Abstract
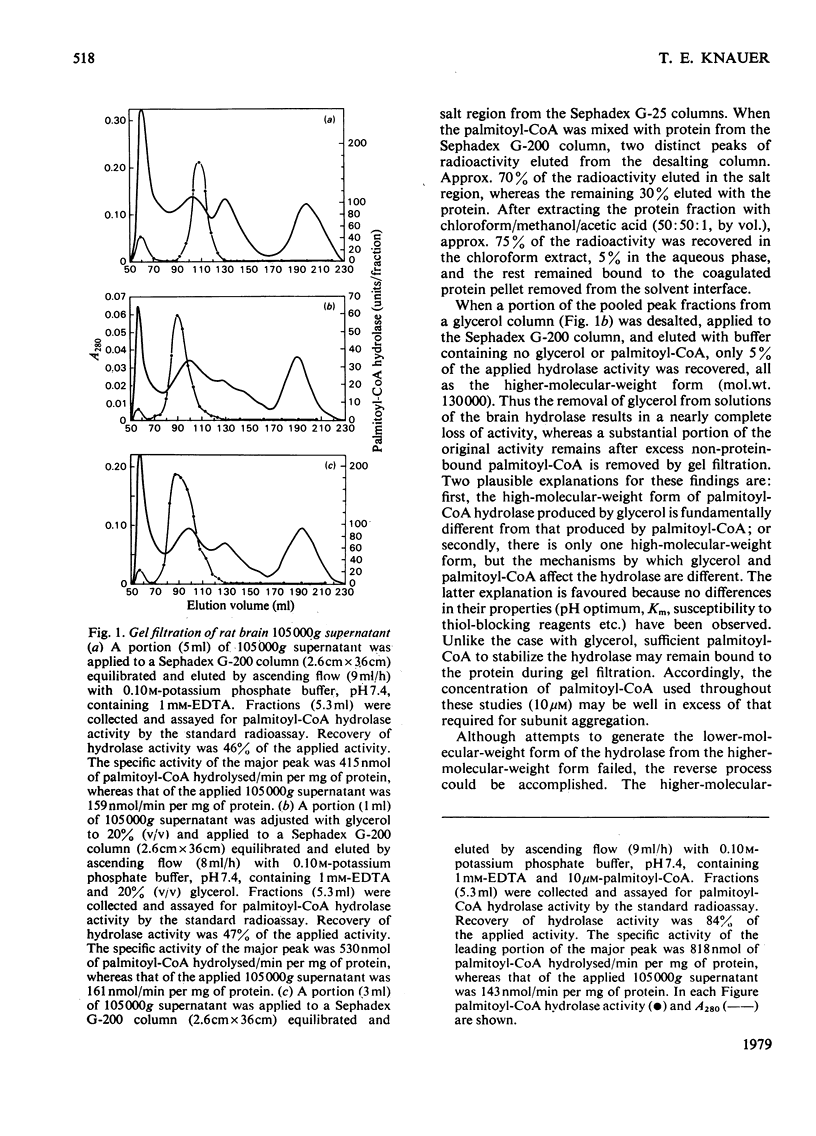
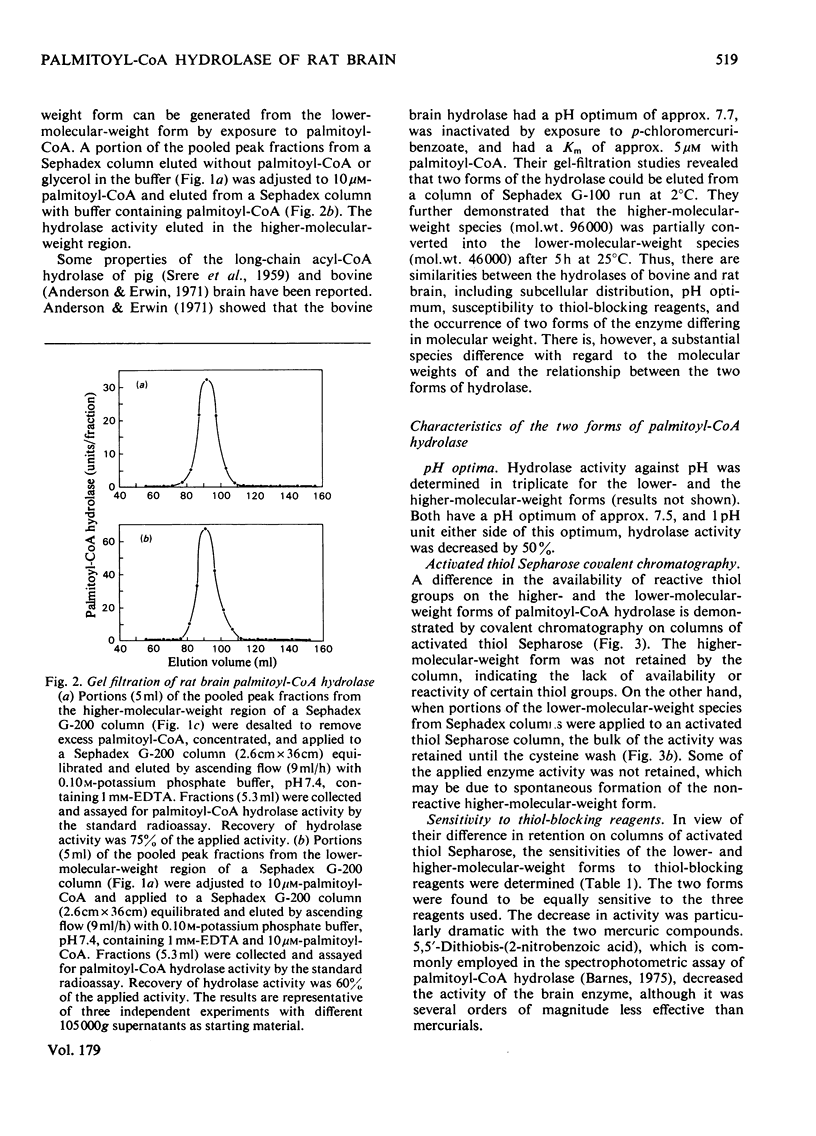
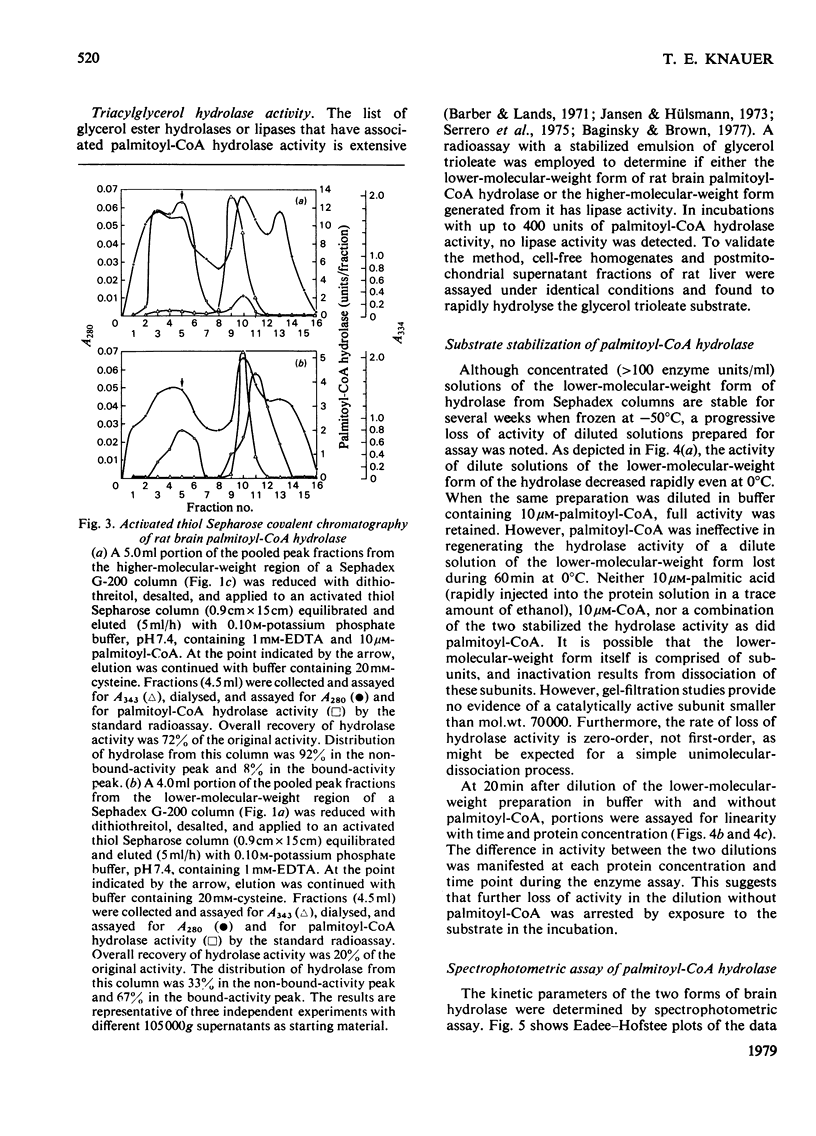
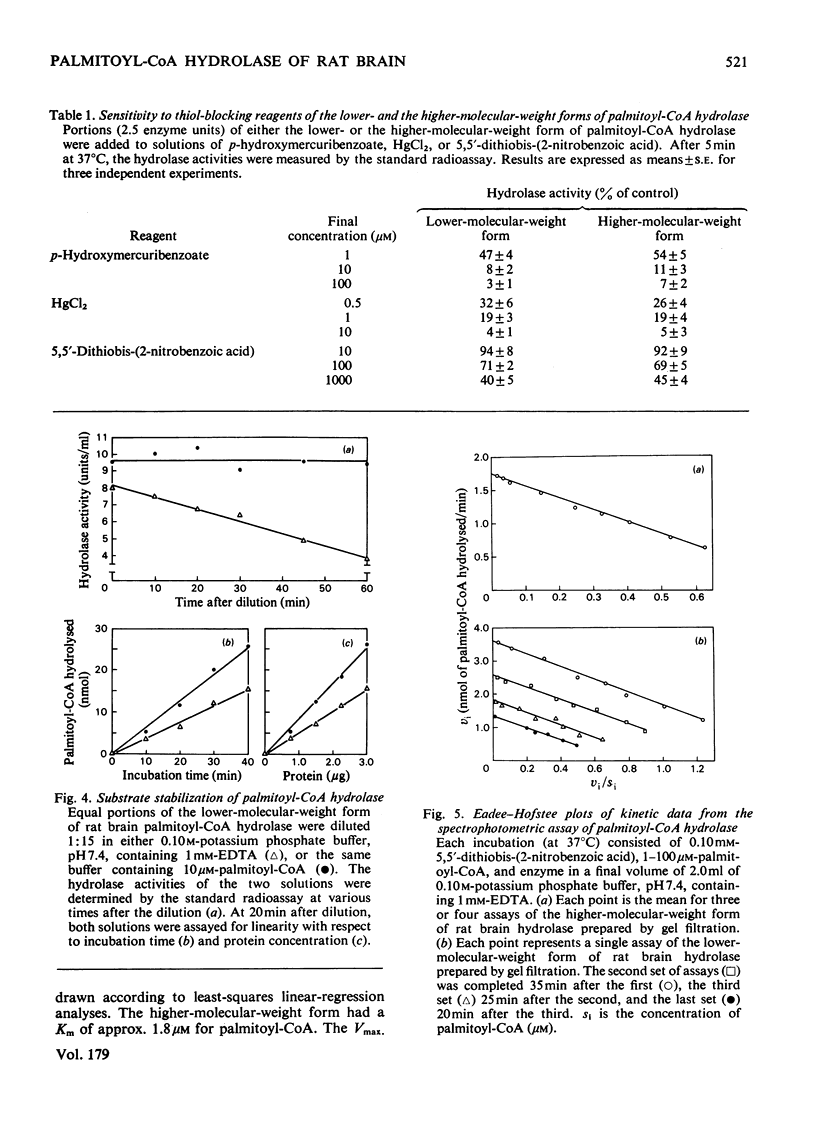
Palmitoyl-CoA hydrolase (EC 3.1.2.2) catalyses the irreversible hydrolysis of long-chain acyl-CoA thioesters. This enzyme is found primarily in the postmicrosomal supernatant fraction prepared from homogenates of rat brain. Either of two forms of the hydrolase, a lower-molecular-weight species of approx. 70000 or a higher-molecular-weight species of approx. 130000 can be isolated by gel filtration. The higher-molecular-weight form is obtained from columns of Sephadex G-200 eluted with buffer containing 10μm-palmitoyl-CoA or 20% (v/v) glycerol, whereas the lower-molecular-weight form is obtained when the eluting buffer does not contain palmitoyl-CoA or glycerol. The two forms of the hydrolase have the same pH optimum of 7.5, are equally sensitive to the thiol-blocking reagents p-hydroxymercuribenzoate, HgCl2, and 5,5′-dithiobis-(2-nitrobenzoic acid), and exhibit the same Km (1.8μm) with palmitoyl-CoA as substrate. The two forms differ in the availability or reactivity of certain external thiol groups, as determined by covalent chromatography with activated thiol Sepharose. Dilute solutions of the lower-molecular-weight form of the hydrolase rapidly lose activity (50% in 60min at 0°C), but there is no change in the Km with palmitoyl-CoA as substrate during this progressive inactivation. Dilutions of the hydrolase in buffer containing 10μm-palmitoyl-CoA retain full activity. However, addition of palmitoyl-CoA to solutions of the lower-molecular-weight form will not restore previously lost hydrolase activity. The evidence supports the conclusion that the substrate palmitoyl-CoA promotes the formation of a relatively stable dimer from two unstable subunits. This process may not be reversible, since the removal of palmitoyl-CoA or glycerol from solutions of the higher-molecular-weight form does not result in the appearance of the lower-molecular-weight form of the hydrolase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. D., Erwin V. G. Brain acyl-coenzyme A hydrolase: distribution, purification and properties. J Neurochem. 1971 Jul;18(7):1179–1186. doi: 10.1111/j.1471-4159.1971.tb00216.x. [DOI] [PubMed] [Google Scholar]
- BORTZ W. M., LYNEN F. ELEVATION OF LONG CHAIN ACYL COA DERIVATIVES IN LIVERS OF FASTED RATS. Biochem Z. 1963 Sep 19;339:77–82. [PubMed] [Google Scholar]
- Baginsky M. L., Brown W. V. Differential characteristics of purified hepatic triglyceride lipase and lipoprotein lipase from human postheparin plasma. J Lipid Res. 1977 Jul;18(4):423–437. [PubMed] [Google Scholar]
- Barber E. D., Lands W. E. Determination of acyl-CoA concentrations using pancreatic lipase. Biochim Biophys Acta. 1971 Nov 13;250(2):361–366. doi: 10.1016/0005-2744(71)90192-6. [DOI] [PubMed] [Google Scholar]
- Barnes E. M., Jr Long-chain fatty acyl thioesterases I and II from Escherichia coli. Methods Enzymol. 1975;35:102–109. doi: 10.1016/0076-6879(75)35144-6. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Carlsson J., Kierstan M. P., Crook E. M. Covalent chromatography. Preparation of fully active papain from dried papaya latex. Biochem J. 1973 Jul;133(3):573–584. doi: 10.1042/bj1330573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheema-Dhadli S., Halperin M. L. The role of the mitochondrial citrate transporter in the regulation of fatty acid synthesis: effect of fasting and diabetes. Can J Biochem. 1973 Nov;51(11):1542–1544. doi: 10.1139/o73-205. [DOI] [PubMed] [Google Scholar]
- Goodridge A. G. Hormonal regulation of the activity of the fatty acid synthesizing system and of the malic enzyme concentration in liver cells-1,2. Fed Proc. 1975 Feb;34(2):117–123. [PubMed] [Google Scholar]
- Goodridge A. G. Regulation of fatty acid synthesis in isolated hepatocytes. Evidence for a physiological role for long chain fatty acyl coenzyme A and citrate. J Biol Chem. 1973 Jun 25;248(12):4318–4326. [PubMed] [Google Scholar]
- Hsu K. H., Powell G. L. Inhibition of citrate synthase by oleoyl-CoA: a regulatory phenomenon. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4729–4733. doi: 10.1073/pnas.72.12.4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob E. J., Butterworth P. H., Porter J. W. Studies on the substrate binding sites of the pigeon liver fatty acid synthetase. Arch Biochem Biophys. 1968 Mar 20;124(1):392–400. doi: 10.1016/0003-9861(68)90343-3. [DOI] [PubMed] [Google Scholar]
- Jansen H., Hülsmann W. C. Long-chain acyl-CoA hydrolase activity in serum: identity with clearing factor lipase. Biochim Biophys Acta. 1973 Jan 19;296(1):241–248. doi: 10.1016/0005-2760(73)90064-7. [DOI] [PubMed] [Google Scholar]
- Kawaguchi A., Bloch K. Inhibition of glutamate dehydrogenase and malate dehydrogenases by palmitoyl coenzyme A. J Biol Chem. 1976 Mar 10;251(5):1406–1412. [PubMed] [Google Scholar]
- Kumar S. Functional deacylases of pigeon liver fatty acid synthetase complex. J Biol Chem. 1975 Jul 10;250(13):5150–5158. [PubMed] [Google Scholar]
- Kurooka S., Hosoki K., Yoshimura Y. Increase in long fatty acyl-CoA hydrolase activity in the liver and kidney of alloxan diabetic rat. J Biochem. 1971 Jan;69(1):247–249. doi: 10.1093/oxfordjournals.jbchem.a129454. [DOI] [PubMed] [Google Scholar]
- Kurooka S., Hosoki K., Yoshimura Y. Some properties of long fatty acyl-coenzyme A thioesterase in rat organs. J Biochem. 1972 Apr;71(4):625–634. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamb R. G., Fallon H. J. The formation of monoacylglycerophosphate from sn-glycerol 3-phosphate by a rat liver particulate preparation. J Biol Chem. 1970 Jun;245(12):3075–3083. [PubMed] [Google Scholar]
- Lerner E., Shug A. L., Elson C., Shrago E. Reversible inhibition of adenine nucleotide translocation by long chain fatty acyl coenzyme A esters in liver mitochondria of diabetic and hibernating animals. J Biol Chem. 1972 Mar 10;247(5):1513–1519. [PubMed] [Google Scholar]
- Lornitzo F. A., Qureshi A. A., Porter J. W. Subunits of fatty acid synthetase complexes. Enzymatic activities and properties of the half-molecular weight nonidentical subunits of pigeon liver fatty acid synthetase. J Biol Chem. 1975 Jun 25;250(12):4520–4529. [PubMed] [Google Scholar]
- Nordlie R. C., Hanson T. L., Johns P. T. Differential effects of palmityl coenzyme A on liver microsomal inorganic pyrophosphate-glucose phosphotransferase and glucose 6-phosphate phosphohydrolase. J Biol Chem. 1967 Sep 25;242(18):4144–4148. [PubMed] [Google Scholar]
- Phillips G. T., Nixon J. E., Dorsey J. A., Butterworth P. H., Chesterton C. J., Porter J. W. The mechanism of synthesis of fatty acids by the pigeon liver enzyme system. Arch Biochem Biophys. 1970 Jun;138(2):380–391. doi: 10.1016/0003-9861(70)90360-7. [DOI] [PubMed] [Google Scholar]
- Pittman R. C., Khoo J. C., Steinberg D. Cholesterol esterase in rat adipose tissue and its activation by cyclic adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1975 Jun 25;250(12):4505–4511. [PubMed] [Google Scholar]
- SRERE P. A., SEUBERT W., LYNEN F. Palmityl coenzyme A deacylase. Biochim Biophys Acta. 1959 Jun;33(2):313–319. doi: 10.1016/0006-3002(59)90118-0. [DOI] [PubMed] [Google Scholar]
- Seitz H. J., Müller M. J., Krone W., Tarnowski W. Rapid conversion by insulin of hepatic intermediary metabolism from glucose production to glucose utilization in the liver of alloxan-diabetic rats. Diabetes. 1977 Dec;26(12):1159–1174. doi: 10.2337/diab.26.12.1159. [DOI] [PubMed] [Google Scholar]
- Serrero G., Négrel R., Ailhaud G. Characterization and partial purification of an intestinal lipase. Biochem Biophys Res Commun. 1975 Jul 8;65(1):89–99. doi: 10.1016/s0006-291x(75)80065-9. [DOI] [PubMed] [Google Scholar]
- Shafrir E., Ruderman N. B. Enzymes of carbohydrate and fat metabolism in anti-insulin serum diabetes; inactivation by free fatty acids and the protective effect of cellular protein. Diabetologia. 1974 Dec;10(6):731–742. doi: 10.1007/BF01219535. [DOI] [PubMed] [Google Scholar]
- Srere P. A. Palmityl-coenzyme A inhibition of the citrate-condensing enzyme. Biochim Biophys Acta. 1965 Dec 2;106(3):445–455. doi: 10.1016/0005-2760(65)90061-5. [DOI] [PubMed] [Google Scholar]
- Taketa K., Pogell B. M. The effect of palmityl coenzyme A on glucose 6-phosphate dehydrogenase and other enzymes. J Biol Chem. 1966 Feb 10;241(3):720–726. [PubMed] [Google Scholar]
- Tubbs P. K., Garland P. B. Variations in tissue contents of coenzyme A thio esters and possible metabolic implications. Biochem J. 1964 Dec;93(3):550–557. doi: 10.1042/bj0930550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veloso D., Veech R. L. Stoichiometric hydrolysis of long chain acyl-CoA and measurement of the CoA formed with an enzymatic cycling method. Anal Biochem. 1974 Dec;62(2):449–450. doi: 10.1016/0003-2697(74)90177-8. [DOI] [PubMed] [Google Scholar]
- Wititsuwannakul D., Kim K. H. Mechanism of palmityl coenzyme A inhibition of liver glycogen synthase. J Biol Chem. 1977 Nov 10;252(21):7812–7817. [PubMed] [Google Scholar]


