Abstract
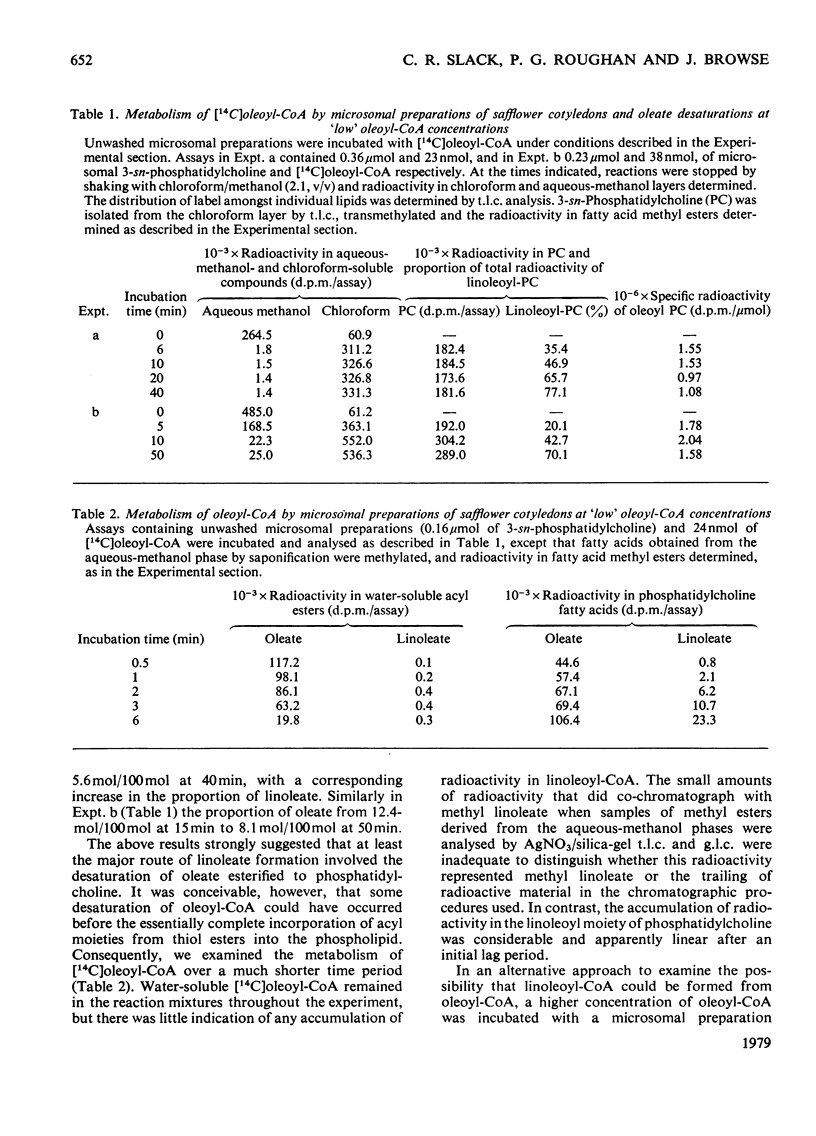
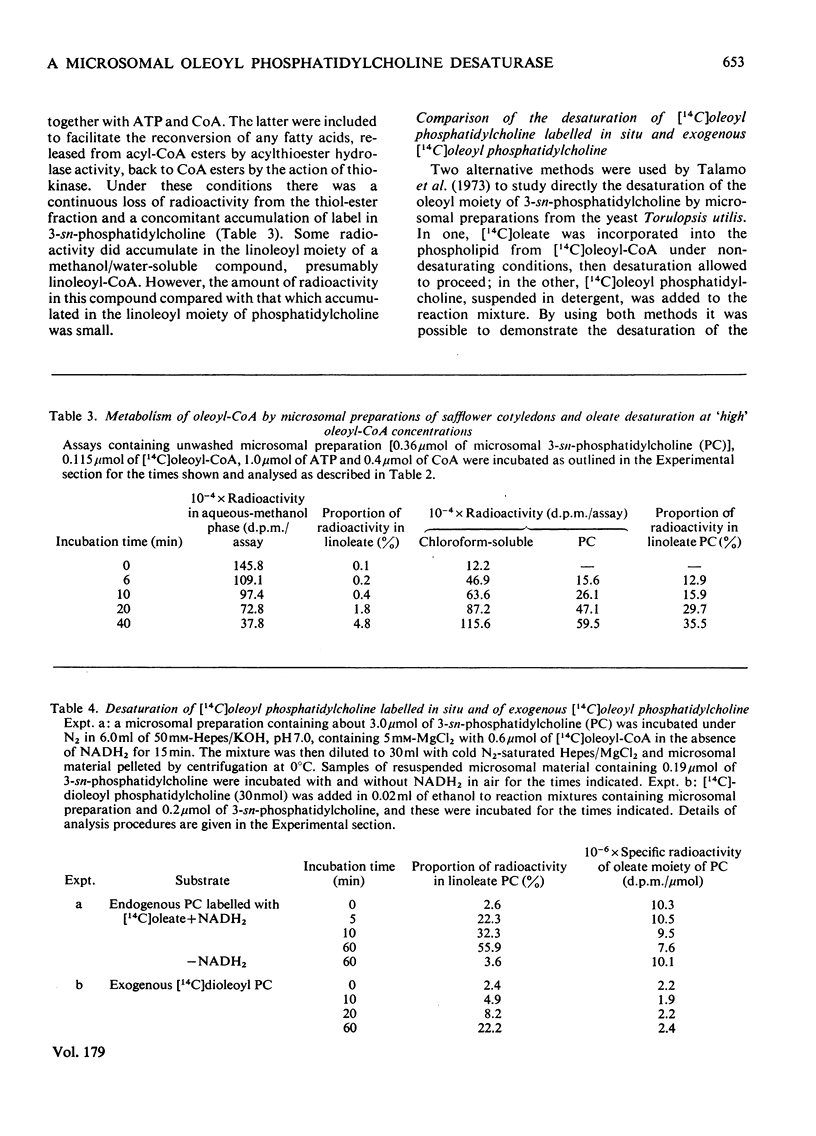
1. [14C]Oleoyl-CoA was metabolized rapidly and essentially completely by microsomal preparations from developing safflower (Carthamus tinctorius) cotyledons, and most of the [14C]oleate was incorporated into 3-sn-phosphatidylcholine. 2. In aerobic reaction mixtures containing NADH2 the [14C]oleate in 3-sn-phosphatidylcholine was converted into [14C]linoleate without any change in the specific radioactivity of the lipid. Over a 60 min incubation period the extent of conversion of [14C]oleoyl phosphatidylcholine into [14C]linoleoyl phosphatidylcholine was generally greater than 60%. The rate of desaturation of endogenous [14C]oleoyl phosphatidylcholine labelled from [14C]oleoyl-CoA was much greater that of exogenous [14C]dioleoyl phosphatidylcholine the specific radioactivity of the oleoyl moiety of the lipid remained constant, indicating that labelled and unlabelled oleate were desaturated at the same rate. On this assumption an initial rate of desaturation of about 15 nmol of oleate desaturated/min per mumol of 3-sn-phosphatidylcholine was estimated. 4. [14C]Oleate esterified at positions 1 and 2 of both endogenous and exogenous 3-sn-phosphatidylcholine was desaturated. 5. Attempts to demonstrate the presence of an oleoyl-CoA desaturase in safflower microsomal fractions by the appearance of linoleoyl-CoA in reaction mixtures were inconclusive.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelkader A. B., Cherif A., Demandre C., Mazliak P. The oleyl-coenzyme-A desaturase of potato tubers. Enzymatic properties, intracellular localization and induction during "aging" of tuber slices. Eur J Biochem. 1973 Jan 3;32(1):155–165. doi: 10.1111/j.1432-1033.1973.tb02592.x. [DOI] [PubMed] [Google Scholar]
- Arvidson G. A. Structural and metabolic heterogeneity of rat liver glycerophosphatides. Eur J Biochem. 1968 May;4(4):478–486. doi: 10.1111/j.1432-1033.1968.tb00237.x. [DOI] [PubMed] [Google Scholar]
- Baker N., Lynen F. Factors involved in fatty acyl CoA desaturation by fungal microsomes. The relative roles of acyl CoA and phospholipids as substrates. Eur J Biochem. 1971 Mar 11;19(2):200–210. doi: 10.1111/j.1432-1033.1971.tb01305.x. [DOI] [PubMed] [Google Scholar]
- Barber E. D., Lands W. E. Determination of acyl-CoA concentrations using pancreatic lipase. Biochim Biophys Acta. 1971 Nov 13;250(2):361–366. doi: 10.1016/0005-2744(71)90192-6. [DOI] [PubMed] [Google Scholar]
- Devor K. A., Mudd J. B. Control of fatty acid distribution in phosphatidylcholine of spinach leaves. J Lipid Res. 1971 Jul;12(4):412–419. [PubMed] [Google Scholar]
- Kader J. C. Cyanide sensitivity and induction of the microsomal oleoyl-CoA desaturase of potato tuber. Biochim Biophys Acta. 1977 Mar 25;486(3):429–436. doi: 10.1016/0005-2760(77)90092-3. [DOI] [PubMed] [Google Scholar]
- Morris L. J. Separations of lipids by silver ion chromatography. J Lipid Res. 1966 Nov;7(6):717–732. [PubMed] [Google Scholar]
- Nichols B. W., Safford R. Conversion of lipids to fatty alcohols and lysolipids by NaBH4. Chem Phys Lipids. 1973 Oct;11(3):222–227. doi: 10.1016/0009-3084(73)90024-8. [DOI] [PubMed] [Google Scholar]
- Pugh E. L., Kates M. Characterization of a membrane-bound phospholipid desaturase system of candida lipolytica. Biochim Biophys Acta. 1975 Mar 24;380(3):442–453. doi: 10.1016/0005-2760(75)90112-5. [DOI] [PubMed] [Google Scholar]
- Shine W. E., Mancha M., Stumpf P. K. Fat metabolism in higher plants. Differential incorporation of acyl-coenzymes A and acyl-acyl carrier proteins into plant microsomal lipids. Arch Biochem Biophys. 1976 Apr;173(2):472–479. doi: 10.1016/0003-9861(76)90284-8. [DOI] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Balasingham N. Labelling of glycerolipids in the cotyledons of developing oilseeds by [1-14C] acetate and [2-3H] glycerol. Biochem J. 1978 Feb 15;170(2):421–433. doi: 10.1042/bj1700421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G. Rapid temperature-induced changes in the fatty acid composition of certain lipids in developing linseed and soya-bean cotyledons. Biochem J. 1978 Feb 15;170(2):437–439. doi: 10.1042/bj1700437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Terpstra J. Some properties of a microsomal oleate desaturase from leaves. Biochem J. 1976 Apr 1;155(1):71–80. doi: 10.1042/bj1550071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf P. K., Porra R. J. Lipid biosynthesis in developing and germinating soybean cotyledons. The formation of oleate by a soluble stearyl acyl carrier protein desaturase. Arch Biochem Biophys. 1976 Sep;176(1):63–70. doi: 10.1016/0003-9861(76)90141-7. [DOI] [PubMed] [Google Scholar]
- Stumpf P. K., Weber N. Uptake and metabolism of fatty acids by soybean suspension cells. Lipids. 1977 Jan;12(1):120–124. doi: 10.1007/BF02532983. [DOI] [PubMed] [Google Scholar]
- Stymne S., Appelqvist L. A. The biosynthesis of linoleate from oleoyl-CoA via oleoyl-phosphatidylcholine in microsomes of developing safflower seeds. Eur J Biochem. 1978 Oct;90(2):223–229. doi: 10.1111/j.1432-1033.1978.tb12594.x. [DOI] [PubMed] [Google Scholar]
- Sánchez M., Nicholls D. G., Brindley D. N. [The relationship between palmitoyl-coenzyme A synthetase activity and esterification of sn-glycerol 3-phosphate in rat liver mitochondria]. Biochem J. 1973 Apr;132(4):697–706. doi: 10.1042/bj1320697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talamo B., Chang N., Bloch K. Desaturation of oleyl phospholipid to linoleyl phospholipid in Torulopsis utilis. J Biol Chem. 1973 Apr 25;248(8):2738–2742. [PubMed] [Google Scholar]
- Vijay I. K., Stumpf P. K. Fat metabolism in higher plants. XLVI. Nature of the substrate and the product of oleyl coenzyme A desaturase from Carthamus tinctorius. J Biol Chem. 1971 May 10;246(9):2910–2917. [PubMed] [Google Scholar]


