Abstract
Double-strand breaks (DSBs) of chromosomal DNA trigger the cellular response that activates the pathways for DNA repair and cell-cycle checkpoints, and sometimes the pathways leading to cell death if the damage is too severe to be tolerated. Evidence indicates that, upon generation of DNA DSBs, many nuclear proteins that are involved in DNA repair and checkpoints are recruited to chromatin around the DNA lesions. In the present study we used a proteomics approach to identify DNA-damage-induced chromatin-binding proteins in a systematic way. Two-dimensional gel analysis for protein extracts of chromatin from DNA-damage-induced and control HeLa cells identified four proteins as the candidates for DNA-damage-induced chromatin-binding proteins. MALDI–TOF (matrix-assisted laser-desorption ionization–time-of-flight) MS analysis identified these proteins to be NPM (nucleophosmin), hnRNP (heterogeneous nuclear ribonucleoprotein) C1, hnRNP C2 and 37-kDa laminin-receptor precursor, and the identity of these proteins was further confirmed by immunoblot analysis with specific antibodies. We then demonstrated with chromatin-binding assays that NPM and hnRNP C1/C2, the abundant nuclear proteins with pleiotropic functions, indeed bind to chromatin in a DNA-damage-dependent manner, implicating these proteins in DNA repair and/or damage response. Immunofluorescence experiments showed that NPM, normally present in the nucleoli, is mobilized into the nucleoplasm after DNA damage, and that neither NPM nor hnRNP C1/C2 is actively recruited to the sites of DNA breaks. These results suggest that NPM and hnRNP C1/C2 may function at the levels of the global context of chromatin, rather than by specifically targeting the broken DNA.
Keywords: chromatin, DNA damage, heterogeneous nuclear ribonucleoprotein (hnRNP), nucleophosmin, proteomics, radiation
Abbreviations: 2-D, two-dimensional; DNA-PK, DNA-dependent protein kinase; DSB, double-strand break; GFP, green fluorescent protein; H2AX, histone H2A variant X; γ-H2AX, phosphorylated H2AX; hnRNP, heterogeneous nuclear ribonucleoprotein; IRIF, ionizing-radiation-induced foci; 37LRP, 37-kDa laminin-receptor precursor; MALDI–TOF, matrix-assisted laser-desorption ionization–time-of-flight; NHEJ, non-homologous end-joining; NP40, Nonidet P40; NPM, nucleophosmin; TSA, trichostatin A
INTRODUCTION
DNA double-strand breaks (DSBs) are generated by exogenous factors, such as ionizing radiation and chemotherapeutic drugs, and also can occur during cellular processes, such as DNA replication, meiotic recombination and the programmed rearrangement of antigen-receptor genes. DSBs are the most destructive form of DNA damage in that, if left unrepaired, they can result in loss of genetic information, chromosomal rearrangements, including translocations, and unstable chromosomes, all of which can potentially lead to cancer development [1–3]. Cells have evolved mechanisms to detect DNA breaks and trigger the pathways for activation of DNA repair and cell-cycle checkpoints, and sometimes for cell death if the damage is too severe to be tolerated [4,5].
DNA in eukaryotic cells is organized into nucleosomes and a higher-order chromatin structure, the physiological substrate for all processes requiring a DNA template, including DNA repair. In general, chromatin, from the levels of individual nucleosomes to more complex folding structure, presents a fundamental problem in access of proteins to their DNA target [6,7]. This inhibitory effect of chromatin is therefore counteracted in order for repair machinery to carry out their tasks properly. It is thought that the process of DNA repair and propagation of damage signals requires the proteins carrying out this process, as well as the proteins responsible for remodelling and modifying chromatin structure, to be recruited to the chromatin at the sites of damaged DNA [8,9].
Studies have shown that many proteins involved in DNA repair and checkpoints are recruited to the chromatin at the sites of DNA DSBs, resulting in the formation of immunostainable nuclear foci, called IRIF (ionizing-radiation-induced foci). IRIF are thought to serve as the platform in which repair and checkpoint proteins accumulate to both facilitate DNA repair and propagate the damage signals [4]. H2AX (histone H2A variant X) is rapidly phosphorylated on Ser-139 at the C-terminal tail upon DSB generation, and the phosphorylated H2AX (γ-H2AX), occurring on a Mbase of chromatin around broken DNA, is thought to serve as the nucleating site for the formation of IRIF [10,11]. A number of well-characterized repair and checkpoint proteins, such as ATM (ataxia telangiectasia mutated), ATR (ataxia telangiectasia-related), Mre11–Rad50–Nbs1 complex, Brca1 (breast-cancer susceptibility gene 1), Chk2 (checkpoint kinase 2), the p53-interacting protein 53BP1, Rad51 and Rad52, have been shown to co-localize with γ-H2AX foci, suggesting that they bind to chromatin around the DSB sites [4,10,11].
Efforts have so far been focused on examining the particular proteins that have been characterized to participate in DNA repair and checkpoint activation with respect to their association with IRIF. In the present study, we used a proteomics approach to systematically analyse the proteins that inducibly bind to chromatin in response to generation of DNA DSBs. In the present study we report that NPM (nucleophosmin) and hnRNP (heterogeneous nuclear ribonucleoprotein) C1/C2, abundant nuclear proteins with pleiotropic functions, are identified as DNA-damage-induced chromatin-binding proteins. NPM is of particular interest in that this protein has recently emerged as an important player in DNA-damage response and cell-cycle regulation, in addition to its long-standing role in ribosome biogenesis.
EXPERIMENTAL
Antibodies
The following primary antibodies were used; anti-γ-H2AX (mouse monoclonal, clone JBW301; Upstate Biotechnology, Lake Placid, NY, U.S.A.), anti-H2A (acidic patch, rabbit polyclonal; Upstate Biotechnology), anti-Ku70 (sc-9033, rabbit polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.), anti-NPM (sc-5564, rabbit polyclonal; Santa Cruz Biotechnology), anti-hnRNP C1/C2 (sc-15386, rabbit polyclonal; Santa Cruz Biotechnology). HRP (horseradish peroxidase)-linked rabbit IgG and mouse IgG secondary antibodies were purchased from Amersham Biosciences. Polyclonal anti-37LRP (37-kDa laminin-receptor precursor) antibody was generated by immunization of rabbits with GST (glutathione S-transferase)-fusion proteins, encompassing amino acids 215–295 of human 37LRP.
Generation of DNA DSBs and induction of chromatin structural change
HeLa cells were obtained from the Korean Cell Line Bank (Seoul, Korea) and were maintained in Dulbecco's modified Eagle's medium plus 5% fetal bovine serum (Hyclone), 10000 units/ml antibiotic/antimycotic (Gibco BRL) at 37 °C at an atmosphere with 5% CO2. To generate DNA DSBs, cells were grown to approx. 80% confluence were exposed to γ-radiation (137Cs; Cell Irradiation System, GC 3000 Elan-Model β; MDS Nordion, Ontario, Canada) at a dosage of 25 Gy or treated with 50 μM of etoposide (Sigma). To induce chromatin structural change, cells were treated with 10 μM of TSA (trichostatin A; Sigma) for 12–14 h, or incubated for 1 h in the presence of hypotonic PBS containing 50 mM NaCl.
Nuclei isolation and sequential salt extraction
The whole procedure of nuclei isolation was performed on ice. Cells (2×108) were washed with cold PBS two times and suspended in a 4-ml volume of buffer A containing 20 mM Hepes (pH 7.5), 3 mM MgCl2, 0.25 M sucrose, 0.5% NP40 (Nonidet P40), 3 mM 2-mercaptoethanol, 0.4 mM PMSF, 1 μM pepstatin A, 1 μM leupeptin and 5 μg/ml aprotinin. The cell suspension was homogenized using Wheaton homogenizer and centrifuged at 3000 g (Beckman JA-20 rotor) for 15 min. After repeating this procedure (from suspension to centrifugation) two times, the pellet was resuspended in 4 ml of the buffer B containing 20 mM Hepes (pH 7.5), 3 mM MgCl2, 0.2 mM EGTA, 1 mM 2-mercaptoethanol, 0.4 mM PMSF, 1 μM pepstatin A, 1 μM leupeptin and 5 μg/ml aprotinin, followed by centrifugation at 3000 g (Beckman JA-20 rotor). The nuclei pellet was immediately used for salt extraction or stored at −80 °C until use. The volume of nuclei was approx. 200 μl.
To carry out the 0.1-M salt extraction, 200 μl of nuclei pellet was resuspended in 100 μl of buffer B, followed by stepwise addition of 200 μl of buffer B containing 0.2 M NaCl, and this procedure was repeated (addition of 100 μl of buffer B and then 200 μl of buffer B containing 0.2 M NaCl) in the same manner. This method of nuclei suspension minimizes unnecessary exposure of nuclei to locally elevated salt concentrations. The nuclei suspension at a final volume of 800 μl was incubated at 30 °C for 1 h, centrifuged at 3000 g for 15 min, and the supernatant was removed (0.1-M salt extract). The remaining nuclei pellet was subjected to subsequent extractions with the buffer B containing 0.7 M, 1 M and 1.3 M NaCl in the same way as above to produce 0.35-M, 0.5-M and 0.65-M salt extracts respectively. Supernatants (extracts) were stored at −80 °C until use.
2-D (two-dimensional) electrophoresis and MALDI–TOF (matrix-assisted laser-desorption ionization–time-of-flight) analysis
Protein extracts were deprived of salt through a protein-desalting spin column (Pierce, Rockford, IL, U.S.A.) prior to being subjected to 2-D gel electrophoresis. Desalted protein sample (30 μl) were mixed with 100 μl of rehydration buffer (0.1 M urea, 2.6 M thiourea and 2.6% CHAPS) plus 20 mM dithiothreitol and 0.8% (v/v) ampholyte, and then was vortexed at room temperature for 30 min. The protein sample was subjected to isoelectric focusing with 7-cm Readystrip IPG strips (pH 3-10, non-linear; Bio-Rad) and IPGphor (Amersham Biosciences) under the conditions of rehydration for 12–14 h, 500 V for 1 h, 1000 V for 1 h and 8000 V for 3 h.
Isoelectric-focused strips were equilibrated in the buffer containing 50 mM Tris/HCl (pH 8.8), 6 M urea, 30% glycerol, 2% SDS and a trace amount of Bromophenol Blue at room temperature for 15 min, and further equilibrated for 15 min in the above buffer with 2.5% iodoacetamide. The strips were then transferred on to the 2-D 1-mm thick SDS/PAGE gel (12.5%), and sealed in place using 1% low-melting-temperature agarose gel. SDS/PAGE was performed at 100 V for 5 min, followed by 80 V for 2 h and 120 V for 4 h. SDS/PAGE gels were silver-stained and stored at 4 °C.
Protein spots were excised from silver-stained gels and subjected to trypsin digestion and MALDI–TOF analysis by essentially the same method as described previously [12] using the MS core facility of the Center for Cell Signaling Research, Ewha Woman's University.
Immunoblot analysis
Acid extraction of histones for immunoblot analysis shown below in Figure 1(B) was performed as follows. Cells were washed with PBS and resuspended in the buffer containing 20 mM Tris/HCl (pH 8.0), 150 mM NaCl, 1 mM EDTA (pH 8.0) and 0.5% NP40, and incubated on ice for 10 min. Nuclei were pelleted by centrifugation at 6000 g for 5 min at 4 °C, resuspended in 0.1 M HCl and incubated for 10 min at room temperature. Acid-extracted histones were obtained by centrifugation at 6000 g for 5 min at 4 °C, and the concentrations of histones were measured by the Bradford method. After separation by SDS/PAGE (15% gel), proteins were transferred on to nitrocellulose membrane (Hybond, Amersham Biosciences) using transfer buffer containing 50 mM Caps [3-(cyclohexylamino)propane-1-sulphonic acid; pH 10.0] and 20% methanol and probed with appropriate antibodies. Signals were detected by enhanced chemiluminescence (LabFrontier, Seoul, Korea).
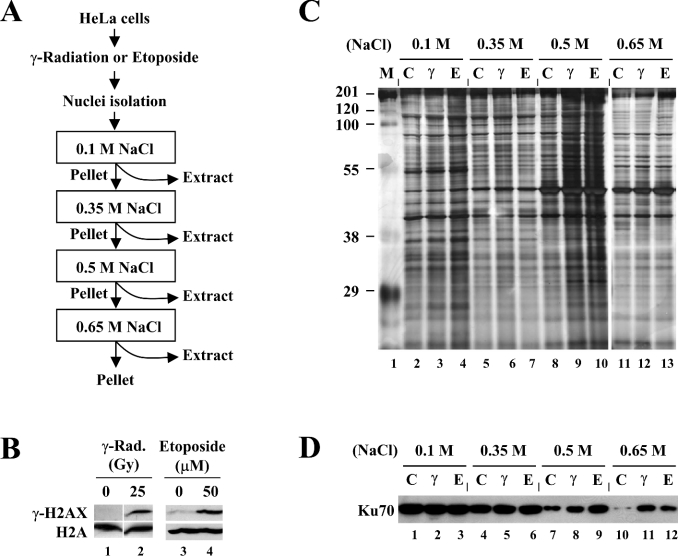
Figure 1. Experimental strategy for sequential salt extraction of chromatin-associated proteins.
(A) Representation of the experimental strategy employing sequential salt extraction of HeLa nuclei with increasing salt concentrations; see the text for details. (B) Establishment of the conditions for generating DNA DSBs. HeLa cells were untreated (lanes 1 and 3), exposed to 25 Gy of γ-radiation (lane 2) or treated with 50 μM of etoposide (lane 4). The cells were collected after 3 h for nuclei isolation, histone extraction and immunoblot analysis for γ-H2AX expression. H2A was used as an internal control. (C) Protein extracts were prepared from HeLa cells in which DNA DSBs were induced as described above (M, molecular-mass markers; C, control; γ, γ-radiation; E, etoposide), and subjected to SDS/PAGE (12.5% gel) followed by silver staining. (D) The validity of the experimental strategy used was examined by immunoblotting for Ku proteins. Increased levels of Ku70 and Ku80 (results not shown), known to bind to the ends of broken DNA, were extracted from DSB-generated nuclei than from control nuclei at 0.5- and 0.65-M salt concentrations (lanes 7–12).
Chromatin-binding assays
Chromatin-binding assays were performed as described previously [13] with slight modifications. Cells (5×106) were washed with PBS and resuspended in 200 μl of the lysis buffer containing 10 mM Hepes (pH 7.5), 10 mM KCl, 3 mM MgCl2, 0.35 M sucrose, 0.1% NP40, 3 mM 2-mercaptoethanol, 0.4 mM PMSF, 1 μM pepstatin A, 1 μM leupeptin and 5 μg/ml aprotinin. The cells were incubated on ice for 5 min. Cytoplasmic proteins were removed from nuclei by centrifugation at 1300 g for 5 min. The nuclei pellet was resuspended in lysis buffer and nuclei were spun down by centrifugation. Isolated nuclei were resuspended in 200 μl of solution containing 3 mM EDTA, 0.2 mM EGTA, 1 mM dithiothreitol, 100 mM NaCl and 0.8% NP40. The nuclei were incubated on ice for 60 min, and soluble nuclear proteins (soluble fraction) were separated from chromatin by centrifugation at 1700 g for 5 min. The chromatin pellet was washed twice with the same solution as the above and spun down at high speed (10000 g for 1 min), and the chromatin was resuspended in SDS sample buffer and sheared by sonication (chromatin fraction). Both fractions were subjected to SDS/PAGE and immunoblot analysis.
Plasmid construction and transfection
cDNAs encoding full-length NPM and hnRNP C1 were PCR amplified from HeLa cDNA library using the following primers: NPM forward, 5′-GAT ATT GCT AGC ATG GAA GAT TCG ATG GAC ATG-3′; NPM reverse, 5′-CGG TGG ATC CCG AAG AGA CTT CCT CCA CTG CCA-3′; hnRNP C1 forward, 5′-GAT ATT GCT AGC ATG GCC AGC AAC GTT ACC AAC-3′; hnRNP C1 reverse, 5′-CGG TGG ATC CCG TCC TCC ATT GGC GCT GTC TCT-3′. PCR products were digested with NheI and BamHI, and ligated into the same sites of pEGFP-N1 (BD Biosciences Clontech) to generate the constructs expressing NPM–GFP (green fluorescent protein) or hnRNP-C1–GFP fusion proteins. Transfection into HeLa cells was performed using Lipofectamine™ reagent (Life Technologies) according to the manufacturer's instructions.
Immunocytochemistry
Cells were seeded on to glass coverslips or 30-mm culture dishes, fixed with 4% paraformaldehyde for 15 min and washed for 10 min in PBS three times. After quenching in 50 mM NH4Cl for 10 min, cells were permeabilized with 0.2% Triton X-100 for 5 min, and blocked with 1% BSA for 1 h at room temperature. Cells were washed for 10 min in PBS four times, and incubated with the antibodies against γ-H2AX, NPM or hnRNP C1/C2 overnight at 4 °C followed by incubation with Alexa Fluor 568-conjugated anti-mouse (Molecular Probes) or FITC-conjugated anti-rabbit (Zymed) antibodies for 30 min at 30 °C. Cells were washed for 10 min with PBS four times and mounted using Vectashield mounting medium with DAPI (4,6-diamidino-2-phenylindole; Vector Laboratories). Fluorescence images were photographed by using Axiovert 100M microscope.
RESULTS
Sequential salt extraction of chromatin-associated proteins
To perform proteomic analysis with the smallest group of proteins as possible, we employed the strategy in which chromatin-associated proteins are sequentially extracted using the buffers containing increasing salt concentrations as depicted in Figure 1(A). We rationalized that proteins bound to chromatin with different affinities require different salt concentrations to be dissociated such that, at a certain salt concentration, loosely bound proteins will be easily dissociated from chromatin, whereas tightly bound proteins will not. This strategy would not only reduce proteome size to be analysed, but also increase the possibility of identifying the proteins that are differentially bound to the chromatin in DSB-induced versus non-induced conditions.
We have chosen two different sources for generating DNA DSBs, ionizing radiation and etoposide; the former breaks DNA through the cell cycle by generating hydroxyl radicals, and the latter, a topoisomerase II inhibitor, induces DSBs by blocking DNA replication during S-phase. Actively proliferating HeLa cells were exposed to 25 Gy of γ-radiation or treated with 50 μM etoposide, and after 3 h, cells were harvested for analysing γ-H2AX expression, which is induced by DSB generation and thereby can be used as a DSB indicator. γ-H2AX was induced in the cells that had been exposed to γ-radiation or treated with etoposide, whereas it was barely detectable from untreated cells (Figure 1B), indicating that DNA DSBs are properly generated under these conditions.
Nuclei isolated from HeLa cells that had been left untreated or induced to generate DNA DSBs were subjected to the sequential extraction with the buffers containing NaCl at the concentrations of 0.1, 0.35, 0.5 and 0.65 M, as shown in Figure 1(A). Protein extraction using the salt concentrations higher than 0.65 M could not be performed, because nuclear suspension in such buffers became extremely viscous due to chromatin disruption. The obtained protein extracts were subjected to SDS/PAGE (12.5% gel) and the silver-stained gel is shown in Figure 1(C). Whereas some proteins appeared to be extracted equally in all salt concentrations, many proteins were differentially extracted depending on the salt concentrations used, indicating that the proteins bound to chromatin with different affinities can be dissociated by different salt concentrations. When extraction was performed at 0.1-M or 0.35-M salt concentrations, the extracted proteome was not significantly different (at least on a one-dimensional gel) between control and DSB-generated nuclei (Figure 1C, lanes 2–4 and lanes 5–7 respectively). At 0.5-M salt concentration, however, significantly more proteins were extracted from DSB-generated nuclei compared with control nuclei (Figure 1C, compare lane 8 with lanes 9 and 10). This difference was not observed from the 0.65-M extracts (lanes 11–13), indicating that most of the proteins that had bound to chromatin upon DSB generation were dissociated at 0.5-M salt concentration.
To determine whether the strategy employed is suitable for our experimental goals, we tested Ku proteins as a positive control for DNA-damage-dependent salt extraction. Ku proteins, existing as a Ku70–Ku80 heterodimer in the DNA-PK (DNA-dependent protein kinase) complex, are the essential components of the NHEJ (non-homologous end-joining) pathway of DNA DSB repair [14], and are known to bind to the ends of DNA both in free form and in the context of chromatin [15]. Indeed, both Ku70 (Figure 1D) and Ku80 (results not shown) showed higher levels of extraction from DSB-generated nuclei than control nuclei at 0.5-M salt concentration (lanes 7–9), whereas they were equally extracted independent of DNA damage at 0.1-M and 0.35-M (lanes 1–6). The DNA-damage-dependent salt extraction of Ku was more dramatic at 0.65-M salt (lanes 10–12), likely due to its unusually high affinity of DNA binding [14]. These results led us to conclude that higher levels of proteins in the extracts from DSB-generated nuclei compared with control nuclei at certain salt concentrations reflect more binding of those proteins to chromatin after DNA damage.
2-D gel analysis identified several proteins as the candidates for DNA-damage-induced chromatin-binding proteins
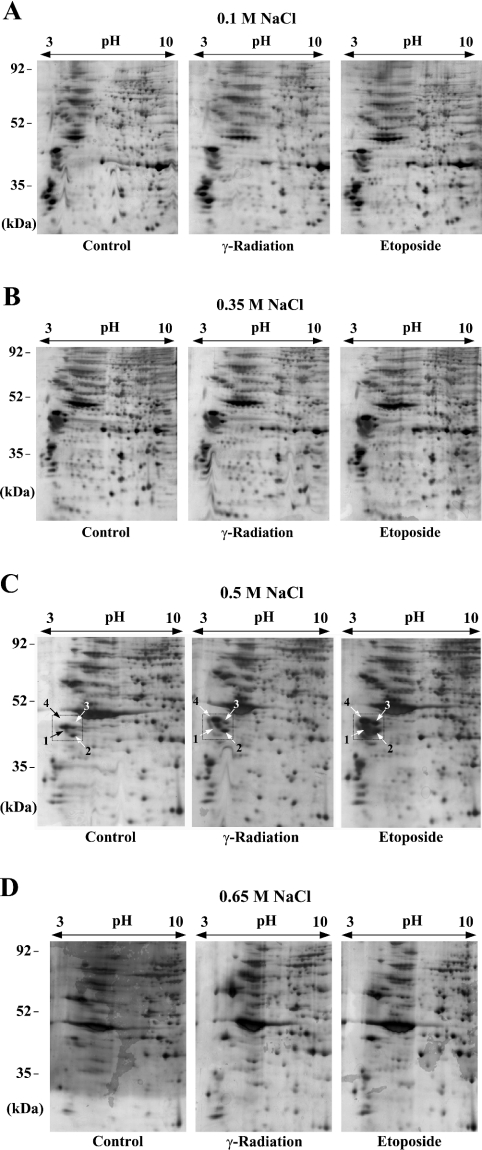
Next we performed 2-D gel electrophoresis for the protein extracts prepared as described above with pI range from 3 to 10, and the results are shown in Figure 2. For the 0.1-M or 0.35-M extracts, as could be expected from the one-dimensional gel in Figure 1(C), no protein spot on the 2-D gel showed a significant intensity change by DNA damage (Figures 2A and 2B). 2-D gel analysis for the 0.5-M extracts showed that, whereas many other protein spots showed subtle changes in their intensity, the levels of a group of proteins with low pI migrating at the range of 35–52 kDa were largely increased by DNA damage (Figure 2C, the protein spots are numbered in the box). These proteins were also present in the 0.1- or 0.35-M extracts, but their levels were not affected by DNA damage (Figures 2A and 2B), and they were largely absent in the 0.65-M extracts (Figure 2D). These results indicate that some portion of each of these proteins binds to chromatin after DNA damage such that 0.5-M or higher salt concentrations are required for the bound proteins to be dissociated from chromatin.
Figure 2. 2-D gel electrophoresis for protein extracts from DNA-damage-induced nuclei.
Protein extracts, prepared as described in Figure 1, were subjected to 2-D gel electrophoresis with a pI range from 3 to 10 and 12.5% gels. The scanned images of silver-stained wet gels are shown. The salt concentrations used for protein extraction are indicated above each group of gels. On each of the gels in (C), the protein spots showing a large difference in their intensity between control and DSB-induced samples are numbered within a box. The indicated protein spots of the third gel (Etoposide) in (C) were excised for trypsin digestion and MALDI–TOF MS analysis.
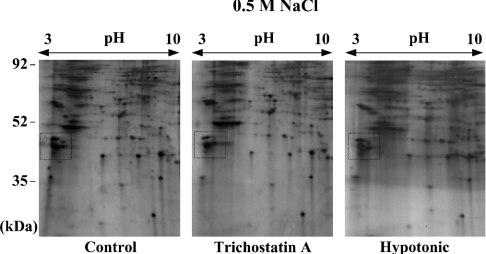
Although the intensity increase of the proteins described above could reflect the actual binding to chromatin, it is also possible that DNA-breakage-induced chromatin structural change causes a release in the constitutive chromatin-bound proteins during 0.5-M salt extraction. To test this possibility, we prepared 0.5-M salt extracts by the same procedure as before, except using HeLa cells in which chromatin structural change was induced by treatment with TSA, an inhibitor of histone acetylase, or treatment with hypotonic buffer. We then performed 2-D gel anlaysis for those extracts. As shown in Figure 3 (see the boxed area), the levels of the corresponding proteins were not affected by merely changing chromatin structure, suggesting that the increased levels of these proteins on the 2-D gels of Figure 2(C) reflect actual binding to chromatin after DNA damage. We therefore pursued further investigation of these proteins.
Figure 3. 2-D gel electrophoresis for protein extracts from the nuclei in which chromatin structural change, not DNA damage, was induced.
Protein extracts were prepared in 0.5-M NaCl salt solution as described in Figure 1, except for using the nuclei from HeLa cells that had been untreated (left-hand panel), treated with 10 μM TSA for 16 h (middle panel) or treated with hypotonic PBS containing 50 mM NaCl (right-hand panel) for 1 h. The extracts were separated on 2-D gels, as described in Figure 2. The intensity of the corresponding protein spots within the box, which was largely changed in Figure 2(C), was not changed by the treatment with TSA or hypotonic buffer.
MALDI–TOF analysis to identify the candidate proteins
To determine the identity of the four candidate proteins, the protein spots were excised from the gel and subjected to trypsin digestion and MALDI–TOF MS analysis. The proteins were identified to be NPM, hnRNP C1, hnRNP C2 and 37LRP. Accession number, functions, theoretical molecular masses and pI values, the number of matched peptides, the percentage of sequence coverage and MOWSE (molecular weight search) score for each protein are listed in Table 1. Reproducible results were obtained from the three gels analysed. The identity of the proteins was confirmed by immunoblot analysis with antibodies specific for each protein (results not shown, and Figure 4).
Table 1. Proteins identified by MALDI–TOF MS.
Mdm2, murine double minute clone 2 oncoprotein; MOWSE, molecular weight search (see http://www.hgmp.mrc.ac.uk/Bioinformatics/Webapp/mowse/).
| Spot | Protein | Accession number | Functions | Molecular mass (kDa) | pI | Number of matched peptides | Coverage (%) | MOWSE score |
|---|---|---|---|---|---|---|---|---|
| 1 | NPM | NP_002511 | Ribosome biogenesis | 30.9 | 4.7 | 12 | 24.0 | 6.52×105 |
| Centrosome duplication | ||||||||
| DNA-damage response | ||||||||
| Regulation of p53 activity | ||||||||
| Interaction with p14ARF and Mdm2 | ||||||||
| Regulation of cell cycle | ||||||||
| Histone chaperone | ||||||||
| Chromatin remodelling | ||||||||
| 2 | hnRNP C1 | NP_004491 | RNA maturation/transport/turnover | 31.9 | 5.1 | 14 | 29.0 | 3.90×105 |
| Telomere/telomerase regulation | ||||||||
| 3 | hnRNP C2 | NP_112604 | RNA maturation/transport/turnover | 33.3 | 5.1 | 14 | 29.0 | 3.90×105 |
| Telomere/telomerase regulation | ||||||||
| 4 | 37LRP | NP_002286 | Translation | 32.8 | 4.8 | 9 | 40.0 | 1.00×104 |
| Ribosomal structure and biogenesis |
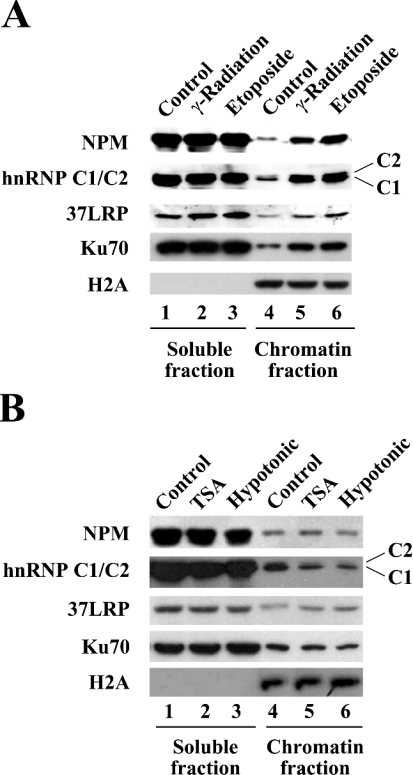
Figure 4. NPM and hnRNP C1/C2 bind to chromatin after DNA damage.
Chromatin-binding assays were performed. The soluble (lanes 1–3) and chromatin (lanes 4–6) fractions were separated using the nuclei from the cells in which DNA damage was induced as described in Figure 1(B) (A), or from the cells in which chromatin structural change was induced by treatment with TSA or hypotonic buffer (B). The distribution of the indicated proteins with soluble versus chromatin fractions was determined by immunoblotting with the antibodies indicated.
NPM and hnRNP C1/C2 bind to chromatin in response to DNA damage
Next, we wanted to determine whether the identified proteins indeed bind to chromatin in response to DNA damage using chromatin-binding assays. Chromatin fractionation was performed to separate soluble fractions from insoluble chromatin fractions, which were then analysed for distribution of NPM, hnRNP C1/C2 and 37LRP with immunoblotting. As shown in Figure 4(A), the majority of NPM and hnRNP C1/C2 proteins were present in the soluble fractions with their levels being independent of DNA damage (lanes 1–3), whereas, in the chromatin fractions, much more NPM and hnRNP C1/C2 were found in the samples from DSB-induced nuclei compared with control nuclei (lanes 4–6). The levels of 37LRP were only slightly increased by DNA damage in both soluble and chromatin fractions (lanes 1–6), indicating that, unlike what would be expected from the 2-D gel analysis in Figure 2(C), 37LRP may not be considered as a DNA-damage-induced chromatin-binding protein. Ku70, used as a positive control, showed a very similar pattern to that seen for NPM and hnRNP C1/C2. Histone H2A was detected in the chromatin fractions, but not in the soluble fractions, ensuring that the chromatin fractionations were properly done.
Again, to exclude the possibility of a contribution of mere chromatin structural change to the observed chromatin association of NPM and hnRNP C1/C2 proteins, we performed similar chromatin-binding assays with the nuclei from the cells that had been treated with TSA or hypotonic buffer. As shown in Figure 4(B), inducing chromatin structural change without introducing exogenous DNA damage does not cause NPM and hnRNP C1/C2 to bind to chromatin (lanes 4–6). Taken together, these results demonstrated that NPM and hnRNP C1/C2 are DNA-damage-induced chromatin-binding proteins.
Subcellular localization of NPM and hnRNP C1/C2 after DNA damage
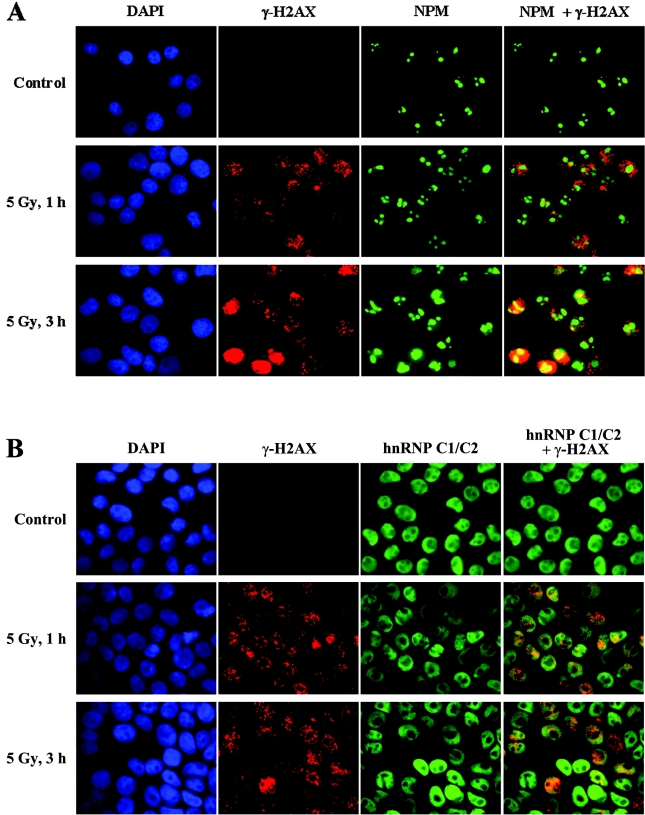
Having established the activity of DNA-damage-dependent chromatin-binding for NPM and hnRNP C1/C2, we wanted to examine whether these proteins form IRIF and/or are recruited to γ-H2AX foci after DNA damage. HeLa cells were either left untreated or exposed to a low dosage of irradiation (5 Gy), and further incubated for 1 or 3 h before performing immunofluorescence dual labelling using antibodies against γ-H2AX and NPM. Although barely detectable in the untreated cells, γ-H2AX was clearly detected at 1 h after irradiation, with its levels somewhat increased after 3 h (Figure 5A). Importantly, the immunostaining of γ-H2AX showed the typical distinctive punctate pattern, which is indicative of DNA DSB-induced formation of IRIF. The pattern of NPM immunostaining showed that NPM was exclusively present in the nucleoli under the normal conditions, and that it gradually diffused into the nucleoplasm following DNA damage by irradiation (Figure 5A). This DNA-damage-induced mobilization of NPM has also been observed when cells were subjected to UV irradiation [16,17], suggesting that NPM mobilization is a general mechanism for responding to DNA damage. However, NPM was neither concentrated in speckles nor co-localized with γ-H2AX foci, indicating that NPM is not actively recruited to γ-H2AX foci following DNA damage.
Figure 5. Examination of subcellular localization of NPM and hnRNP C1/C2 after DNA damage.
HeLa cells were exposed to 5 Gy of γ-radiation or treated with 50 μM etoposide (results not shown), and incubated for 1 or 3 h as indicated. The control cells were left untreated. Cells were then collected and subjected to dual immunostaining with antibodies against γ-H2AX (red) and NPM (green) (A), or γ-H2AX (red) and hnRNP C1/C2 (green) (B) before the immunofluorescence images were taken. The images of anti-γ-H2AX and anti-NPM staining, or anti-γ-H2AX and anti-(hnRNP C1/C2) staining were merged to examine co-localization (shown in the right-hand column in each row). The nuclei were visualized by DAPI staining (blue).
We next performed similar experiments to examine subcellular localization of hnRNP C1/C2. In sharp contrast with NPM, hnRNP C1/C2 was present throughout the nucleoplasm, excluding the nucleoli, under the normal conditions (Figure 5B), which agrees with a previous study [18]. However, the staining pattern of hnRNP C1/C2 was not apparently changed by radiation-induced DNA damage, whereas γ-H2AX was properly induced to form foci (Figure 5B). We observed virtually the same results as thus far described about the subcellular localization of NPM and hnRNP C1/C2 when we subjected cells to DNA damage using etoposide (results not shown). We also investigated subcellular localization of NPM and hnRNP C1/C2 by transfection experiments with expression vectors for NPM–GFP and hnRNPC1–GFP fusion proteins, and observed generally the same results as the above (results not shown).
Taken together, the results indicate that NPM, normally present in the nucleolus, is mobilized into nucleoplasm in response to DNA damage, whereas hnRNP C1/C2, the major component of the nuclear matrix, does not appear to change in its subcellular localization after DNA damage. In addition, neither of these proteins is accumulated into IRIF or recruited to γ-H2AX foci in response to DNA damage.
DISCUSSION
In an effort to identify the proteins that bind to chromatin in response to the DNA damage of DSBs, we used a proteomics approach in combination with the strategy employing sequential extractions of chromatin-associated proteins with a variety of salt concentrations. In the present study we report the identification of NPM and hnRNP C1/C2, the abundant nuclear proteins with pleiotropic functions, as DNA-damage-induced chromatin-binding proteins. We confirmed, using chromatin-binding assays, that these proteins indeed bind to chromatin following DNA damage. We also showed that NPM is mobilized from the nucleoli into the nucleoplasm in response to DNA damage. However, neither NPM nor hnRNP C1/C2 was actively recruited to the sites of DNA breaks, suggesting that they may respond to DNA damage through some other mechanisms than directly targeting the DNA lesions.
NPM, also known as B23, numatrin and NO38, is an acidic nucleolar phosphoprotein, which is thought to be involved in various biological processes, including mRNA processing, ribosome biogenesis, cell proliferation and centrosome duplication [19–22]. Recent experimental evidence strongly suggests that NPM is also implicated in regulation of the cell cycle and the cellular response to DNA damage. For example, studies showed that NPM functions as an important regulator of the p53 tumour suppressor by directly interacting with p53 or by modulating the key regulators of p53, such as Mdm2 (murine double minute clone 2 oncoprotein) and p14ARF [16,23–29]. These studies also showed that NPM plays a positive role in the cellular response to DNA damage triggered by UV or ionizing radiation [16,17,23,24,26,30,31]. Interestingly, NPM, together with nucleolin, which is another nucleolar protein involved in ribosome biogenesis, has been identified as a genotoxic-stress-induced RNA-binding protein [32]. In this respect, the possibility is plausible that the association of NPM with chromatin following DNA damage is mediated by its activity to bind to nascent RNA transcripts still tethered to the chromatin, whereby transcription can be regulated. Alternatively, it is also possible that NPM directly binds to histones to modulate chromatin structure in response to DNA damage, as NPM has been shown to have histone-chaperone and chromatin-remodelling activities [33–35].
Our results, showing that NPM is diffused from the nucleolus into the nucleoplasm in response to DNA damage, are consistent with the recently proposed hypothesis about the role of the nucleolus in the DNA-damage response. This hypothesis postulates that the structure of the nucleolus is disrupted by a variety of DNA-damaging agents, and that this nucleolar disruption plays a key role in triggering the DNA-damage response [36]. In this context, it is intriguing to propose that DNA-damaging agents, such as ionizing radiation and etoposide, cause nucleolar disruption, and consequently NPM is released into the nucleoplasm where it binds to chromatin.
hnRNP C1/C2, belonging to the hnRNP family, have been implicated in a variety of biological functions, including many aspects of mRNA metabolism, such as splicing, polyadenylation and turnover, and telomere/telomerase regulation [37,38]. Our results, identifying hnRNP C1/C2 as DNA-damage-induced chromatin-binding proteins, implicate this pleiotropic protein in DNA repair and/or damage response as well. It is intriguing to speculate that a possible role of hnRNP C1/C2 in DNA-damage response might entail its ability to control mRNA maturation/turnover in a way to help regulate overall rate of gene expression after DNA damage. Interestingly, however, a recent study showed that hnRNP C1/C2 binds to Ku in an RNA-dependent manner, and that it can be phosphorylated by DNA-PKcs, the catalytic subunit of the DNA-PK complex [39]. In this respect, it is also possible that hnRNP C1/C2 directly participates in DNA DSB repair through the NHEJ pathway.
The proteomics approach used in the present study was successful to identify the proteins that exist abundantly in the nucleus and largely bind to chromatin after DNA damage, such as NPM and hnRNP C1/C2. However, it should be noted that this approach has limitations in identifying less abundant DNA-damage-induced chromatin-binding proteins, which seems to be case for most of the repair and checkpoint proteins. This is likely to be the reason why only a few proteins were identified as candidates for DSB-induced chromatin-binding proteins from 2-D gel analysis. Given that both NPM and hnRNP C1/C2 are abundant in the nucleus and bind to chromatin to a great extent, we hypothesized that these proteins might play roles in DNA repair and/or damage response through some global regulation of the essential processes, such as modulation of chromatin structure and control of gene expression. Further experiments will be necessary to address the biological significance of the DNA-damage-induced chromatin-binding activity of NPM and hnRNP C1/C2.
Acknowledgments
This work was supported by the Ewha Womans University intramural research program, and also supported by the Nuclear Research and Development Program from the Ministry of Science and Technology of Korea granted to J.K. (M2-0408-00-0028).
References
- 1.Hoeijmakers J. H. Genome maintenance mechanisms for preventing cancer. Nature (London) 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 2.Peterson C. L., Cote J. Cellular machineries for chromosomal DNA repair. Genes Dev. 2004;18:602–616. doi: 10.1101/gad.1182704. [DOI] [PubMed] [Google Scholar]
- 3.Mills K. D., Ferguson D. O., Alt F. W. The role of DNA breaks in genomic instability and tumorigenesis. Immunol. Rev. 2003;194:77–95. doi: 10.1034/j.1600-065x.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- 4.Sancar A., Lindsey-Boltz L. A., Unsal-Kacmaz K., Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 5.Jackson S. P. Sensing and repairing DNA double-strand breaks. Carcinogenesis. 2002;23:687–696. doi: 10.1093/carcin/23.5.687. [DOI] [PubMed] [Google Scholar]
- 6.Narlikar G. J., Fan H. Y., Kingston R. E. Cooperation between complexes that regulate chromatin structure and transcription. Cell (Cambridge, Mass.) 2002;108:475–487. doi: 10.1016/s0092-8674(02)00654-2. [DOI] [PubMed] [Google Scholar]
- 7.Vignali M., Hassan A. H., Neely K. E., Workman J. L. ATP-dependent chromatin-remodeling complexes. Mol. Cell. Biol. 2000;20:1899–1910. doi: 10.1128/mcb.20.6.1899-1910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gontijo A. M., Green C. M., Almouzni G. Repairing DNA damage in chromatin. Biochimie. 2003;85:1133–1147. doi: 10.1016/j.biochi.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Allard S., Masson J. Y., Cote J. Chromatin remodeling and the maintenance of genome integrity. Biochim. Biophys. Acta. 2004;1677:158–164. doi: 10.1016/j.bbaexp.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Capetillo O., Lee A., Nussenzweig M., Nussenzweig A. H2AX: the histone guardian of the genome. DNA Repair. 2004;3:959–967. doi: 10.1016/j.dnarep.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 11.Bassing C. H., Alt F. W. H2AX may function as an anchor to hold broken chromosomal DNA ends in close proximity. Cell Cycle. 2004;3:149–153. doi: 10.4161/cc.3.2.689. [DOI] [PubMed] [Google Scholar]
- 12.Lee J.-W., Kim J.-E., Park E.-J., Kim J.-H., Lee C.-H., Lee S.-R., Kwon J. Two conserved cysteine residues are critical for the enzymic function of the human platelet-derived growth factor receptor-beta: evidence for different roles of Cys-822 and Cys-940 in the kinase activity. Biochem. J. 2004;382:631–639. doi: 10.1042/BJ20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou L., Cortez D., Elledge S. J. Regulation of ATR substrate selection by Rad17-dependent loading of Rad9 complexes onto chromatin. Genes Dev. 2002;16:198–208. doi: 10.1101/gad.950302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Downs J. A., Jackson S. P. A means to a DNA end: the many roles of Ku. Nat. Rev. Mol. Cell Biol. 2004;5:367–378. doi: 10.1038/nrm1367. [DOI] [PubMed] [Google Scholar]
- 15.Park E.-J., Chan D. W., Park J.-H., Oettinger M. A., Kwon J. DNA-PK is activated by nucleosomes and phosphorylates H2AX within the nucleosomes in an acetylation-dependent manner. Nucleic Acids Res. 2003;31:6819–6827. doi: 10.1093/nar/gkg921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurki S., Peltonen K., Latonen L., Kiviharju T. M., Ojala P. M., Meek D., Laiho M. Nucleolar protein NPM interacts with HDM2 and protects tumor suppressor protein p53 from HDM2-mediated degradation. Cancer Cell. 2004;5:465–475. doi: 10.1016/s1535-6108(04)00110-2. [DOI] [PubMed] [Google Scholar]
- 17.Wu M. H., Yung B. Y. UV stimulation of nucleophosmin/B23 expression is an immediate-early gene response induced by damaged DNA. J. Biol. Chem. 2002;277:48234–48240. doi: 10.1074/jbc.M206550200. [DOI] [PubMed] [Google Scholar]
- 18.Mattern K. A., van der Kraan I., Schul W., de Jong L., van Driel R. Spatial organization of four hnRNP proteins in relation to sites of transcription, to nuclear speckles, and to each other in interphase nuclei and nuclear matrices of HeLa cells. Exp. Cell Res. 1999;246:461–470. doi: 10.1006/excr.1998.4267. [DOI] [PubMed] [Google Scholar]
- 19.Borer R. A., Lehner C. F., Eppenberger H. M., Nigg E. A. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell (Cambridge, Mass.) 1989;56:379–390. doi: 10.1016/0092-8674(89)90241-9. [DOI] [PubMed] [Google Scholar]
- 20.Yung B. Y., Busch H., Chan P. K. Translocation of nucleolar phosphoprotein B23 (37 kDa/pI 5.1) induced by selective inhibitors of ribosome synthesis. Biochim. Biophys. Acta. 1985;826:167–173. doi: 10.1016/0167-4781(85)90002-8. [DOI] [PubMed] [Google Scholar]
- 21.Chan W. Y., Liu Q. R., Borjigin J., Busch H., Rennert O. M., Tease L. A., Chan P. K. Characterization of the cDNA encoding human nucleophosmin and studies of its role in normal and abnormal growth. Biochemistry. 1989;28:1033–1039. doi: 10.1021/bi00429a017. [DOI] [PubMed] [Google Scholar]
- 22.Okuda M., Horn H. F., Tarapore P., Tokuyama Y., Smulian A. G., Chan P. K., Knudsen E. S., Hofmann I. A., Snyder J. D., Bove K. E., Fukasawa K. Nucleophosmin/B23 is a target of CDK2/cyclin E in centrosome duplication. Cell (Cambridge, Mass.) 2000;103:127–140. doi: 10.1016/s0092-8674(00)00093-3. [DOI] [PubMed] [Google Scholar]
- 23.Kurki S., Peltonen K., Laiho M. Nucleophosmin, HDM2 and p53: players in UV damage incited nucleolar stress response. Cell Cycle. 2004;3:976–979. [PubMed] [Google Scholar]
- 24.Colombo E., Marine J. C., Danovi D., Falini B., Pelicci P. G. Nucleophosmin regulates the stability and transcriptional activity of p53. Nat. Cell Biol. 2002;4:529–533. doi: 10.1038/ncb814. [DOI] [PubMed] [Google Scholar]
- 25.Brady S. N, Yu Y., Maggi L. B., Jr, Weber J. D. ARF impedes NPM/B23 shuttling in an Mdm2-sensitive tumor suppressor pathway. Mol. Cell. Biol. 2004;24:9327–9338. doi: 10.1128/MCB.24.21.9327-9338.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maiguel D. A., Jones L., Chakravarty D., Yang C., Carrier F. Nucleophosmin sets a threshold for p53 response to UV radiation. Mol. Cell. Biol. 2004;24:3703–3711. doi: 10.1128/MCB.24.9.3703-3711.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertwistle D., Sugimoto M., Sherr C. J. Physical and functional interactions of the Arf tumor suppressor protein with nucleophosmin/B23. Mol. Cell. Biol. 2004;24:985–996. doi: 10.1128/MCB.24.3.985-996.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y. The ARF–B23 connection: implications for growth control and cancer treatment. Cell Cycle. 2004;3:259–262. [PubMed] [Google Scholar]
- 29.Itahana K., Bhat K. P., Jin A., Itahana Y., Hawke D., Kobayashi R., Zhang Y. Tumor suppressor ARF degrades B23, a nucleolar protein involved in ribosome biogenesis and cell proliferation. Mol. Cell. 2003;12:1151–1164. doi: 10.1016/s1097-2765(03)00431-3. [DOI] [PubMed] [Google Scholar]
- 30.Wu M. H., Chang J. H., Yung B. Y. Resistance to UV-induced cell-killing in nucleophosmin/B23 over-expressed NIH 3T3 fibroblasts: enhancement of DNA repair and up-regulation of PCNA in association with nucleophosmin/B23 over-expression. Carcinogenesis. 2002;23:93–100. doi: 10.1093/carcin/23.1.93. [DOI] [PubMed] [Google Scholar]
- 31.Wu M. H., Chang J. H., Chou C. C., Yung B. Y. Involvement of nucleophosmin/B23 in the response of HeLa cells to UV irradiation. Int. J. Cancer. 2002;97:297–305. doi: 10.1002/ijc.1606. [DOI] [PubMed] [Google Scholar]
- 32.Yang C., Maiguel D. A., Carrier F. Identification of nucleolin and nucleophosmin as genotoxic stress-responsive RNA-binding proteins. Nucleic Acids Res. 2002;30:2251–2260. doi: 10.1093/nar/30.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okuwaki M., Iwamatsu A., Tsujimoto M., Nagata K. Identification of nucleophosmin/B23, an acidic nucleolar protein, as a stimulatory factor for in vitro replication of adenovirus DNA complexed with viral basic core proteins. J. Mol. Biol. 2001;311:41–55. doi: 10.1006/jmbi.2001.4812. [DOI] [PubMed] [Google Scholar]
- 34.Okuwaki M., Matsumoto K., Tsujimoto M., Nagata K. Function of nucleophosmin/B23, a nucleolar acidic protein, as a histone chaperone. FEBS Lett. 2001;506:272–276. doi: 10.1016/s0014-5793(01)02939-8. [DOI] [PubMed] [Google Scholar]
- 35.Szebeni A., Olson M. O. Nucleolar protein B23 has molecular chaperone activities. Protein Sci. 1999;8:905–912. doi: 10.1110/ps.8.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubbi C. P., Milner J. Disruption of the nucleolus mediates stabilization of p53 in response to DNA damage and other stresses. EMBO J. 2003;22:6068–6077. doi: 10.1093/emboj/cdg579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krecic A. M., Swanson M. S. hnRNP complexes: composition, structure, and function. Curr. Opin. Cell Biol. 1999;11:363–371. doi: 10.1016/S0955-0674(99)80051-9. [DOI] [PubMed] [Google Scholar]
- 38.Ford L. P., Wright W. E., Shay J. W. A model for heterogeneous nuclear ribonucleoproteins in telomere and telomerase regulation. Oncogene. 2002;21:580–583. doi: 10.1038/sj.onc.1205086. [DOI] [PubMed] [Google Scholar]
- 39.Zhang S., Schlott B., Gorlach M., Grosse F. DNA-dependent protein kinase (DNA-PK) phosphorylates nuclear DNA helicase II/RNA helicase A and hnRNP proteins in an RNA-dependent manner. Nucleic Acids Res. 2004;32:1–10. doi: 10.1093/nar/gkg933. [DOI] [PMC free article] [PubMed] [Google Scholar]