Abstract
IFNγ (interferon-γ) binding to its cognate receptor results, through JAK (Janus kinase), in direct activation of receptor-bound STAT1 (signal transducer and activator of transcription 1), although there is evidence for additional activation of a MAPK (mitogen-activated protein kinase) pathway. In the present paper, we report IFNγ-dependent activation of the MEKK4 (MAPK/extracellular-signal-regulated kinase kinase kinase 4) pathway in HaCaT human keratinocytes. MEKK4 is tyrosine-phosphorylated and the IFNγ-dependent phosphorylation requires intracellular calcium. Calcium-dependent phosphorylation of MEKK4 is mediated by Pyk2. Moreover, MEKK4 and Pyk2 co-localize in an IFNγ-dependent manner in the perinuclear region. Furthermore, the calcium-binding protein, annexin II, and the calcium-regulated kinase, Pyk2, co-immunoprecipitate with MEKK4 after treatment with IFNγ. Immunofluorescence imaging of HaCaT cells shows an IFNγ-dependent co-localization of annexin II with Pyk2 in the perinuclear region, suggesting that annexin II mediates the calcium-dependent regulation of Pyk2. Tyrosine phosphorylation of MEKK4 correlates with its activity to phosphorylate MKK6 (MAPK kinase 6) in vitro and subsequent p38 MAPK activation in an IFNγ-dependent manner. Additional studies demonstrate that the SH2 (Src homology 2)-domain-containing tyrosine phosphatase SHP2 co-immunoprecipitates with MEKK4 in an IFNγ-dependent manner and co-localizes with MEKK4 after IFNγ stimulation in the perinuclear region in HaCaT cells. Furthermore, we provide evidence that SHP2 dephosphorylates MEKK4 and Pyk2, terminating the MEKK4-dependent branch of the IFNγ signalling pathway.
Keywords: annexin II, interferon-γ (IFNγ), mitogen-activated protein kinase/extracellular-signal-regulated kinase kinase kinase 4 (MEKK4), Pyk2, Src-homology-2-domain-containing phosphatase (SHP2), STAT1 (signal transducer and activator of transcription 1)
Abbreviations: ATF2, activating transcription factor 2; BAPTA/AM, bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid tetrakis(acetoxymethyl ester); COX2, cyclo-oxygenase 2; CSK, C-terminal Src kinase; DMEM, Dulbecco's minimal essential medium; DTT, dithiothreitol; FBS, foetal bovine serum; GST, glutathione S-transferase; HA, haemagglutinin; IFNγ, interferon-γ; IFNGR, IFNγ receptor; JAK, Janus kinase; MAPK, mitogen-activated protein kinase; MEKK, MAPK/extracellular-signal-regulated protein kinase kinase kinase); MKK6, MAPK kinase 6; NP40, Nonidet P40; SH2, Src homology 2; SHP2, SH2-domain-containing tyrosine phosphatase; STAT1, signal transducer and activator of transcription 1; TBST, Tris-buffered saline with Tween 20
INTRODUCTION
Interferons, especially the type II cytokine IFNγ (interferon-γ), exhibit antiviral, immunoregulatory and growth-inhibitory activities [1–4]. The IFNGR (IFNγ receptor) is expressed on almost all cell types. IFNγ activates the IFNGR, consisting of two heterodimers, the IFNGR1 (α) and the IFNGR2 (β) subunits. Upon ligand binding to the IFNGR1 subunits, an active complex assembles by associating the α subunits with the two smaller β subunits. Activated JAK (Janus kinase) 2 that is associated with the α subunit transphosphorylates both JAK1, which binds to the β subunit, and IFNGR1. The tyrosylphosphate of IFNGR1 recruits STAT1 (signal transducer and activator of transcription 1), which itself becomes activated by tyrosine phosphorylation, subsequently dimerizes and translocates to the nucleus [1]. IFNγ has been shown to elicit a Ca2+ flux, which has been investigated in certain cell types, such as neutrophils [5], promyelocytic leukaemia cells [6], thyroid cells [7], lymphocytes [8], microglia [9], fibroblasts [10] and gastric carcinoma cells [11], suggesting that this increase of intracellular calcium is involved in IFNγ signalling.
To transduce a signal from an activated receptor into the cell to produce a physiological response, a cascade of protein kinases is often utilized, and the MAPK (mitogen-activated protein kinase) cascade represents such a signal transduction module. While STAT1 activation via IFNGR-activated JAK has been studied extensively, there is also evidence for the activation of MAPKs, although there is little knowledge about the upstream proteins in these signalling pathways. MEKKs (MAPK/extracellular-signal-regulated kinase kinase kinases) were identified based on their homology with the yeast MAPKKK (MAPK kinase kinase), STE11 [12]. MEKK1–4 have been identified, and their role in signal transduction pathways is only partially known. With regard to MEKK4, little is known about the agonist-dependent regulation of this kinase, although MEKK4 is predicted to function as a serine/threonine protein kinase [13] upstream of MKK6 (MAPK kinase 6), the regulating kinase for the p38 MAPK [14–16]. Based on the position of MEKK4 in the p38 MAPK pathway, it is reasonable to suggest that IFNγ could signal through MEKK4, but the details of how IFNγ regulates the p38 MAPK are not known (reviewed in [17]).
Pyk2 is a calcium-regulated tyrosine kinase that functions in G-protein-coupled receptor signalling [18–21], as well as in IFNGR signalling [22]. Pyk2 is a signalling protein that links G-protein and cytokine receptor signalling to the activation of MAPK cascades [23,24]. However, Pyk2 substrates are not well defined, and, moreover, the mechanism for calcium-dependent regulation of Pyk2 remains unclear.
The SH2 (Src homology 2)-domain-containing protein tyrosine phosphatase, SHP2, is widely expressed [25–27], and direct interaction of SHP2 with Pyk2 has been reported [28]. In a quiescent state, SHP2 phosphatase activity is regulated by autoinhibition achieved by binding of the N-terminal SH2 domain to its catalytic domain, thereby preventing access of substrate to the active site [29,30]. Upon agonist stimulation, SHP2 can be activated by at least two mechanisms. Tyrosine phosphorylation of the C-terminal tyrosines Tyr542 and Tyr580 results in intramolecular binding of the N-terminal SH2 domain(s) to the C-terminal tyrosylphosphates or the N-terminal SH2 domains bind to another protein which frees the catalytic cleft for substrate access. A few substrates for SHP2 have been investigated, with SHP2 having positive (i.e. signal-enhancing) and negative effects on signal transduction pathways. In order for SHP2 to bind to Pyk2, Tyr906 of Pyk2 is essential, and, surprisingly, the two N-terminal SH2 domains of SHP2 alone are not sufficient for binding to Pyk2 [28]. Moreover, deletion of the two N-terminal SH2 domains, leaving only the phosphatase domain, resulted in an unregulated, more active phosphatase [31,32] which inhibited angiotensin-II-stimulated tyrosine phosphorylation of Pyk2 [28,33].
The present study shows, using HaCaT cells, IFNγ-dependent tyrosine phosphorylation of MEKK4. We provide evidence that Pyk2 tyrosine phosphorylates MEKK4 and that Pyk2 functions in a Ca2+-dependent manner, involving annexin II. We demonstrate that tyrosine phosphorylation of MEKK4 correlates with MEKK4-dependent phosphorylation of MKK6. Furthermore, we provide evidence that SHP2 regulates this signalling pathway by dephosphorylating MEKK4 and its activating kinase, Pyk2.
MATERIALS AND METHODS
Materials
Monoclonal antibodies against Pyk2, annexin II and SHP2 were obtained from BD Transduction Laboratories (San Diego, CA, U.S.A.); antibodies against phosphotyrosine (clone 4G10) were from Upstate Biotechnology (Lake Placid, NY, U.S.A.); monoclonal anti-v-Src antibodies were obtained from Calbiochem (San Diego, CA, U.S.A.); polyclonal anti-(annexin II) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.); monoclonal antibodies against p38 and phospho-specific p38 were obtained from Cell Signaling Technology (Beverly, MA, U.S.A.). Specific anti-MEKK4 antibodies were described by Derbyshire et al. [34]. Alexa Fluor®-conjugated secondary fluorescence antibodies were obtained from Molecular Probes (Eugene, OR, U.S.A.). Protein A–Sepharose, Myc–agarose, anti-FLAG Matrix and Hoechst 33342 were obtained from Sigma (St. Louis, MO, U.S.A.). Haemagglutinin (HA)–agarose and FuGENE 6 were from (Roche, Indianapolis, IN, U.S.A.). Ni-NTA (Ni2+-nitrilotriacetate)–Sepharose was obtained from Invitrogen (Carlsbad, CA, U.S.A.). Recombinant human IFNγ was obtained from R&D systems (Minneapolis, MN, U.S.A.).
Cell culture
Immortalized human keratinocytes, HaCaT cells, were maintained in DMEM (Dulbecco's minimal essential medium) (Gibco, Carlsbad, CA, U.S.A.) supplemented with 10% (v/v) FBS (foetal bovine serum) (Invitrogen) and 50 μg/ml penicillin/streptomycin (Invitrogen) at 37 °C under 5% CO2. HEK-293 (human embryonic kidney) cells were grown in DMEM, with 10% (v/v) FBS, 100 μg/ml gentamicin (Invitrogen) and 250 μg/ml geneticin (Invitrogen). Sf9 insect cells were maintained in Grace's complete insect media (Gibco), supplemented with 10% (v/v) FBS, 50 μg/ml penicillin/streptomycin, 0.25 μg/ml fungizone, 50 μg/ml gentamicin and 0.1% pluronic acid, with gentle shaking at 27 °C.
HEK-293 transient transfections
To optimize transfections, the culture medium was exchanged with Opti-MEM® serum-free medium (Gibco). FuGENE 6 transfection reagent was used according to the manufacturer's instructions at a ratio of 2 μl:1 μg of DNA. For a 10-cm-diameter plate of HEK-293 cells, 3 μg of plasmid DNA was used, while, for co-transfection, a ratio of 1:3 (Pyk2/SHP2) was used. After 5 h, 10% (v/v) FBS and 50% complete DMEM were added. Cells were allowed to recover for 1 day and were deprived of serum for 14 h before treatment and analysis.
Preparation of cell lysates, immunoprecipitation
Cells were deprived of serum (DMEM, 0.1% BSA) for 14 h, were agonist-treated and washed twice with ice-cold PBS. Cells were harvested on ice in MAPK-lysis buffer [70 mM 2-glycerophosphate, 2 mM MgCl2, 1 mM EGTA, 1 mM DTT (dithiothreitol), 0.5% (v/v) Triton X-100, and, freshly added, 1% aprotonin, 5 μg/ml leupeptin and 2 mM Na3VO4]. Proteins were immunoprecipitated from cleared lysates with appropriate antibodies, as indicated, and Protein A–Sepharose under constant rotation at 4 °C for 3 h. Immunoprecipitates were washed twice with 1 ml PAN-NP40 buffer [PAN (10 mM Pipes, pH 7, 100 mM NaCl and 1% aprotonin) and 0.5% (v/v) NP40 (Nonidet P40)] followed with 1 ml of PAN. Immunoprecipitates were resuspended in Laemmli-DTT loading buffer, denatured for 5 min at 100 °C and separated by SDS/8% PAGE.
Bead pull-down
Anti-epitope (Myc, FLAG and HA)-coupled beads were used to purify transiently expressed proteins with the respective tags. HEK-293 cells were lysed with extraction buffer [10 mM Tris/HCl, pH 7.4, 50 mM NaCl, 1% (v/v) Triton X-100, 5 mM EDTA, 1% aprotonin, 50 mM NaF, 2.5 μg/ml leupeptin, 1mM PMSF and 2 mM Na3VO4]. Cleared lysates were incubated with appropriate anti-epitope-coupled beads under constant rotation for 2 h, washed twice with PAN-NP40 and once with PAN.
Immunoblotting
SDS/polyacrylamide gels were transferred on to nitrocellulose by total immersion electrotransfer {10 mM Caps [3-(cyclohexylamino)propane-1-sulphonic acid], pH 11, and 10% methanol}. The membrane was blocked with 2.5% (w/v) non-fat dried milk in TBST (Tris-buffered saline with Tween 20) and incubated with primary antibodies under constant rocking at room temperature (22 °C). The membrane was washed three times with TBST and incubated with HRP (horseradish peroxidase)-conjugated secondary antibodies (Cell Signaling Technology). Subsequently, the membrane was washed again three times with TBST, and subjected to ECL® (enhanced chemiluminescence detection) (Amersham Biosciences, Piscataway, NJ, U.S.A.).
In vitro kinase assay
Immunoprecipitates were washed once with RIPA buffer (150 mM NaCl, 50 mM Tris/HCl, pH 8.0, 5 mM EDTA, 1% NP40, 0.5% sodium deoxycholate, 10 mM NaF, 10 mM disodium pyrophosphate and 0.1% SDS), twice with PAN-NP40 and once with PAN. The precipitates were in 40 μl PAN supplemented with 4 μl 10× UKB (universal kinase buffer; 20 mM Pipes, pH 7, 20 mM MnCl, 20 mM MgCl2 and 0.01% aprotonin), 50 μM ATP or 5 μCi of [γ-32P]ATP was added, and the reaction mixture was incubated at 30 °C for 20 min. Subsequently, the reaction was stopped by adding 2× loading buffer and boiling the sample for 5 min. In the case of the MEKK4 assay, the immunoprecipitates were washed with kinase buffer (25 mM Tris/HCl, pH 7.4, 25 mM MgCl2, 25 mM 2-glycerophosphate, 1 mM EGTA, 2 mM DTT and 500 μM Na3VO4) before the in vitro kinase assay.
Phosphatase assay
Immunoprecipitates or protein-coupled beads were washed with phosphatase buffer (100 mM Hepes, pH 7.4, 150 mM NaCl, 1 mM EDTA and 1 mM 2-mercaptoethanol), and the supernatant was removed, leaving a volume of 40 μl. The phosphatase reaction mixture was incubated for 30 min at 30 °C, and was stopped by adding 2× loading buffer and boiling the sample for 5 min.
Immunofluorescence staining
HaCaT cells were grown on glass coverslips that were pre-treated with HaCaT-conditioned growth medium for a few hours. Cells were deprived of serum and were agonist-treated. Coverslips were washed twice with ice-cold PBS and fixed with ice-cold methanol/acetone (1:1) for 90 s, washed again three times and blocked in 10% goat serum in PBS for 1 h. Incubation with primary antibodies was in 1% goat serum simultaneously for 90 min with gentle rocking in a humidified chamber. Subsequently, coverslips were washed three times for 5–15 min with PBS and incubated with the secondary antibodies, Alexa-Fluor®-488-coupled anti-mouse and Alexa-Fluor®-594-coupled anti rabbit IgG (1:1500), for a further 1 h in the dark with gentle rocking, followed by three washes with PBS. Nuclear staining was performed with Hoechst 33342 (1:10000) at the end of the secondary antibody incubation for 3 min. Coverslips were mounted on glass slides using Cytoseal, low viscosity (Richard-Allan Scientific, Kalamazoo, MI, U.S.A.). The staining was visualized with an Olympus IX70 inverted microscope equipped with 40× (9Uapo/340), 60× (PlanAPO) and 100× (UPlanAPO) oil-immersion objectives and a cooled charge-coupled device camera, controlled by SoftWoRX software (Applied Precision, Issaquah, WA, U.S.A.).
RESULTS
MEKK4 is tyrosine-phosphorylated in an IFNγ-dependent manner
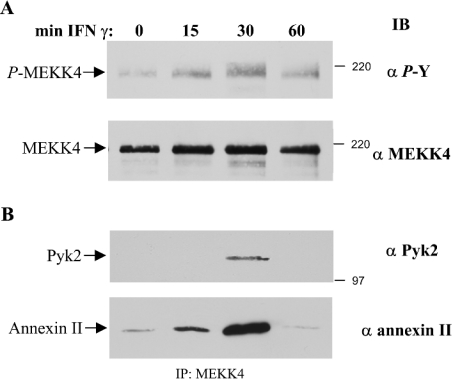
Studies have demonstrated that the levels of the pro-inflammatory mediator IFNγ increase in cancers of the skin and in inflammatory diseases [3]. We show that MEKK4 is expressed in keratinocytes (Figure 1A). We used immortalized HaCaT human keratinocytes to determine whether activation of a cytokine receptor leads to tyrosine phosphorylation of MEKK4, as observed in aortic smooth muscle cells in response to angiotensin II [34]. HaCaT cells were deprived of serum for 14 h and were treated with 200 units/ml IFNγ for 15–60 min. Subsequently, MEKK4 was immunoprecipitated from lysates prepared from untreated or IFNγ-treated cells and tyrosine-phosphorylation of MEKK4 was determined by immunoblotting with anti-phosphotyrosine specific antibodies. Maximal tyrosine phosphorylation of MEKK4 was observed following 30 min of IFNγ treatment, while the level of tyrosine phosphorylation returned to near basal levels after 60 min (Figure 1A, upper panel). This result is consistent with a previous study in which Derbyshire et al. [34] showed that MEKK4 is expressed in aortic smooth muscle cells and is phosphorylated on tyrosine in response to angiotensin II, which acts through a G-protein-coupled receptor. Derbyshire et al. [34] also reported that angiotensin II stimulated the association of the calcium-binding protein, annexin II, with MEKK4 in smooth muscle cells. Therefore we wanted to determine whether activation of a cytokine receptor would also promote the association of annexin II with MEKK4. Indeed, annexin II was observed to co-immunoprecipitate with MEKK4 in an agonist- and time-dependent manner, correlating with the level of tyrosine phosphorylation of MEKK4, with maximal association of annexin II at 30 min (Figure 1B). Furthermore, prompted by results using smooth muscle cells in which we demonstrated a direct binding of the tyrosine kinase, Pyk2, to MEKK4 [34], we investigated whether IFNγ also stimulated the binding of Pyk2 to MEKK4. Again, when probing the same membrane with a monoclonal antibody directed against Pyk2, we observed a co-association of Pyk2 with MEKK4 in a similar manner, with the maximal amount at 30 min after cytokine treatment (Figure 1B). Taken together, these results demonstrate that activation of the IFNGR promotes the association of the calcium-dependent proteins, annexin II and Pyk2, with MEKK4, and that IFNγ affects tyrosine phosphorylation of MEKK4. Thus MEKK4 functions downstream of cytokine receptors, such as the IFNGR, as well as G-protein-coupled receptors, such as the angiotensin II receptor [34].
Figure 1. MEKK4 is tyrosine-phosphorylated in an IFNγ-dependent manner in HaCaT cells.
(A) HaCaT cells deprived of serum were treated with 200 units/ml IFNγ as indicated, and MEKK4 was immunoprecipitated with antibodies directed against amino acids 18–139 of MEKK4 and immunoblotted (IB) for phosphotyrosine (α P-Y; upper panel). The total amount of protein was determined by re-probing with antibodies directed against amino acids 1102–1255 of MEKK4 (α MEKK4; lower panel). (B) Co-immunoprecipitation of Pyk2 and annexin II with MEKK4. The immunoblot was probed with monoclonal antibodies against Pyk2 (α Pyk2; upper panel) and annexin II (α annexin II; lower panel).
Selective calcium requirements for tyrosine phosphorylation of MEKK4
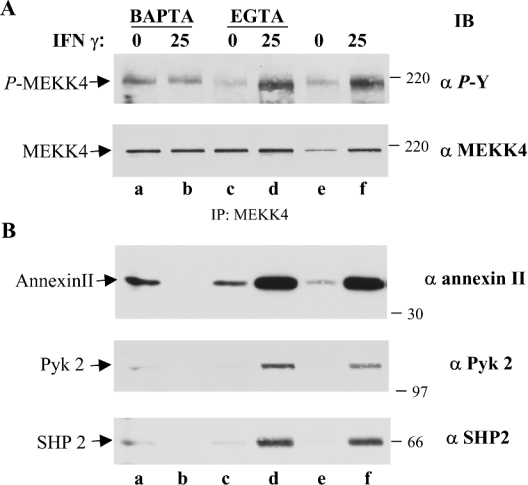
We employed calcium chelators to investigate the potential role of calcium on MEKK4 tyrosine phosphorylation. HaCaT cells were pre-treated with EGTA to reduce the availability of extracellular calcium or with BAPTA/AM [bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid tetrakis(acetoxymethyl ester)] to chelate intracellular calcium. As described above, MEKK4 was immunoprecipitated, and tyrosine phosphorylation was determined by immunoblotting. Chelating extracellular calcium followed by IFNγ treatment had no effect on MEKK4 tyrosine phosphorylation (Figure 2A, compare lanes d and f). In addition, chelating extracellular calcium reduced the basal level of tyrosine phosphorylation of MEKK4 (considering slightly lower amounts of immunoprecipitated MEKK4 in lane e as compared with lane c of Figure 2A). However, depleting intracellular calcium by applying BAPTA/AM resulted in slightly elevated levels of MEKK4 tyrosine phosphorylation over basal levels (Figure 2A, compare lanes a and e), but prevented further stimulation by IFNγ (Figure 2A, lanes a and b). Moreover, chelation of intracellular, but not extracellular calcium, abolished the IFNγ-stimulated association of annexin II and Pyk2 with MEKK4 (Figure 2B, compare lane b with lanes d and f). These results indicate that intracellular calcium is the primary source of calcium utilized to mediate calcium-dependent tyrosine phosphorylation of MEKK4 by IFNγ, most likely by the calcium-dependent tyrosine kinase, Pyk2. Furthermore, the effects of calcium chelation on the co-association pattern of annexin II suggest that this calcium-binding protein mediates the calcium-dependent effect. Although the time course of IFNγ stimulation seemed to suggest that co-association of annexin II coupled with the increase of tyrosine phosphorylation of MEKK4 (see Figure 1), using the BAPTA/AM pre-treatment (Figure 2A, lanes a and b) demonstrates that annexin II co-association is not coupled with tyrosine phosphorylation of MEKK4, as MEKK4 is tyrosine-phosphorylated to a similar extent (Figure 2A, lanes a and b), but the presence of annexin II is greatly diminished following chelation of intracellular calcium and IFNγ stimulation (Figure 2B, lane b). Thus the IFNγ-dependent association of annexin II and Pyk2 with MEKK4 requires intracellular calcium, and not tyrosine phosphorylation of MEKK4.
Figure 2. Calcium-dependent tyrosine phosphorylation of MEKK4.
(A) Effect of calcium chelation by BAPTA/AM and EGTA on MEKK4 tyrosine phosphorylation. HaCaT cells were pre-treated for 15 min with 10 μM BAPTA/AM to chelate intracellular calcium or for 20 min with 3 mM EGTA to deplete extracellular calcium, followed by IFNγ stimulation as indicated. Controls were harvested after chelator pre-treatment. MEKK4 was immunoprecipitated from lysates and immunoblotted (IB) against phosphotyrosine (α P-Y; upper panel). The membrane was stripped and re-probed with polyclonal antibody directed against MEKK4 (α MEKK4; lower panel to show total amounts of protein, as described in the legend to Figure 1(A) (lower panel). (B) Co-immunoprecipitating proteins are affected by depletion of intracellular calcium. Appropriate parts of the same immunoblot were probed with monoclonal antibodies against annexin II (α annexin II; top panel), Pyk2 (α Pyk 2; middle panel) and SHP2 (α SHP2; bottom panel) to visualize the calcium dependency of proteins co-immunoprecipitating with MEKK4. IFNγ stimulation following BAPTA/AM pre-treatment resulted in abolished induction of tyrosine phosphorylation of MEKK4 and eradicated co-immunoprecipitation of annexin II, Pyk2 and SHP2.
IFNγ-dependent co-localization of MEKK4 with Pyk2 and annexin II
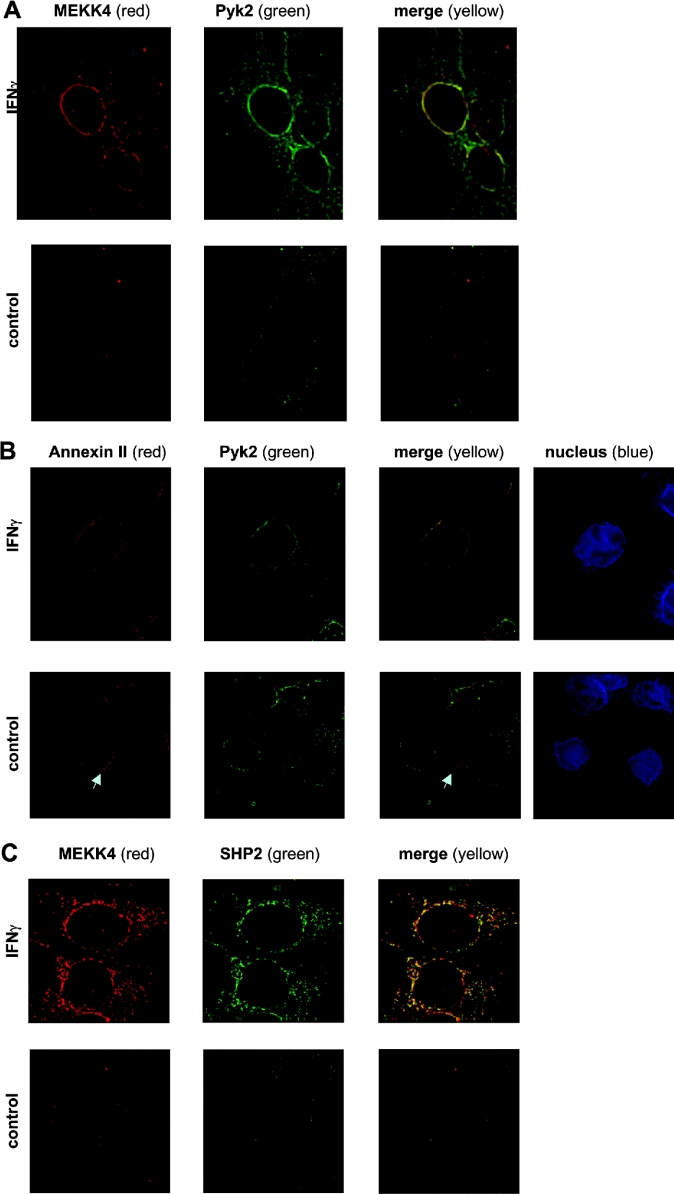
To corroborate the co-association of MEKK4, Pyk2 and annexin II, the cellular distribution of these proteins was determined by immunofluorescence microscopy. HaCaT cells were stained simultaneously with anti-MEKK4 and anti-Pyk2 antibodies, and optical sections were obtained by confocal microscopy. Figure 3(A) shows IFNγ-dependent co-localization of MEKK4 with Pyk2 in the perinuclear region, as indicated by the yellow staining when the individual images were merged together. In contrast, very little co-localization was observed in untreated cells. The calcium-dependency of Pyk2 prompted us to investigate the spatial distribution of Pyk2 and annexin II. Indeed, co-staining with anti-Pyk2 and anti-(annexin II) antibodies displayed an IFNγ-dependent perinuclear co-localization pattern (Figure 3B), similar to that observed with MEKK4 and Pyk2. In addition, Pyk2 and annexin II were distributed independently throughout the cell. Annexin II also appears to accumulate at points of cell–cell contact, suggesting that annexin II may also play a role in intercellular communication. These immunohistological data substantiate the biochemical results of Pyk2 and annexin II co-immunoprecipitating with MEKK4.
Figure 3. IFNγ-induced co-localization of MEKK4 and Pyk2, annexin II and Pyk2, and MEKK4 and SHP2 in the perinuclear region in HaCaT cells.
HaCaT cells, untreated or treated for 25 min with 200 units/ml IFNγ, were simultaneously stained with antibodies against MEKK4 and Pyk2 (A), annexin II (polyclonal) and Pyk2 (B), MEKK4 and SHP2 (C). The secondary antibody against MEKK4 (amino acids 18–139) and annexin II was Alexa-Fluor®-594-coupled anti-rabbit IgG, while Alexa-Fluor®-488-coupled anti-mouse was used against Pyk2 and SHP2 antibodies. Deconvoluted images of selected z-sections of immunofluorescence microscopy are shown, with MEKK4 and annexin II in red (first column) and Pyk2 and SHP2 in green (second column) and co-localization signals in yellow (third column). (B) Hoechst 33342 staining (blue) visualized the nucleus (fourth column). An arrow points to the accumulation of annexin II at points of cell–cell contact. Note that images in (A) are taken in a lower z-section showing more of the distribution in the cell body (closer to the cell-attachment area on the coverslip) than in (B), where most of the cell body was out of focus.
IFNγ activates the tyrosine kinase Pyk2 to phosphorylate MEKK4
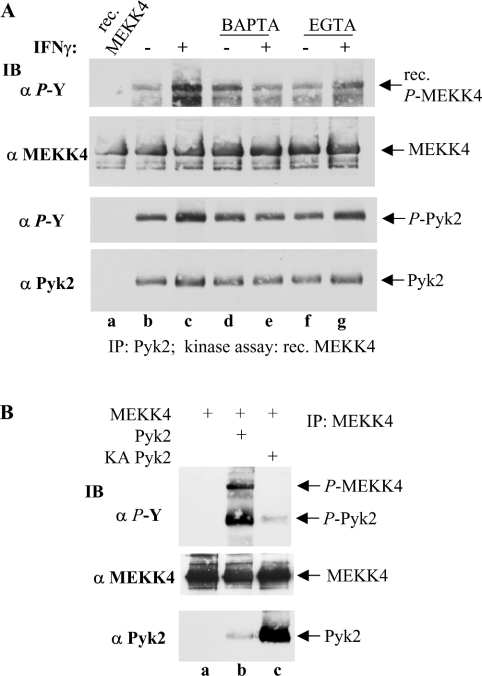
To investigate further the IFNγ-mediated activation of Pyk2 catalytic activity towards MEKK4, Pyk2 was immunoprecipitated and subsequently utilized in an in vitro kinase assay with recombinant, catalytically inactive KM (K1361M) MEKK4 as a substrate. Recombinant KM MEKK4 was expressed and purified from Sf9 insect cells and, importantly, was not phosphorylated on tyrosine in these cells (Figure 4A, lane a). IFNγ treatment of HaCaT cells stimulated Pyk2 to tyrosine phosphorylate MEKK4 in an in vitro kinase assay (Figure 4A, lane c), while no tyrosine phosphorylation was detected with recombinant MEKK4 alone under kinase assay conditions (Figure 4A, lane a). Pyk2 itself had increased levels of tyrosine phosphorylation as compared with the basal level (Figure 4A, third panel, lanes b and c). Furthermore, to substantiate that the calcium-dependent tyrosine phosphorylation of MEKK4 (see Figure 2) resulted from altered Pyk2 activity, we employed calcium chelators. Thus pre-treatment of the cells with BAPTA/AM abolished IFNγ-induced elevated phosphorylation and activation of Pyk2, as demonstrated by basal levels of MEKK4 tyrosine phosphorylation (Figure 4A, compare lanes c and e), while BAPTA/AM treatment alone resulted in a slightly elevated level of tyrosine phosphorylation over basal (Figure 4A, compare lanes b and d). EGTA treatment, however, did not affect basal levels of Pyk2 activity (Figure 4A, lane f), which were, however, only slightly induced upon IFNγ stimulation (Figure 4A, lane g). These in vitro kinase assay data of calcium-dependent Pyk2 activity towards MEKK4 are in accordance with the results from the in vivo tyrosine-phosphorylation pattern of MEKK4 displayed in Figure 2, supporting further the conclusion that Pyk2 phosphorylates MEKK4 in HaCaT cells.
Figure 4. IFNγ activates the tyrosine kinase Pyk2 to phosphorylate MEKK4.
(A) Pyk2 was immunoprecipitated from HaCaT cells that were pre-treated with BAPTA/AM or EGTA, followed by IFNγ treatment for 2 min as indicated, and subjected to in vitro kinase assay conditions with recombinant (rec.) KM MEKK4, produced in Sf9 cells, as a substrate. Subsequently, tyrosine phosphorylation of KM MEKK4 and Pyk2 were determined by immunoblotting (IB) against phosphotyrosine (α P-Y; top panel). For a loading control, the membrane was stripped and reprobed with anti-MEKK4 antibodies (α MEKK4; second panel). Tyrosine phosphorylation of Pyk2 (α P-Y; third panel) and total amount of Pyk2 (α Pyk2; bottom panel) are shown. IFNγ-induced tyrosine phosphorylation of MEKK4 by Pyk2 was abolished by BAPTA/AM pre-treatment. (B) Sf9 cells were infected or co-infected as indicated. MEKK4 was immunoprecipitated (IP), and tyrosine phosphorylation was determined for MEKK4 and Pyk2 with anti-phosphotyrosine antibody (α P-Y; top panel). The membrane was reprobed for MEKK4 and Pyk2 with antibodies against MEKK4 (α MEKK4; middle panel) and Pyk2 (α Pyk2; bottom panel) respectively. Pyk2, but not KA Pyk2, tyrosylphosphorylates MEKK4.
The Sf9 insect cell system provides a powerful tool not only to obtain recombinant proteins for in vitro assays, but also to study the effect of the activity of a protein by selecting and analysing specific members of a multi-protein complex. Therefore this system provides a unique opportunity to study the interaction of specific proteins and their activity without having a background of mammalian proteins that could complicate the interpretation of some experiments, as would occur in mammalian cells.
We therefore used the Sf9 insect cell system to corroborate Pyk2 kinase activity towards MEKK4 in an in vivo system. Sf9 cells were co-infected with baculoviruses that encode MEKK4 and Pyk2. Immunoprecipitation of MEKK4 and subsequent immunoblotting for phosphotyrosine revealed that co-infection of MEKK4 and Pyk2 resulted in tyrosine phosphorylation of MEKK4 (Figure 4B, lane b), while MEKK4 expressed alone was not phosphorylated on tyrosine (Figure 4B, lane a). When Pyk2 was replaced with a kinase-inactive form, MEKK4 tyrosine phosphorylation was abolished (Figure 4B, lane c), demonstrating that Pyk2 provided the kinase activity responsible for MEKK4 tyrosine phosphorylation. Pyk2 expressed in Sf9 cells was activated, as is evident from its high levels of tyrosine phosphorylation and catalytic activity towards MEKK4. This activity may be due to growth factors present in the growth medium, since no agonist was used to stimulate the Sf9 insect cells. This would also explain the low amount of tyrosyl-KA (K457A) Pyk2 (Figure 4B, lane c). On the other hand, overexpressed Pyk2 may activate itself, as reported recently [35]. Interestingly, re-probing the membrane with anti-Pyk2 antibody to assess the total amounts of Pyk2 co-immunoprecipitating with MEKK4 showed that highly increased amounts of kinase-inactive Pyk2 was retained with MEKK4, suggesting that the inactive kinase, Pyk2, was trapped with MEKK4 (Figure 4B, compare lanes b and c). These data, utilizing the Sf9 expression system, provide further evidence that Pyk2 phosphorylates MEKK4 on tyrosine in vivo.
Tyrosine phosphorylation of MEKK4 results in elevated kinase activity towards MKK6
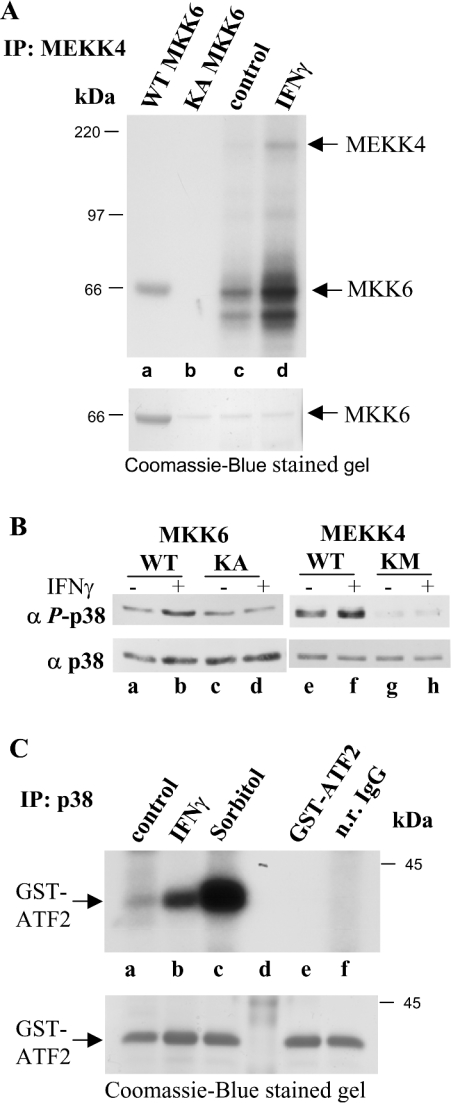
To investigate whether IFNγ-dependent tyrosine phosphorylation of MEKK4 affected its catalytic activity, we tested MEKK4 kinase activity. MKK6 has previously been shown to be a substrate for MEKK4 in an in vitro kinase assay [14–16]. MEKK4 immunoprecipitated from IFNγ-stimulated cells was subjected to an in vitro kinase assay with recombinant kinase-inactive MKK6 as substrate in the presence of [γ-32P]ATP. Indeed, treatment of HaCaT cells with IFNγ activated MEKK4, as measured by increased phosphorylation of MKK6 (Figure 5A, upper panel, lanes c and d). MEKK4 autophosphorylation was elevated as well, indicating that MEKK4 was activated by IFNγ (Figure 5A, lane d). Figure 5A (lane a) shows autophosphorylated wild-type MKK6, while KA (K82A) MKK6 had no residual kinase activity (Figure 5A, lane b). To visualize protein amounts, the same SDS/polyacrylamide gel was stained with Coomassie Blue before subjecting it to autoradiography (Figure 5A, lower panel).
Figure 5. IFNγ regulates MEKK4 activity towards MKK6, the activating kinase for p38 MAPK.
(A) MEKK4 was immunoprecipitated (IP) from HaCaT cells untreated and treated for 25 min with IFNγ, and subjected to in vitro kinase assay conditions in the presence of [γ-32P]ATP with recombinant KA MKK6 as substrate (lanes c and d). Note, in lanes c and d, the band below the indicated MKK6 signal is probably a breakdown product of KA MKK6. As controls, MKK6 and kinase-inactive KA MKK6 were also incubated under kinase assay conditions (lanes a and b). The amount of MKK6 used in each assay was visualized by Coomassie-Blue-staining the same gel before autoradiography. (B) p38 MAPK activation is dependent on MKK6 and MEKK4 activity. HEK-293 cells were transfected with MKK6 and KA MKK6 or with MEKK4 and KM MEKK4, and were stimulated with IFNγ as indicated. Cell lysates were probed for p38 activation with a monoclonal phospho-specific anti-p38 antibody (α P-p38; upper panel). The membrane was reprobed with a monoclonal p38 antibody (α p38; lower panel) to show total p38 protein amounts. (C) p38 is activated by IFNγ. p38 was immunoprecipitated from HaCaT cells, untreated or treated for 15 min with IFNγ and subjected to in vitro kinase assay conditions in the presence of [γ-32P]ATP with a recombinant GST–ATF2 fragment. Incubation with 0.3 M sorbitol for 20 min was used as control, while GST–ATF2 alone and normal rabbit (n.r.) IgG served as negative controls. Shown is an autoradiograph, while the amounts of GST–ATF2 was visualized by Coomassie-Blue-staining the same gel.
MKK6 has been shown to be an activating kinase for the p38 MAPK, and to demonstrate an MKK6-dependent activation of p38 in an IFNγ-dependent manner, HEK-293 cells were transfected with MKK6 or dominant-negative kinase-inactive KA MKK6. Indeed, immunoblotting with a p38 phospho-specific antibody showed that KA MKK6 overexpression abolished IFNγ-dependent p38 activation, while MKK6 expression resulted in elevated p38 phosphorylation upon IFNγ stimulation (Figure 5B, lanes c and d, compared with lanes a and b). To verify that this p38 activation is MEKK4-dependent, MEKK4 and dominant-negative KM MEKK4 were also overexpressed, and the cell lysates were tested for p38 phosphorylation. Again, overexpression of KM MEKK4 abolished p38 activation, while MEKK4 expression stimulated IFNγ-dependent elevated p38 phosphorylation (Figure 5B, lanes g and h, compared with lanes e and f).
To corroborate IFNγ-induced activation of the p38 signalling cascade in HaCaT cells, we tested the effect of IFNγ treatment on the catalytic activity of p38. p38 was immunoprecipitated and incubated with recombinant GST (glutathione S-transferase)–ATF2 (activating transcription factor 2) as substrate in the presence of [γ-32P]ATP under in vitro kinase assay conditions as described previously [13]. As expected, IFNγ stimulated p38 to phosphorylate GST–ATF2 (Figure 5C, lane b), while immunoprecipitation with normal rabbit IgG did not result in any substrate phosphorylation (Figure 5C, lane f). Our results demonstrate that IFNγ-stimulated p38 activation is dependent on active MKK6 and MEKK4. Taken together, we have elucidated an IFNγ-stimulated MAPK pathway that involves Pyk2-dependent tyrosine phosphorylation of MEKK4 in a calcium-dependent manner. Tyrosine phosphorylation of MEKK4 correlates with MKK6 phosphorylation, which in turn activates p38, leading to phosphorylation of ATF2.
SHP2 co-immunoprecipitates and co-localizes with MEKK4
So far, we have shown that tyrosine phosphorylation is an initial mediator of signal transduction in the IFNγ-stimulated p38 MAPK pathway. MAPK pathways need to be regulated tightly, while deregulated signalling components are found in many diseases, such as cancer [36]. Consequently, we were interested in the possible presence of a tyrosine phosphatase and its potential role in down-regulating this signal transduction pathway. Indeed, we identified SHP2 as co-immunoprecipitating with MEKK4 by immunoblotting the same membrane used to produce the data that is presented in Figure 2. We showed an agonist-dependent co-immunoprecipitation of SHP2 (Figure 2B, lanes e and f), which was also affected by the availability of intracellular calcium, as demonstrated by utilizing calcium chelators (Figure 2B, lanes a–d). Interestingly, SHP2 co-immunoprecipitated with MEKK4 in a pattern that correlates with the presence of Pyk2 (Figure 2B). In addition, we corroborated the co-association of SHP2 with MEKK4 by immunofluorescence microscopy in HaCaT cells. We observed an IFNγ-stimulated co-localization of MEKK4 and SHP2 mainly in the perinuclear area (Figure 3C). In contrast, we found little co-localization of SHP2 with MEKK4 in untreated cells.
MEKK4 does not recognize SHP2 as a substrate
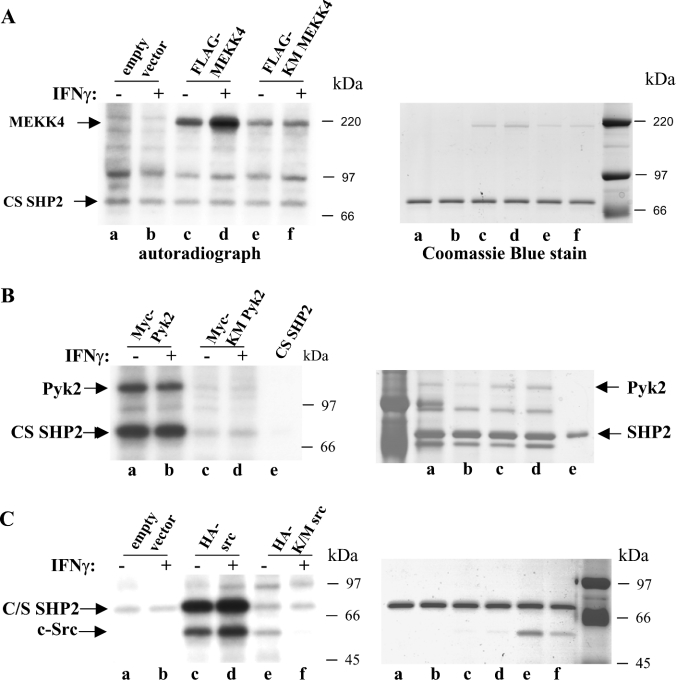
Phosphorylation of two C-terminal tyrosine residues (Tyr542 and Tyr580) has been reported to activate the SHP2 phosphatase by releasing it from its autoinhibitory conformation [30]. In vitro phosphorylation of SHP2 on serine and threonine residues by MAPK and PKC (protein kinase C) respectively have been reported [32]; however, the functional consequence of serine phosphorylation on SHP2 activity is controversial. One study reports that serine phosphorylation has no effect on SHP2 activity [37]. In another report, serine phosphorylation of SHP2 by PKA (protein kinase A) seems to contribute to SHP2 activation [38]. The presence of SHP2 in the MEKK4 multimeric complex prompted us to investigate whether MEKK4 would phosphorylate SHP2. In order to discriminate non-specific activity, kinase-inactive proteins were chosen additionally as controls. To facilitate the use of an in vivo agonist, HEK-293 cells were transiently transfected with the appropriate plasmids encoding the proteins with an epitope tag as indicated in Figure 6. Those proteins were isolated from IFNγ-treated or untreated cells utilizing the corresponding anti-epitope-coupled beads. FLAG-tagged MEKK4 subjected to a kinase assay with recombinant phosphatase-inactive CS (C459S) SHP2 protein did not recognize SHP2 as a substrate (Figure 6A, lanes c and d), as there was only a background signal that was comparable with that of assays with kinase-inactive MEKK4 and the empty vector control (Figure 6A, lanes e and f, and a and b respectively). However, MEKK4 displayed IFNγ-dependent autophosphorylation, demonstrating that MEKK4 was an active kinase in this assay (Figure 6A, lanes c and d). Recombinant SHP2 and MEKK4 were visualized by Coomassie Blue staining of the same gel (Figure 6A, right-hand panel). As a positive control, we used HA-tagged Src which strongly phosphorylated SHP2 [27,39], while KM Src and an empty vector control showed no activity (Figure 6C). In parallel, we used Myc-tagged Pyk2, which also phosphorylated SHP2, and, again, no activity was obtained with the kinase-inactive Pyk2 (Figure 6B). These experiments demonstrate that the serine/threonine kinase, MEKK4, does not phosphorylate recombinant SHP2 in vitro.
Figure 6. MEKK4 does not phosphorylate SHP2.
(A) HEK-293 cells were transfected with plasmids coding for FLAG–MEKK4, or FLAG–KM MEKK4 and vector as controls. Proteins were FLAG–bead affinity-purified from cells untreated or treated for 25 min with IFNγ and subjected to in vitro kinase assays in the presence of [γ-32P]ATP with recombinant SHP2 that was produced in Sf9 cells. Proteins were separated by SDS/PAGE, Coomassie-Blue-stained (right-hand panel) and autoradiographed (left-hand panel). MEKK4 autophosphorylation was IFNγ-dependent, and MEKK4 did not phosphorylate SHP2. (B) Myc–Pyk2 and Myc–KM Pyk2 were used as positive controls, produced in HEK-293 cells untreated or treated for 2 min with IFNγ and subjected to in vitro kinase assay conditions. Pyk2 phosphorylates SHP2 strongly. (C) HA–Src and corresponding HA–KM Src were utilized as additional positive controls for in vitro kinase activity on SHP2.
SHP2 recognizes MEKK4 as a substrate for dephosphorylation
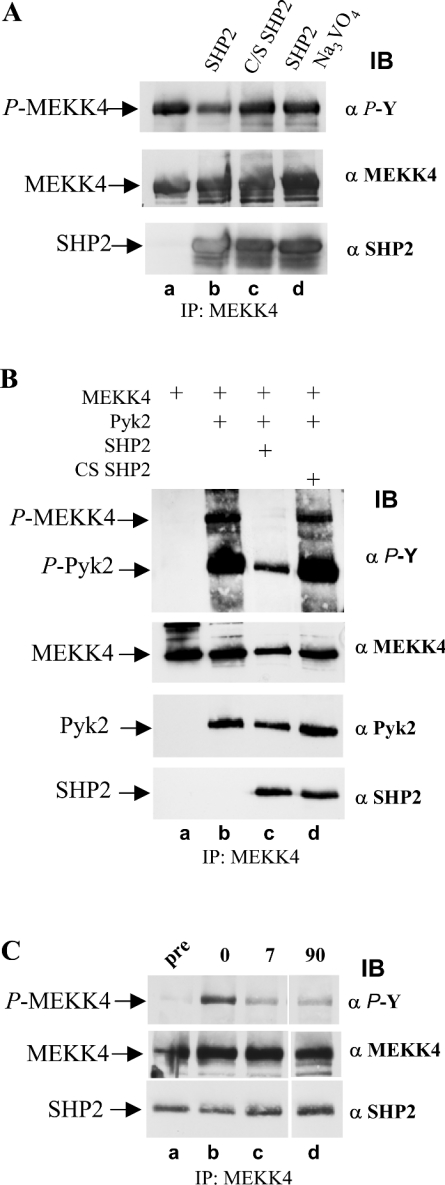
To determine whether tyrosine-phosphorylated MEKK4 is a substrate for SHP2, we utilized the Sf9 cell expression system to obtain tyrosine-phosphorylated MEKK4. Recombinant MEKK4, purified after co-infection with Pyk2 (see Figure 4B, lane b) was subjected to an in vitro phosphatase assay with recombinant SHP2 that had also been separately expressed and purified from Sf9 cells. Incubation with SHP2 resulted in a modest loss of phosphotyrosine on MEKK4 as compared with the untreated sample (Figure 7A, lanes a and b). In addition, an in vitro phosphatase assay allowed us to employ phosphatase-inactive CS SHP2 as a control. Using inactive SHP2 or inhibiting active SHP2 with the phosphatase inhibitor Na3VO4, resulted in levels of phosphotyrosine comparable with the untreated sample (Figure 7A, compare lane a with lanes c and d), demonstrating that the loss of phosphotyrosine seen in lane b of Figure 7(A) is due to SHP2 enzymatic activity and not to non-specific phosphatase activity, indicating that SHP2 dephosphorylates MEKK4.
Figure 7. Co-expression of SHP2 in Sf9 cells results in loss of phosphotyrosine on MEKK4.
(A) In vitro phosphatase assay with SHP2 on tyrosine-phosphorylated MEKK4. MEKK4 and Pyk2 were co-expressed in Sf9 cells and tyrosine-phosphorylated MEKK4 was immunoprecipitated (IP) with antibodies against MEKK4 (α MEKK4). Equal amounts of immunoprecipitated MEKK4 were incubated with purified SHP2, phosphatase-inactive CS SHP2, and SHP2 in the presence of 2 mM Na3VO4 under phosphatase assay conditions (lanes b–d), and, subsequently, MEKK4 tyrosine phosphorylation was determined by immunoblotting (IB) with anti-phosphotyrosine antibody (α P-Y). Active SHP2 leads to loss of phosphotyrosine of MEKK4. (B) Co-expression of SHP2, but not CS SHP2, results in greatly decreased amounts of tyrosylphosphate on MEKK4 and Pyk2. Sf9 cells were co-infected with MEKK4 and Pyk2 without and with SHP2. Substitution with inactive CS SHP2 served as a control. MEKK4 was immunoprecipitated, and tyrosine phosphorylation was determined by immunoblotting with anti-phosphotyrosine antibody (α P-Y; top panel). Amounts of proteins were visualized by re-probing the membrane with antibodies against MEKK4 (α MEKK4; second panel), Pyk2 (α Pyk2; third panel) and SHP2 (α SHP2; bottom panel). (C) SHP2 dephosphorylates MEKK4 directly. Sf9 suspension cell culture co-expressing MEKK4, Pyk2 and SHP2 were supplemented with 2 mM Na3VO4 for 1 h, and subsequently washed out. MEKK4 was immunoprecipitated from aliquots taken before (pre), after treatment (0) and after Na3VO4 washout (7 and 90 min) and were analysed for phosphotyrosine (α P-Y; top panel). Amounts of proteins, MEKK4 and co-immunoprecipitating SHP2 were determined by re-probing the immunoblot with antibodies against MEKK4 (α MEKK4; middle panel) and monoclonal SHP2 (α SHP2; bottom panel). SHP2 dephosphorylates MEKK4 directly as well as its activating kinase Pyk2.
Since the SHP2 activity in vitro was modest, we wanted to corroborate SHP2 activity in an in vivo cell system, hence SHP2 or CS SHP2 were co-infected with MEKK4 and Pyk2 in Sf9 cells. The presence of active SHP2 abolished tyrosine phosphorylation of MEKK4 (Figure 7B, top panel, lane c), and resulted in reduced tyrosine phosphorylation of Pyk2 as compared with the amount of phosphotyrosine observed when MEKK4 and Pyk2 were co-expressed (Figure 7B, lane b). However, when inactive CS SHP2 was substituted for wild-type SHP2, the amount of tyrosine phosphorylation of MEKK4 and Pyk2 was unaffected (Figure 7B, compare lanes b and d), demonstrating that the loss of phosphotyrosine on MEKK4 and Pyk2 was an SHP2-specific effect. This experiment showed that the presence of SHP2 prevented MEKK4 phosphorylation. But, since Pyk2 is the activating kinase for MEKK4, and since Pyk2 is also dephosphorylated by SHP2 and presumably deactivated, we were unable to conclude whether SHP2 indeed dephosphorylated MEKK4 or whether Pyk2 never had a chance to phosphorylate MEKK4 in the first place, because it was dephosphorylated by SHP2.
The following experiment was designed to address this issue. An Sf9 suspension culture was co-infected with baculoviruses that encoded MEKK4, Pyk2 and SHP2. After 36 h, allowing time for protein expression, an aliquot of the cells (2×106) was removed, and MEKK4 was immunoprecipitated and, as expected, showed negligible amounts of tyrosine phosphorylation (Figure 7C, lane a), which was similar to the result seen in Figure 7(B), lane c. Subsequently, the culture was supplemented for 1 h with the tyrosine phosphatase inhibitor, Na3VO4. Then, an aliquot of Sf9 cells was harvested and MEKK4 tyrosine phosphorylation was assessed. Inhibiting SHP2 with Na3VO4 resulted in restored levels of phosphotyrosine of MEKK4 (Figure 7C, lane b), comparable with that seen in experiments where only MEKK4 and Pyk2 were expressed (see Figure 7B, lane b) without affecting SHP2 co-association with MEKK4 (Figure 7C, bottom panel). Subsequently, the Na3VO4 was washed out to restore SHP2 activity, and, after 7 min, an additional aliquot of cells was taken, and the proteins were analysed for phosphotyrosine. MEKK4 showed loss of phosphotyrosine (Figure 7C, compare lanes b and c), suggesting that SHP2 dephosphorylated MEKK4. This clarified whether SHP2 dephosphorylated MEKK4 directly or only Pyk2, its activating kinase, and therefore prevented MEKK4 tyrosine phosphorylation as a consequence of Pyk2 inactivation. Analysis of an aliquot taken after 90 min showed that loss of phosphotyrosine on MEKK4 remained constant (Figure 7C, lane d). Taken together, these data provide strong evidence that SHP2 dephosphorylates MEKK4 directly, as well as its activating kinase Pyk2.
SHP2 dephosphorylates MEKK4 and Pyk2 from HaCaT cells
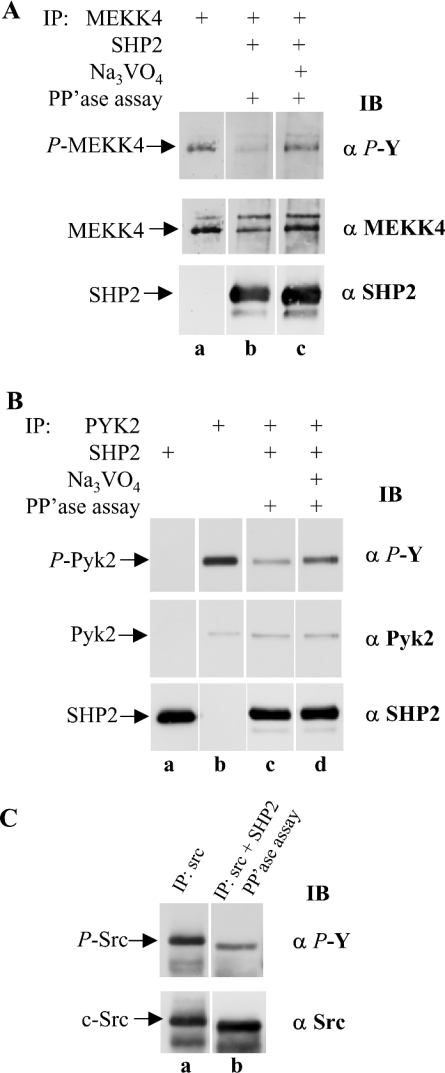
Next, we investigated the role of SHP2 in keratinocytes in the multi-protein complex of MEKK4, Pyk2 and annexin II. MEKK4 and SHP2 were each immunoprecipitated separately from lysates prepared from HaCaT cells stimulated with IFNγ. Then the MEKK4 and SHP2 immunoprecipitates were combined and subjected to in vitro phosphatase assay conditions. The presence of SHP2 resulted in loss of phosphotyrosine from MEKK4 (Figure 8A, lane b), compared with immunoprecipitated MEKK4 that was not incubated with SHP2 (Figure 8A, lane a). When the assay was performed in the presence of Na3VO4, SHP2 phosphatase activity towards MEKK4 was greatly diminished, as visualized by greater amounts of tyrosine phosphorylation of MEKK4 (Figure 8A, lane c), demonstrating that the loss of phosphotyrosine observed in lane b of Figure 8(A) was due to SHP2 enzymatic activity and not due to non-specific phosphatase activity. Furthermore, the second panel, showing total amounts of MEKK4, confirmed that the reduced phosphotyrosine signal in Figure 8(A), lane b, was not from lesser amounts of MEKK4. This experiment demonstrates that SHP2 from HaCaT cells recognizes MEKK4 as a substrate.
Figure 8. SHP2 recognizes MEKK4 from HaCaT cells as substrate.
(A) MEKK4 and SHP2 were immunoprecipitated (IP) separately from HaCaT cells treated for 25 min with IFNγ. To assay SHP2 phosphatase activity, MEKK4 and SHP2 immunoprecipitates were combined and incubated under phosphatase (PP'ase) assay conditions (lanes b and c). SHP2 activity was blocked by adding 2 mM Na3VO4 to the reaction mixture (lane c). SHP2 activity was monitored by phosphotyrosine (α P-Y) immunoblot (IB) analysis. The presence of SHP2 resulted in decreased tyrosylphosphate on MEKK4. For a loading control, the membrane was reprobed against MEKK4 (α MEKK4) and SHP2 (α SHP2). Similarly, (B) immunoprecipitation of Pyk2, and combined immunoprecipitation of Pyk2 and SHP2, with immunoblotting with anti-phosphotyrosine (α P-Y), anti-Pyk2 (α Pyk2) or anti-SHP2 (α SHP2) antibodies; (C) immunoprecipitation of Src, and combined immunoprecipitation of Src and SHP2 from HaCaT cells treated for 2 min with IFNγ and subjected to phosphatase assay conditions as described in (A) and were then immunoblotted for phosphotyrosine (α P-Y) or Src (α Src). Active SHP2 dephosphorylates Pyk2 and c-Src as compared with Na3VO4-inhibited SHP2.
SHP2 activity on Pyk2 has been implicated in different cell types with overexpression and in vitro experiments [28,33,35]. Therefore, to provide a comparable experimental control, we subjected Pyk2 and SHP2, simultaneously immunoprecipitated from HaCaT cell extracts, to phosphatase assay conditions. Similarly, a loss of tyrosine phosphorylation of Pyk2 was observed when incubated with SHP2 (Figure 8B, lane c) as compared with Pyk2 alone (Figure 8B, lane b). Again, to confirm that this result was due to phosphatase activity, including Na3VO4 in the assay resulted in more phosphotyrosine (Figure 8B, lane d). The total amount of Pyk2 and SHP2 were visualized by reprobing the same membrane for total Pyk2 and SHP2 (Figure 8B, lower panels). These data show that SHP2 can dephosphorylate Pyk2 and the Pyk2 substrate, MEKK4, from HaCaT cells.
Src is differentially tyrosine phosphorylated, and phosphorylation by the C-terminal Src kinase, CSK, at Tyr529 results in an autoinhibitory conformation. Tyrosine phosphorylation in the activation loop at Tyr418, however, activates its kinase activity [40]. Since c-Src is known to interact with activated Pyk2 and then to stimulate Pyk2 further by additional tyrosine phosphorylation [23], we tested the effect of SHP2 on c-Src. Again, when both c-Src and SHP2 were immunoprecipitated and subjected to phosphatase assay conditions, a dramatic loss of phosphotyrosine was observed in a phosphotyrosine immunoblot (Figure 8C, compare lanes a and b). Taken together, dephosphorylation of Pyk2 and c-Src affirm the validity of the dephosphorylation of MEKK4 using the same assay conditions, and demonstrate that SHP2 dephosphorylates MEKK4, as well as Pyk2 and c-Src.
DISCUSSION
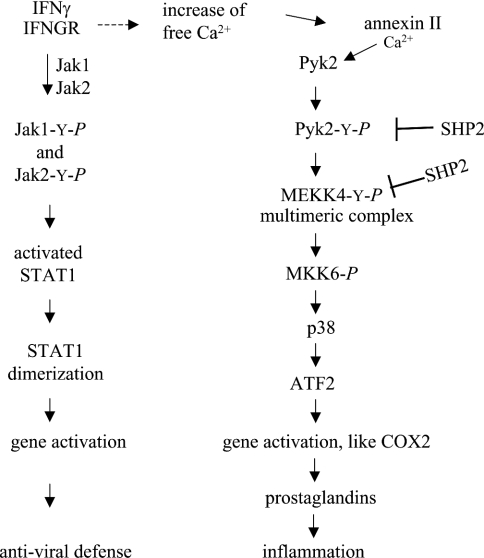
In the present paper, we have reported that the inflammatory mediator IFNγ applied to immortalized HaCaT human keratinocytes induces tyrosine phosphorylation of MEKK4 mediated by Pyk2 in a time- and calcium-dependent manner. Furthermore, the tyrosine phosphatase SHP2, which is present in the MEKK4 multiprotein complex, dephosphorylates MEKK4, as well as its activating kinase, Pyk2, deactivating this IFNγ signalling pathway. We show that IFNγ-induced tyrosine phosphorylation of MEKK4 correlates with its catalytic activity, as it increases its ability to phosphorylate MKK6. Following IFNγ treatment, p38 MAPK becomes activated, which is dependent on active MEKK4, and can then phosphorylate transcription factors, such as ATF2. Derbyshire et al. [34] demonstrated that MEKK4 tyrosine phosphorylation in aortic smooth muscle cells, induced by angiotensin II, results in COX2 (cyclo-oxygenase 2) activation, while IFNγ-stimulated transcriptional activation of COX2 has also been reported in keratinocytes [41]. Those studies and the data of the present study may suggest that IFNγ-induced activation of MEKK4 in keratinocytes also results in COX2 activation and associated responses that involve prostaglandin synthesis (Scheme 1).
Scheme 1. Proposed schema for IFNγ-induced MEKK4 pathway.
IFNγ activation of the IFNGR in keratinocytes results in activation of STAT1 via JAK2 phosphorylation and leads to antiviral defence response (left-hand side). IFNγ activation also leads to a flux of free calcium. Annexin II binding calcium contributes to Pyk2's calcium-dependent activation. MEKK4 is tyrosine-phosphorylated by Pyk2 and activates MKK6, which activates p38 MAPK and results in activation of transcription factors for prostaglandin responses (right-hand side).
Immunofluorescence microscopy of HaCaT cells revealed colocalization of MEKK4, Pyk2 and annexin II in the perinuclear region which was IFNγ-dependent, confirming the biochemical as- sociation of these proteins. MEKK4 localized to perinuclear vesicular structures has been reported in breast carcinoma cells and HEK-293 cells [13]; moreover, perinuclear localization has also been shown for Pyk2 in the presence of fibronectin in fibroblasts [42]. Subcellular localization in a spatiotemporal manner is correlated with a functional association of participating proteins. Interestingly, IFNγ stimulation resulted in the association of annexin II with the MEKK4 multimeric complex (Figures 1 and 2), which also contained the small subunit, p11 (results not shown). The perinuclear region contains the nuclear membrane and endoplasmic reticulum. Calcium-dependent membrane-binding of the annexin II–p11 heterotetramer has been shown to require a lower micromolar calcium concentration, such as that present at cellular localizations, facilitating its membrane association under physiological conditions [43]. Taken together, the intracellular calcium-dependent association of the MEKK4 complex and the calcium-dependent membrane-binding properties of the annexin II complex may provide membrane anchoring of the MEKK4 multimeric complex through annexin II that is initiated by an IFNγ-dependent localized increase of free calcium.
The perinuclear localization of the proteins may suggest that activation of the MEKK4 multiprotein complex leads to the activation of a transcription factor which then translocates into the nucleus. We have shown that the activated MEKK4 signalling complex can lead to p38 MAPK activation and phosphorylation of ATF2. Although STAT1 is directly activated at the IFNGR upon IFNγ stimulation, STAT1-independent, and IFNγ-dependent, gene activation has also been reported [44]. Thus placing MEKK4 in the IFNγ pathway may account for STAT1-independent gene transcription and may provide a molecular mechanism by which IFNγ activates both anti-viral defences (STAT1-dependent) and MEKK4-dependent inflammatory responses in the same cell (Scheme 1).
Genetic and mutational studies have shown the regulatory involvement of SHP2 in many pathways, but its specific targets are still mostly under investigation [30]. We have presented strong evidence that SHP2 regulates the MEKK4 signalling pathway, as is demonstrated by its co-association with the MEKK4 complex, its co-localization with MEKK4 in the perinuclear region and its phosphatase activity towards MEKK4 and Pyk2. Our data showing direct dephosphorylation of MEKK4 and Pyk2 by SHP2 represent a negative-regulatory function of SHP2 in the IFNγ pathway, terminating this signalling pathway. In contrast, cytokine-dependent activation has been claimed to require SHP2 as a positive, signal-enhancing, factor [45]. To this end, Zhang et al. [46] have shown that SHP2 regulates Src family kinases by controlling their inhibitory tyrosine phosphorylation by dephosphorylating the upstream docking protein for the inhibitory c-Src kinase CSK, preventing recruitment of CSK, and therefore ensuring Src activation. The Pyk2–MEKK4 multi-protein complex also contains Src (results not shown), which is most likely to be recruited to activated Pyk2 with its SH2 domain binding to pTyr402 of Pyk2 subsequently contributing to further phosphorylation of Pyk2 [23]. This initial effect of SHP2, securing activated Src, results via activated Pyk2 in IFNγ-dependent activation of MEKK4 and is a positive, signal-enhancing, event. However, subsequently, SHP2 has a negative, deactivating, effect on this pathway by dephosphorylating Pyk2 and MEKK4, thus ending the signal transduction pathway. In summary, SHP2 serves a dual role. First, SHP2 activity leads to activation of Src [46] and increased Pyk2 activity, and, secondly, SHP2 dephosphorylates and inactivates MEKK4 and Pyk2. The complex role of SHP2 may explain why disruption of the SHP2 gene is lethal in mice [47]. We showed in Figure 8(C) SHP2 activity on immunoprecipitated Src in an in vitro phosphatase assay. Zhang et al. [46] provide evidence that SHP2-dependent activation of Src is achieved not by directly dephosphorylating the inhibitory tyrosylphosphate Tyr529 of human c-Src. Moreover, the phosphatase RPTPα (receptor protein tyrosine phosphatase α) is suggested to be active on Tyr529 of Src [48,49]. Thus we probably observed SHP2 dephosphorylating Tyr418 in the activation loop.
IFNγ has been shown to elicit a Ca2+ flux in certain cell types [5–11], suggesting that this increase of free calcium is involved in IFNγ signalling. CaMK (Ca2+/calmodulin-dependent kinase) II has been implicated in the mediation of the calcium effect in one study [10]; however, the mechanism by which Ca2+ acts as a second messenger in IFNγ signalling is mostly unclear. Furthermore, Pyk2, which has been reported to be activated by IFNγ [22], is a calcium-dependent tyrosine kinase, but Pyk2 itself has no calcium binding motifs, so the mechanism of its calcium regulation is largely unknown [21]. In the present study, we have shown the calcium-binding protein, annexin II, co-immunoprecipitates with MEKK4 (Figures 1 and 2) and co-localizes with Pyk2 (Figure 3B). This co-association is stimulated by IFNγ and is dependent on calcium. Annexin II probably provides the calcium regulation of Pyk2 which was monitored by calcium-dependent tyrosine phosphorylation of MEKK4 (Figure 4).
We have provided evidence that IFNγ-stimulated tyrosine phosphorylation of MEKK4 is dependent on intracellular calcium (BAPTA/AM-affected; Figures 2 and 4). Conversely, employing angiotensin II to stimulate smooth muscle cells, Derbyshire et al. [34] found MEKK4 tyrosine phosphorylation is strongly decreased by chelating extracellular calcium with EGTA. Interestingly, activating the purinergic receptor (P2Y) in keratinocytes with ATP also requires extracellular calcium to activate tyrosylphosphorylation of MEKK4 (U. M. Halfter and R. R. Vaillancourt, unpublished work). These data suggest that the G-protein-coupled receptors, AT1A (angiotensin II receptor 1A) and P2Y, both stimulate the increase of free calcium from extracellular sources, while the IFNGR pathway requires intracellular calcium. Furthermore, basal levels of MEKK4 tyrosine phosphorylation in keratinocytes may be stimulated by extracellular calcium, as EGTA application reduces that level (Figure 2A).
Although BAPTA/AM inhibits further stimulation of tyrosylphosphorylation on MEKK4 by IFNγ, the basal level is higher than that in untreated cells (Figure 2A, compare lanes a and b with e). This could indicate that a particular tyrosine, which is different from that stimulated by IFNγ in an intracellular-calcium-dependent manner, is usually inhibited by intracellular calcium, but in the absence of intracellular calcium becomes phosphorylated.
Our data showing treatment of keratinocytes with IFNγ and/or calcium chelators, or inhibitors, resulted in a similar pattern of Pyk2 and SHP2 co-immunoprecipitation with MEKK4 (Figure 2, and results not shown). Direct binding of SHP2 to Pyk2 has been shown by far-Western blot analysis [28]. A few substrates for SHP2 have been investigated. Pyk2 dephosphorylation by SHP2 has been documented in in vitro assays and also in overexpression experiments [28,33,35]. We now demonstrate that MEKK4 is a substrate for SHP2. Although this result is observed easily in Sf9 insect cells, where we can manipulate expression of the various mammalian proteins, we have also observed this result with MEKK4 from HaCaT keratinocytes. Our results demonstrate that the use of the Sf9 insect cell expression system is a powerful tool to dissect complex interactions and reactions that occur in mammalian cells.
IFNγ signalling results in direct tyrosine phosphorylation of STAT1 by the receptor-associated tyrosine kinase JAK. However, a MAPK pathway also seems to play a major role in IFNγ signalling. We have demonstrated the activation of the MEKK4 signalling complex by IFNγ. Pyk2 is activated by G-protein-coupled receptor or receptor tyrosine kinase pathways in different cell types [23,34]. In fibroblasts, Takaoka et al. [22] have shown that Pyk2 tyrosine kinase activity is increased upon IFNγ stimulation. IFNGR-associated STAT1 becomes tyrosine-phosphorylated, dimerizes and translocates to the nucleus. Taken together, IFNγ has the potential to activate parallel pathways simultaneously: (i) JAK activating STAT1 and (ii) MEKK4 leading to ATF2 activation (Scheme 1).
The skin is the outermost organ in contact with the environment, being exposed to biotic and abiotic agents, thus it has protective functions. In this context, IFNγ stimulation also leads to activation of antiviral defences at the molecular level. IFNγ expressed in keratinocytes is particularly important for response to antiviral diseases in the skin [50]. Activation of STAT1 is critical for IFNγ-stimulated antiviral immune defence [51,52]. Thus, as summarized in Scheme 1, IFNγ activating its cognate receptor could result in STAT1-dependent antiviral defence. However, IFNγ activating Pyk2 in a calcium-dependent manner results in p38-dependent activation of COX2, consequently leading to prostaglandin-mediated responses. We believe that these signalling pathways can account for the pleiotropic effects of IFNγ.
Acknowledgments
We are grateful to Dr Carol Gregorio and Michael Urquhart (Department of Cell Biology and Anatomy) for providing training and time on the deconvolution confocal microscope. We thank Dr Woodgett (Ontario Cancer Institute, Toronto, Canada) for the gift of the wild-type and dominant-negative MKK6 cDNA, Dr Earp (Lineberger Cancer Research Center, Chapel Hill, NC, U.S.A.) for the wild-type and KA Pyk2 cDNAs, and Dr Lefkowitz (Duke Medical Center, Durham, NC, U.S.A.) for wild-type and KM c-Src cDNAs. We thank Suzanne Regan for technical assistance. This work was supported, in part, by grants from the National Institutes of Health (P42 ES04940, ES12007 and AG19710) and the Southwest Environmental Health Sciences Center (P30 ES06694). Z.E.D. was supported by a predoctoral fellowship from the American Heart Association.
References
- 1.Bach E. A., Aguet M., Schreiber R. D. The IFNγ receptor: a paradigm for cytokine receptor signaling. Annu. Rev. Immunol. 1997;15:563–591. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- 2.Stark G. R., Kerr I. M., Williams B. R., Silverman R. H., Schreiber R. D. How cells respond to interferons. Annu. Rev. Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 3.Ikeda H., Old L. J., Schreiber R. D. The roles of IFNγ in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 4.Boehm U., Klamp T., Groot M., Howard J. C. Cellular responses to interferon-γ. Annu. Rev. Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 5.Aas V., Larsen K., Iversen J. G. Interferon-γ elicits a G-protein-dependent Ca2+ signal in human neutrophils after depletion of intracellular Ca2+ stores. Cell. Signalling. 1999;11:101–110. doi: 10.1016/s0898-6568(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 6.Koide Y., Ina Y., Nezu N., Yoshida T. O. Calcium influx and the Ca2+–calmodulin complex are involved in interferon-γ-induced expression of HLA class II molecules on HL-60 cells. Proc. Natl. Acad. Sci. U.S.A. 1988;85:3120–3124. doi: 10.1073/pnas.85.9.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kung A. W., Lau K. S., Wong N. S. Interferon-γ increases intracellular calcium and inositol phosphates in primary human thyroid cell culture. Endocrinology. 1995;136:5028–5033. doi: 10.1210/endo.136.11.7588238. [DOI] [PubMed] [Google Scholar]
- 8.Buntinx M., Ameloot M., Steels P., Janssen P., Medaer R., Geusens P., Raus J., Stinissen P. Interferon-γ-induced calcium influx in T lymphocytes of multiple sclerosis and rheumatoid arthritis patients: a complementary mechanism for T cell activation? J. Neuroimmunol. 2002;124:70–82. doi: 10.1016/s0165-5728(01)00495-7. [DOI] [PubMed] [Google Scholar]
- 9.Franciosi S., Choi H. B., Kim S. U., McLarnon J. G. Interferon-γ acutely induces calcium influx in human microglia. J. Neurosci. Res. 2002;69:607–613. doi: 10.1002/jnr.10331. [DOI] [PubMed] [Google Scholar]
- 10.Nair J. S., DaFonseca C. J., Tjernberg A., Sun W., Darnell J. E., Jr, Chait B. T., Zhang J. J. Requirement of Ca2+ and CaMKII for Stat1 Ser-727 phosphorylation in response to IFN-γ. Proc. Natl. Acad. Sci. U.S.A. 2002;99:5971–5976. doi: 10.1073/pnas.052159099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beppu K., Morisaki T., Matsunaga H., Uchiyama A., Ihara E., Hirano K., Kanaide H., Tanaka M., Katano M. Inhibition of interferon-γ-activated nuclear factor-κB by cyclosporin A: a possible mechanism for synergistic induction of apoptosis by interferon-γ and cyclosporin A in gastric carcinoma cells. Biochem. Biophys. Res. Commun. 2003;305:797–805. doi: 10.1016/s0006-291x(03)00853-2. [DOI] [PubMed] [Google Scholar]
- 12.Lange-Carter C. A., Pleiman C. M., Gardner A. M., Blumer K. J., Johnson G. L. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science. 1993;260:315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- 13.Gerwins P., Blank J. L., Johnson G. L. Cloning of a novel mitogen-activated protein kinase kinase kinase, MEKK4, that selectively regulates the c-Jun amino terminal kinase pathway. J. Biol. Chem. 1997;272:8288–8295. doi: 10.1074/jbc.272.13.8288. [DOI] [PubMed] [Google Scholar]
- 14.Mita H., Tsutsui J., Takekawa M., Witten E. A., Saito H. Regulation of MTK1/MEKK4 kinase activity by its N-terminal autoinhibitory domain and GADD45 binding. Mol. Cell. Biol. 2002;22:4544–4555. doi: 10.1128/MCB.22.13.4544-4555.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takekawa M., Posas F., Saito H. A human homolog of the yeast Ssk2/Ssk22 MAP kinase kinase kinases, MTK1, mediates stress-induced activation of the p38 and JNK pathways. EMBO J. 1997;16:4973–4982. doi: 10.1093/emboj/16.16.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takekawa M., Saito H. A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell. 1998;95:521–530. doi: 10.1016/s0092-8674(00)81619-0. [DOI] [PubMed] [Google Scholar]
- 17.Platanias L. C., Fish E. N. Signaling pathways activated by interferons. Exp. Hematol. 1999;27:1583–1592. doi: 10.1016/s0301-472x(99)00109-5. [DOI] [PubMed] [Google Scholar]
- 18.Avraham S., London R., Fu Y., Ota S., Hiregowdara D., Li J., Jiang S., Pasztor L. M., White R. A., Groopman J. E., et al. Identification and characterization of a novel related adhesion focal tyrosine kinase (RAFTK) from megakaryocytes and brain. J. Biol. Chem. 1995;270:27742–27751. doi: 10.1074/jbc.270.46.27742. [DOI] [PubMed] [Google Scholar]
- 19.Lev S., Moreno H., Martinez R., Canoll P., Peles E., Musacchio J. M., Plowman G. D., Rudy B., Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature (London) 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki H., Nagura K., Ishino M., Tobioka H., Kotani K., Sasaki T. Cloning and characterization of cell adhesion kinase β, a novel protein-tyrosine kinase of the focal adhesion kinase subfamily. J. Biol. Chem. 1995;270:21206–21219. doi: 10.1074/jbc.270.36.21206. [DOI] [PubMed] [Google Scholar]
- 21.Yu H., Li X., Marchetto G. S., Dy R., Hunter D., Calvo B., Dawson T. L., Wilm M., Anderegg R. J., Graves L. M., Earp H. S. Activation of a novel calcium-dependent protein-tyrosine kinase: correlation with c-Jun N-terminal kinase but not mitogen-activated protein kinase activation. J. Biol. Chem. 1996;271:29993–29998. doi: 10.1074/jbc.271.47.29993. [DOI] [PubMed] [Google Scholar]
- 22.Takaoka A., Tanaka N., Mitani Y., Miyazaki T., Fujii H., Sato M., Kovarik P., Decker T., Schlessinger J., Taniguchi T. Protein tyrosine kinase Pyk2 mediates the Jak-dependent activation of MAPK and Stat1 in IFN-γ, but not IFN-α, signaling. EMBO J. 1999;18:2480–2488. doi: 10.1093/emboj/18.9.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dikic I., Tokiwa G., Lev S., Courtneidge S. A., Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature (London) 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 24.Tokiwa G., Dikic I., Lev S., Schlessinger J. Activation of Pyk2 by stress signals and coupling with JNK signaling pathway. Science. 1996;273:792–794. doi: 10.1126/science.273.5276.792. [DOI] [PubMed] [Google Scholar]
- 25.Vogel W., Lammers R., Huang J., Ullrich A. Activation of a phosphotyrosine phosphatase by tyrosine phosphorylation. Science. 1993;259:1611–1614. doi: 10.1126/science.7681217. [DOI] [PubMed] [Google Scholar]
- 26.Freeman R. M., Jr, Plutzky J., Neel B. G. Identification of a human src homology 2-containing protein-tyrosine-phosphatase: a putative homolog of Drosophila corkscrew. Proc. Natl. Acad. Sci. U.S.A. 1992;89:11239–11243. doi: 10.1073/pnas.89.23.11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng G. S., Hui C. C., Pawson T. SH2-containing phosphotyrosine phosphatase as a target of protein-tyrosine kinases. Science. 1993;259:1607–1611. doi: 10.1126/science.8096088. [DOI] [PubMed] [Google Scholar]
- 28.Chauhan D., Pandey P., Hideshima T., Treon S., Raje N., Davies F. E., Shima Y., Tai Y. T., Rosen S., Avraham S., et al. SHP2 mediates the protective effect of interleukin-6 against dexamethasone-induced apoptosis in multiple myeloma cells. J. Biol. Chem. 2000;275:27845–27850. doi: 10.1074/jbc.M003428200. [DOI] [PubMed] [Google Scholar]
- 29.Hof P., Pluskey S., Dhe-Paganon S., Eck M. J., Shoelson S. E. Crystal structure of the tyrosine phosphatase SHP-2. Cell. 1998;92:441–450. doi: 10.1016/s0092-8674(00)80938-1. [DOI] [PubMed] [Google Scholar]
- 30.Neel B. G., Gu H., Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 31.Zhao R., Zhao Z. J. Tyrosine phosphatase SHP-2 dephosphorylates the platelet-derived growth factor receptor but enhances its downstream signalling. Biochem. J. 1999;338:35–39. [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Z., Larocque R., Ho W., Fischer E., Shen S. Purification and characterization of PTP2C, a widely distributed protein tyrosine phosphatase containing two SH2 domains. J. Biol. Chem. 1994;269:8780–8785. [PubMed] [Google Scholar]
- 33.Tang H., Zhao Z. J., Landon E. J., Inagami T. Regulation of calcium-sensitive tyrosine kinase Pyk2 by angiotensin II in endothelial cells: roles of Yes tyrosine kinase and tyrosine phosphatase SHP-2. J. Biol. Chem. 2000;275:8389–8396. doi: 10.1074/jbc.275.12.8389. [DOI] [PubMed] [Google Scholar]
- 34.Derbyshire Z. E., Halfter U. M., Heimark R. L., Sy T., Vaillancourt R. R. Angiotensin II stimulated transcription of cyclooxygenase II is regulated by a novel kinase cascade involving Pyk2, MEKK4 and annexin II. Mol. Cell. Biochem. 2005 doi: 10.1007/s11010-005-5386-9. in the press. [DOI] [PubMed] [Google Scholar]
- 35.Meyer A. N., Gastwirt R. F., Schlaepfer D. D., Donoghue D. J. The cytoplasmic tyrosine kinase pyk2 as a novel effector of fibroblast growth factor receptor 3 activation. J. Biol. Chem. 2004;279:28450–28457. doi: 10.1074/jbc.M403335200. [DOI] [PubMed] [Google Scholar]
- 36.Martin G. S. Cell signaling and cancer. Cancer Cell. 2003;4:167–174. doi: 10.1016/s1535-6108(03)00216-2. [DOI] [PubMed] [Google Scholar]
- 37.Strack V., Krutzfeldt J., Kellerer M., Ullrich A., Lammers R., Haring H. U. The protein-tyrosine-phosphatase SHP2 is phosphorylated on serine residues 576 and 591 by protein kinase C isoforms α, β1, β2, and η. Biochemistry. 2002;41:603–608. doi: 10.1021/bi011327v. [DOI] [PubMed] [Google Scholar]
- 38.Rocchi S., Gaillard I., van Obberghen E., Chambaz E. M., Vilgrain I. Adrenocorticotrophic hormone stimulates phosphotyrosine phosphatase SHP2 in bovine adrenocortical cells: phosphorylation and activation by cAMP-dependent protein kinase. Biochem. J. 2000;352:483–490. [PMC free article] [PubMed] [Google Scholar]
- 39.Peng Z. Y., Cartwright C. A. Regulation of the Src tyrosine kinase and Syp tyrosine phosphatase by their cellular association. Oncogene. 1995;11:1955–1962. [PubMed] [Google Scholar]
- 40.Liu X., Brodeur S. R., Gish G., Songyang Z., Cantley L. C., Laudano A. P., Pawson T. Regulation of c-Src tyrosine kinase activity by the Src SH2 domain. Oncogene. 1993;8:1119–1126. [PubMed] [Google Scholar]
- 41.Matsuura H., Sakaue M., Subbaramaiah K., Kamitani H., Eling T. E., Dannenberg A. J., Tanabe T., Inoue H., Arata J., Jetten A. M. Regulation of cyclooxygenase-2 by interferon γ and transforming growth factor α in normal human epidermal keratinocytes and squamous carcinoma cells: role of mitogen-activated protein kinases. J. Biol. Chem. 1999;274:29138–29148. doi: 10.1074/jbc.274.41.29138. [DOI] [PubMed] [Google Scholar]
- 42.Sieg D. J., Ilic D., Jones K. C., Damsky C. H., Hunter T., Schlaepfer D. D. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK− cell migration. EMBO J. 1998;17:5933–5947. doi: 10.1093/emboj/17.20.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerke V., Moss S. E. Annexins: from structure to function. Physiol. Rev. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- 44.Ramana C. V., Gil M. P., Schreiber R. D., Stark G. R. Stat1-dependent and -independent pathways in IFN-γ-dependent signaling. Trends Immunol. 2002;23:96–101. doi: 10.1016/s1471-4906(01)02118-4. [DOI] [PubMed] [Google Scholar]
- 45.Neel B. G., Tonks N. K. Protein tyrosine phosphatases in signal transduction. Curr. Opin. Cell Biol. 1997;9:193–204. doi: 10.1016/s0955-0674(97)80063-4. [DOI] [PubMed] [Google Scholar]
- 46.Zhang S. Q., Yang W., Kontaridis M. I., Bivona T. G., Wen G., Araki T., Luo J., Thompson J. A., Schraven B. L., Philips M. R., Neel B. G. Shp2 regulates SRC family kinase activity and Ras/Erk activation by controlling Csk recruitment. Mol. Cell. 2004;13:341–355. doi: 10.1016/s1097-2765(04)00050-4. [DOI] [PubMed] [Google Scholar]
- 47.Qu C.-K., Yu W.-M., Azzarelli B., Feng G.-S. Genetic evidence that Shp-2 tyrosine phosphatase is a signal enhancer of the epidermal growth factor receptor in mammals. Proc. Natl. Acad. Sci. U.S.A. 1999;96:8528–8533. doi: 10.1073/pnas.96.15.8528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su J., Muranjan M., Sap J. Receptor protein tyrosine phosphatase α activates Src-family kinases and controls integrin-mediated responses in fibroblasts. Curr. Biol. 1999;9:505–511. doi: 10.1016/s0960-9822(99)80234-6. [DOI] [PubMed] [Google Scholar]
- 49.Ponniah S., Wang D. Z., Lim K. L., Pallen C. J. Targeted disruption of the tyrosine phosphatase PTPα leads to constitutive downregulation of the kinases Src and Fyn. Curr. Biol. 1999;9:535–538. doi: 10.1016/s0960-9822(99)80238-3. [DOI] [PubMed] [Google Scholar]
- 50.Banno T., Adachi M., Mukkamala L., Blumenberg M. Unique keratinocyte-specific effects of interferon-γ that protect skin from viruses, identified using transcriptional profiling. Antivir. Ther. 2003;8:541–554. [PubMed] [Google Scholar]
- 51.Meraz M. A., White J. M., Sheehan K. C., Bach E. A., Rodig S. J., Dighe A. S., Kaplan D. H., Riley J. K., Greenlund A. C., Campbell D., et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 52.Durbin J. E., Hackenmiller R., Simon M. C., Levy D. E. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]