Abstract
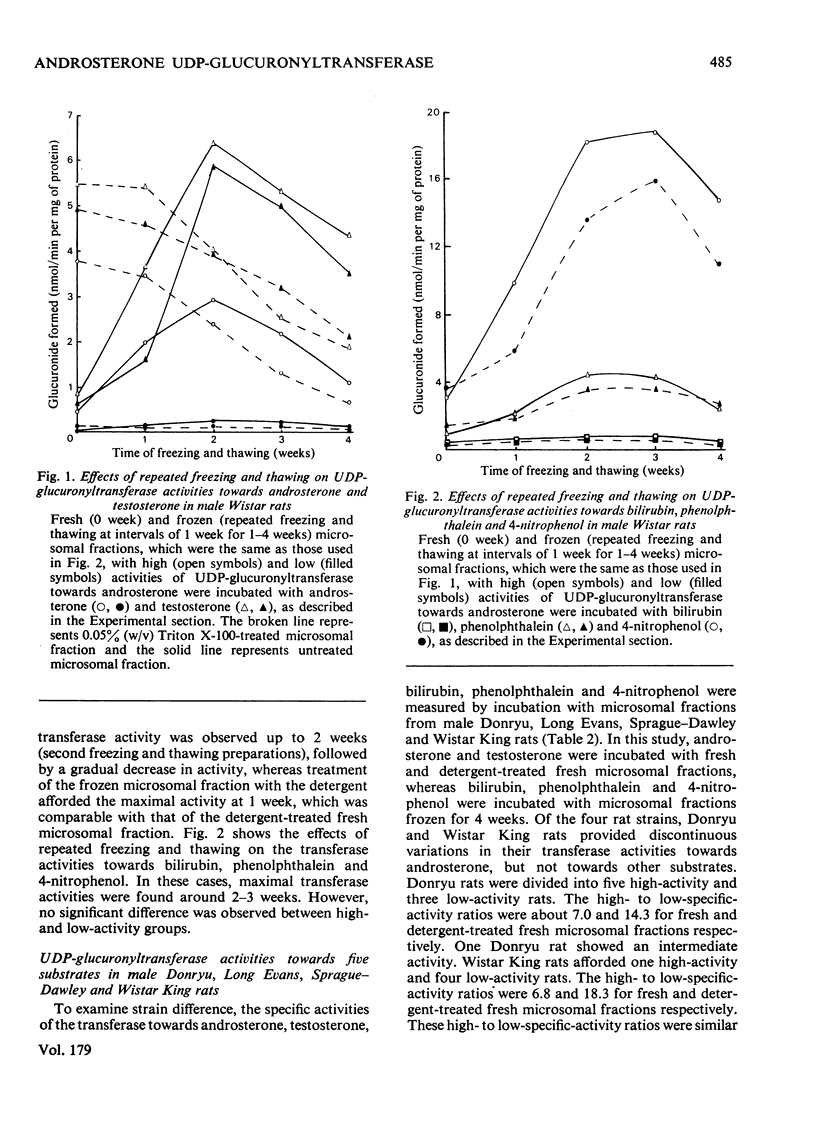
Male Donryu, Wistar King rats showed discontinuous variations in hepatic microsomal UDP-glucuronyltransferase activities towards androsterone, but not towards testosterone, bilirubin, phenolphthalein and 4-nitrophenol. Fresh microsomal fraction with a low transferase activity towards androsterone formed 0.049--0.080 nmole of glucuronide/min per mg of protein, whereas fresh microsomal fraction with a high transferase activity towards androsterone formed 0.335--0.557 nmol of glucuronide/min per mg of protein. The microsomal fraction with low enzyme activity towards androsterone was not stimulated by treatment with Triton X-100 or freezing and thawing. In contrast, male Long Evans and Sprague-Dawley rats did not exhibit such diversity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bock K. W., von Clausbruch U. C., Josting D., Ottenwälder H. Separation and partial purification of two differentially inducible UDP-glucuronyltransferases from rat liver. Biochem Pharmacol. 1977 Jun 1;26(11):1097–1100. doi: 10.1016/0006-2952(77)90254-4. [DOI] [PubMed] [Google Scholar]
- Burchell B., Hallinan T. Phospholipid content and activity of pure uridine diphosphate-glucuronyltransferase from rat liver. Biochem J. 1978 Jun 1;171(3):821–824. doi: 10.1042/bj1710821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchell B. Substrate specificity and properties of uridine diphosphate glucuronyltransferase purified to apparent homogeneity from phenobarbital-treated rat liver. Biochem J. 1978 Sep 1;173(3):749–757. doi: 10.1042/bj1730749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs M., Rao G. S., Rao M. L., Breuer H. Studies on the properties of an enzyme forming the glucuronide of pregnanediol and the pattern of development of steroid glucuronyltransferases in rat liver. J Steroid Biochem. 1977 Mar;8(3):235–241. doi: 10.1016/0022-4731(77)90057-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lucier G. W., McDaniel O. S. Steroid and non-steroid UDP glucuronyltransferase: glucuronidation of synthetic estrogens as steroids. J Steroid Biochem. 1977 Aug;8(8):867–872. doi: 10.1016/0022-4731(77)90096-6. [DOI] [PubMed] [Google Scholar]
- Matsui M., Hakozaki M. Discontinuous variation in hepatic uridine diphosphate glucuronyltransferase toward androsterone in Wistar rats. A regulatory factor for in vivo metabolism of androsterone. Biochem Pharmacol. 1979;28(3):411–415. doi: 10.1016/0006-2952(79)90107-2. [DOI] [PubMed] [Google Scholar]
- Matsui M., Hakozaki M. Variations in biliary metabolites of androsterone in female rats. J Steroid Biochem. 1977 Apr;8(4):319–322. doi: 10.1016/0022-4731(77)90027-9. [DOI] [PubMed] [Google Scholar]
- Matsui M., Kinuyama Y., Hakozaki M. Biliary metabolites of testosterone and testosterone glucosiduronate in the rat. Steroids. 1974 Oct;24(4):557–573. doi: 10.1016/0039-128x(74)90136-6. [DOI] [PubMed] [Google Scholar]
- Rao G. S., Haueter G., Rao M. L., Breuer H. An improved assay for steroid glucuronyltransferase in rat liver microsomes. Anal Biochem. 1976 Jul;74(1):35–40. doi: 10.1016/0003-2697(76)90307-9. [DOI] [PubMed] [Google Scholar]
- Tukey R. H., Billings R. E., Tephly T. R. Separation of oestrone UDP-glucuronyltransferase and p-nitrophenol UDP-glucuronyltransferase activities. Biochem J. 1978 Jun 1;171(3):659–663. doi: 10.1042/bj1710659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Roy F. P., Heirwegh K. P. Determination of bilirubin glucuronide and assay of glucuronyltransferase with bilirubin as acceptor. Biochem J. 1968 Apr;107(4):507–518. doi: 10.1042/bj1070507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherill P. J., Burchell B. Reactivation of a pure defective UDP-glucuronyltransferase from homozygous Gunn rat liver. FEBS Lett. 1978 Mar 15;87(2):207–211. doi: 10.1016/0014-5793(78)80333-0. [DOI] [PubMed] [Google Scholar]
- Wishart G. J. Functional heterogeneity of UDP-glucuronosyltransferase as indicated by its differential development and inducibility by glucocorticoids. Demonstration of two groups within the enzyme's activity towards twelve substrates. Biochem J. 1978 Aug 15;174(2):485–489. doi: 10.1042/bj1740485. [DOI] [PMC free article] [PubMed] [Google Scholar]


