophila. VI. Frequency-property-environment relationships of allelic alcohol dehydrogenases in D. melanogaster. Biochem Genet. 1973 Jul;9(3):213–227. doi: 10.1007/BF00485735. [DOI] [PubMed] [Google Scholar]


