Abstract
We have identified a GDAY motif in the C-terminal domain of guanylyl cyclase (guanylate cyclase)/NPRA (natriuretic peptide receptor A) sequence, which serves a dual role as an internalization signal and a recycling signal. To delineate the role of the GDAY motif in receptor internalization and sequestration, we mutated Gly920, Asp921 and Tyr923 to alanine residues (GDAY/AAAA) in the NPRA cDNA sequence. The cDNAs encoding wild-type and mutant receptors were transfected in HEK-293 cells (human embryonic kidney 293 cells). The internalization studies of ligand–receptor complexes revealed that endocytosis of 125I-ANP by HEK-293 cells expressing G920A, Y923A or GDAY/AAAA mutant receptor was decreased by almost 50% (P<0.001) when compared with cells expressing the wild-type receptor. However, the effect of D921A mutation on receptor internalization was minimal. Ligand-mediated down-regulation of G920A, Y923A and GDAY/AAAA mutant receptors was decreased by 35–40% when compared with wild-type NPRA. Subsequently, the recycling of internalized D921A and GDAY/AAAA mutant receptors from the intracellular pool was decreased by more than 40±4% when compared with wild-type NPRA. Recycling of G920A and Y923A mutant receptors was also decreased, but to a significantly lesser extent compared with the D921A or GDAY/AAAA mutant receptors. We conclude that the Gly920 and Tyr923 residues within the GDAY consensus motif are necessary for internalization, and that residue Asp921 is important for recycling of NPRA. The current results provide new evidence for a dual role of the GDAY sequence motif in ligand-mediated internalization, recycling and down-regulation of a single-transmembrane receptor protein NPRA.
Keywords: cytoplasmic motif, guanylyl cylcase (guanylate cyclase)/natriuretic peptide receptor A, human embryonic kidney 293 cells (HEK-293 cells), ligand binding, receptor internalization and trafficking, site-directed mutagenesis
Abbreviations: ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; CNP, C-type natriuretic peptide; DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum; HBSS, Hanks balanced salt solution; HEK-293 cells, human embryonic kidney 293 cells; LDL, low-density lipoprotein; NPRA, NPRB and NPRC, natriuretic peptide receptors A, B and C
INTRODUCTION
ANP (atrial natriuretic peptide) is a member of the natriuretic peptide hormone family, which comprises ANP, BNP (brain natriuretic peptide) and CNP (C-type natriuretic peptide), each derived from a separate gene [1]. Natriuretic peptides elicit a number of vascular, renal and endocrine effects, resulting in the maintenance of blood pressure and extracellular fluid volume [2–5]. ANP and BNP are primarily synthesized in the granules of heart, circulate in the plasma and display maximum variability in the primary structure, whereas CNP is largely present in the endothelial cells and is highly conserved among the species. Three subtypes of natriuretic peptide receptors have been identified, namely NPRA, NPRB and NPRC (natriuretic peptide receptors A, B and C respectively). NPRA and NPRB contain an extracellular ligand-binding domain, a single transmembrane region and intracellular protein kinase-like and guanylyl cyclase (guanylate cyclase) catalytic domains [6,7]. ANP and BNP activate NPRA, which produces second messenger cGMP in response to hormone binding. CNP activates NPRB, which also produces cGMP; however, all three natriuretic peptides indiscriminately bind to NPRC, which lacks guanylyl cyclase activity [8–10]. NPRA is considered to be the biological receptor of ANP and BNP, since most of the physiological effects of these peptide hormones are triggered by the generation of second messenger cGMP [11,12]. The activity and expression of NPRA, assessed primarily through ANP-dependent guanylyl cyclase activity and cGMP accumulation, are regulated by a number of factors, including the ligand itself [13–15]. Recent studies with Npr1 (coding for NPRA) gene-disruption and gene-duplication mouse models have revealed the hallmark significance of NPRA in lowering arterial pressures and protecting against renal and cardiac pathophysiological functions [16,17].
On ligand binding, NPRA dimerizes and the guanylyl cyclase catalytic domain probably becomes activated [18]. ANP binding also triggers efficient NPRA internalization and trafficking into intracellular compartments [13,15,19,20]. ANP is subsequently delivered, probably through endosomes, to the lysosomal compartments, where it is largely degraded; however, a population of ligand–receptor complexes escapes the lysosomal compartment, and the receptor recycles back to the plasma membrane. So far, the structural determinants of the receptor activation and signalling pathways that control the internalization and subsequent intracellular routing of NPRA are not well understood. We have suggested in a previous study that a sorting mechanism partitions the incoming bound ligand–receptor complexes into one of the two major intracellular routes [15]. First, a degradative route leads to intracellular catabolism and release of the degraded ligand in addition to returning most of the internalized receptors to the cell surface. Secondly, a retroendocytosis pathway determines the release of the intact ligand as well as receptor recycling from intracellular compartments to the plasma membrane. However, the signals in the NPRA sequence that control and specify its endocytosis pathways have not been determined.
In the present study, we have performed mutations in the C-terminal domain of NPRA and we have expressed both wild-type and mutant receptors in HEK-293 cells (human embryonic kidney 293 cells). The region encoded in the C-terminal domain of NPRA contains one copy of the tetrameric sequence GDAY. In the present study, we sought to determine whether these residues play a role in ligand-mediated trafficking and down-regulation of NPRA. Here, we show for the first time that point mutations in the GDAY sequence of NPRA result in defective internalization and subsequent recycling of this receptor protein. Our results reveal that information residing within the GDAY sequence is important for receptor internalization and trafficking from the cell surface to intracellular compartments.
MATERIALS AND METHODS
Materials
ANP (rat-28) was purchased from Peninsula Laboratories (Belmont, CA, U.S.A.). The Quik Change site-directed mutagenesis kit was obtained from Stratagene (La Jolla, CA, U.S.A.), HEK-293 cell line from the A.T.C.C. (Manassas, VA, U.S.A.) and the transfection reagent LT1 was obtained from Panvera (Madison, WI, U.S.A.). 125I-ANP and [35S]methionine were purchased from Amersham Biosciences (Piscataway, NJ, U.S.A.). The mammalian expression vector pcDNA3 was obtained from Invitrogen (Carlsbad, CA, U.S.A.). Lipofectamine™ and tissue culture supplies were purchased from Invitrogen/Life Technologies (Grand Island, NY, U.S.A.). The cGMP immunoassay kit was obtained from Assay Designs (Ann Arbor, MI, U.S.A.). Synthetic oligonucleotide primers were obtained from Midland Scientific (Midland, TX, U.S.A.). All other chemicals were of reagent grade and were obtained from Sigma (St. Louis, MO, U.S.A.).
Site-directed mutagenesis of NPRA
Murine cDNA encoding NPRA [6] was subcloned into the expression vector pcDNA3 as described previously [21]. The individual amino acids in the GDAY sequence along with two additional residues on each side of this motif were mutated as follows: the four amino acids Thr918, Ile919, Gly920 and Asp921 mutated to Ala, Ala922 mutated to Ile and the three amino acids Tyr923, Met924 and Val925 mutated to Ala. The oligonucleotide-mediated mutagenesis was performed using the Quik Change site-directed mutagenesis kit. Specifically, the sequence of the GDAY/AAAA mutagenic sense primer was 5′-AAGGTAGAGACCATTGCTGCTGCTGCTATGGTGGTATCAGGG-3′ and that of the antisense primer was 5′-CCCTGATACCACCATAGCAGCAGCAGCAGCAATGGTCTCTACCTT-3′, where the underlined nucleotides indicate mutations G920A (Gly920→Ala), D921A and Y923A respectively. The residue Ala922 of the GDAY motif was kept unchanged unless otherwise stated. The integrity of the mutated plasmid sequence was confirmed by double-stranded DNA sequencing in the mutagenized region of NPRA. The nucleotide sequence was determined at the Center for Gene Therapy Sequencing Core Facility, Tulane University School of Medicine.
Cell culture and stable transfection of wild-type and mutant NPRA
HEK-293 cells were grown in 6 cm2 culture dishes containing DMEM (Dulbecco's modified Eagle's medium), supplemented with 10% (v/v) FBS (fetal bovine serum). Initially, subconfluent cells were transfected with wild-type and GDAY/AAAA mutant NPRA cDNAs using Lipofectamine™ and LT-1 reagents. To establish the stably expressing receptor cell lines, 400 μg/ml Geneticin was added to the culture medium after transfection. Antibiotic-resistant clones were isolated and established for receptor expression using 125I-ANP binding and the cGMP assay as described in [15]. Cells were maintained in monolayer culture in an atmosphere of 5% CO2 and 95% O2, and the culture medium was replaced on alternate days.
Cell-surface receptor binding assay
Both wild-type and mutant recombinant HEK-293 cells were grown in 6 cm2 culture dishes. After confluence, cells were washed with DMEM containing 0.1% BSA and labelled with 125I-ANP in the absence or presence of 100-fold excess molar concentrations of unlabelled ANP. After completion of binding at 4 °C, the unbound 125I-ANP was removed from the dishes by four washes (2 ml each) with ice-cold assay medium. To determine the cell-surface-associated radioactivity, the acid-wash procedure as described in [21] was used. After binding was completed, each culture dish received 1 ml of ice-cold acidic buffer (50 mM glycine/100 mM NaCl, pH 3.8) at 4 °C. After 2 min, the acid eluate from the dish was collected and each dish received another 1 ml of ice-cold glycine acidic buffer to wash the cells. Both eluate solutions were combined to determine the acid-sensitive radioactivity. Cells were then dissolved in 0.5 M NaOH to determine the acid-resistant radioactivity. Acid-sensitive radioactivity was used as an index of the cell-surface-bound 125I-ANP, and acid-resistant radioactivity was used as a measurement of the internalized ligand–receptor complexes.
Receptor internalization and sequestration
HEK-293 cells expressing either wild-type or mutant NPRAs were allowed to bind 125I-ANP at 4 °C for 60 min. The unbound 125I-ANP was removed by washing the cells with ice-cold assay medium. The total cell-associated radioactivity was determined by dissolving cells in 0.5 M NaOH, and counting the radioactivity in the cell lysate, which represented the initial zero-time control value of 100%. Internalization of ligand–receptor complexes was allowed by quickly warming the cells at 37 °C. At the indicated time periods, the culture dishes were removed from the medium at 37 °C, placed at 4 °C, and the media were collected. As described above, the cell-surface-associated 125I-ANP radioactivity was removed by washing the cells with ice-cold glycine acidic buffer (pH 3.8) at 4 °C. After the acid wash, the internalized 125I-ANP radioactivity was measured by dissolving the cells in 0.5 M NaOH. To quantify the rate of degradation of ligand–receptor complexes in the lysosomal compartments, cells expressing wild-type and mutant receptors were pretreated with lysosomotropic agents such as monensin (50 μM), chloroquine (200 μM) and ammonium chloride (10 mM) at 37 °C for 1 h. Then, cells were allowed to bind 125I-ANP at 4 °C for 1 h, washed with assay medium and re-incubated in the fresh medium at 37 °C. The treatment with lysosomotropic agents was maintained throughout the entire ligand binding and internalization periods of the experiment. It should be noted that lysosomotropic agents did not alter the binding capacity of the ligand to intact HEK-293 cells at 4 °C. Quantitative analyses of intact and degraded ligands released into the culture medium were performed by precipitating the medium with 10% (w/v) trichloroacetic acid containing 200 μg/ml BSA as the carrier by our previously described methods [13]. The 125I-ANP recovered in the trichloroacetic acid precipitate was considered to be the intact ligand, and those in the supernatant were regarded as degraded ligand products.
Recycling of internalized receptors from intracellular compartments to the plasma membrane
The recycling of wild-type and mutant NPRAs was determined by trypsin-dependent loss of cell-surface-binding activity of the receptor molecules. Cells expressing recombinant wild-type or mutant NPRAs were washed with an ice-cold binding assay medium and treated with trypsin (0.025%) for 10 min at 4 °C. To stop the trypsin reaction, cells were treated with a soya-bean trypsin inhibitor (200 μg/ml), washed quickly three times with assay medium and then re-incubated in the fresh medium. In both trypsin-treated and untreated cells, 125I-ANP binding was determined as described above. It should be noted that treatment of cells with trypsin for 10 min at 4 °C essentially abolished the cellsurface binding of 125I-ANP and did not cause any significant cell detachment from the culture dishes.
Quantitative assessment of intracellular wild-type and mutant receptors in solubilized recombinant HEK-293 cells
Total wild-type and mutant receptor contents were quantified by solubilizing HEK-293 cells in a buffer containing 20 mM Hepes (pH 7.4), 1.5 M NaCl, 1% Triton X-100, 15% glycerol, 1 mM EDTA and 10 μg/ml each of leupeptin and aprotinin. The mixture was centrifuged for 5 min at 1000 g to remove the insoluble material and then re-centrifuged at 100000 g for 1 h to obtain a clear supernatant. The 125I-ANP binding activity was assayed at 25 °C for 1 h by adding 50 μl of solubilized supernatant to 400 μl of binding buffer containing 50 mM Tris/HCl (pH 7.4), 0.15 M NaCl, 5 mM MgCl2, 0.1% BSA, 0.5 mg/ml bacitracin and 1 mM 125I-ANP as described previously [15]. Non-specific binding was determined by the addition of 100-fold excess molar concentrations of unlabelled ANP. The radiolabelled ligand bound to solubilized receptors was precipitated by adding 0.25% bovine γ-globulin and 2.5 ml of 10% (w/v) poly(ethylene glycol)-8000 in 20 mM Tris/HCl (pH 7.4) and 0.15 M NaCl. The mixture was filtered under vacuum through Whatman GF/B filters treated with 0.3% (w/v) polyethyleneimine. To quantify only intracellular receptors, cells were first trypsin-treated to degrade total receptor binding activities on the cell surface, followed by quantification of intracellular receptors.
Metabolic labelling and immunoprecipitation
HEK-293 cells expressing wild-type or mutant receptors were plated in 6 cm2 dishes containing DMEM supplemented with 10% FBS. A pulse–chase experiment was performed by washing the subconfluent monolayer cells twice with HBSS (Hanks balanced salt solution) and incubating at 37 °C with 2 ml of methionine-free DMEM, supplemented with 1 μM unlabelled methionine, 10% FBS and 0.14 mCi/ml [35S]methionine. After 1 h, the pulse medium was removed. The cells were washed once with HBSS and then with 2 ml of chase medium consisting of normal DMEM and 10% FBS, and incubated in fresh medium at 37 °C for 18 h. Then, cells were washed once with HBSS and scraped in solubilizing buffer [20 mM Hepes (pH 7.4), 150 mM NaCl, 1% Triton X-100, 10% glycerol, 5 mM EDTA, 1 mM PMSF and 10 μg/ml each of aprotinin and leupeptin]. The cell lysate was stirred for 30 min at 4 °C and then the mixture was centrifuged for 5 min at 1000 g to remove the insoluble material. The supernatant was re-centrifuged at 100000 g for 1 h to obtain a clear solution containing the solubilized receptors by standard methods [21].
For immunoprecipitations, equal amounts of protein (100 μg) obtained from the recombinant HEK-293 cells expressing either wild-type or mutant receptors were incubated with 1:400 dilution of NPRA antiserum at 4 °C for 4 h as described previously [21]. The immunocomplex was precipitated with Protein A–agarose and the non-specific proteins were removed by washing the precipitated material four times with RIPA buffer containing 20 mM Tris/HCl (pH 7.4), 150 mM NaCl, 1% sodium deoxycholate, 1% Triton X-100 and 0.2% SDS as described previously [21]. The content was dissolved in sample buffer, boiled for 5 min, and the immunoprecipitated proteins were analysed by SDS/PAGE (7.5% polyacrylamide) under reducing conditions. Electrophoresis was performed at a constant current of 25 mA until the Bromophenol Blue front reached the bottom of the gel. Proteins in the gel were stained with Coomassie Brilliant Blue R-250. After destaining, the gel was dried and fluorographed at −70 °C using a Kodak X-Omat film and Cronex Lightening Plus intensifying screen (DuPont, Boston, MA, U.S.A.). The proteins used for standard molecular-mass (relative molecular mass) calibration were as follows: myosin (Mr 205000), β-galactosidase (Mr 116000), phosphorylase B (Mr 97000), BSA (Mr 67000), ovalbumin (Mr 45000) and carbonic anhydrase (Mr 29000).
Statistical analysis
The dissociation constant (kd) and the receptor density (Bmax) in recombinant HEK-293 cells expressing wild-type or GDAY/AAAA mutant receptor were determined by Scatchard plot analysis using a computer program (Ligand). Results are presented as means±S.E.M. for triplicate determinations in at least three to four separate sets of experiments. Statistical significance was ascertained by the use of an unpaired t test.
RESULTS
Internalization of NPRA containing alanine mutations at different positions in the C-terminal GDAY motif
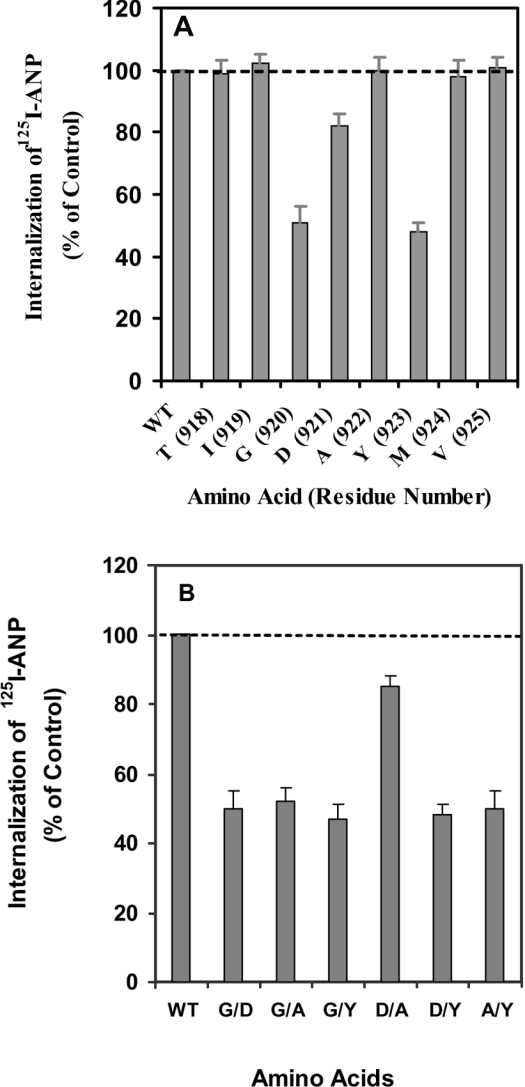
Figure 1 shows the experiment in which alanine was substituted for each of the amino acids in the conserved region from positions 918 to 925 in the C-terminal domain of NPRA. In each experiment, we compared the cells expressing one or more of the NPRA mutations with the cells expressing wild-type receptor. Two of the alanine substitutions lowered internalization by almost 50% of the control value (Figure 1A). Three of the amino acids, including Gly920, Asp921 and Tyr923, seem to be important in the GDAY motif for receptor internalization. When the alanine residue of the GDAY sequence was replaced with a leucine residue, we did not observe any diminution of internalization when compared with the wild-type receptor. The substitution of two amino acids on either side of GDAY motif did not show any effect on the internalization of NPRA (Figure 1A). In addition to a single alanine substitution, we also performed mutations in which two residues were substituted with alanine residues simultaneously (Figure 1B). The substitution of Gly920 or Tyr923 with alanine residues individually or simultaneously decreased the internalization of the mutant receptor by almost 50% when compared with wild-type NPRA. The substitution of Asp921 with an alanine residue decreased the internalization of NPRA by only 20%, indicating that Asp921 has only a minimal effect on internalization; however, Ala922 does not seem to be important for receptor internalization. In subsequent studies, single mutations of Gly920, Asp921 and Tyr923 to alanine (G920A, D921A and Y923A and/or a triple mutation of GDAY (GDAY/AAAA) were utilized to determine the effect of the GDAY motif on the kinetics of internalization and/or recycling of NPRA.
Figure 1. Sequence requirements for internalization of NPRA in HEK-293 cells.
(A) Alanine substitutions at amino acid positions 918–925. (B) Alanine substitutions indicating two residues in different combinations in the GDAY motif. Confluent HEK-293 cells in 6 cm2 dishes expressing either wild-type (WT) or mutant receptors were washed twice with 2 ml of assay medium (DMEM containing 0.1% BSA) and then treated with 125I-ANP at 4 °C for 1 h in the absence or presence of unlabelled ANP. Then, the cells were washed four times with assay medium and re-incubated in 2 ml of fresh medium at 37 °C. After 10 min of internalization and incubation, the internalization of ligand–receptor complexes was quantified as described in the Materials and methods section. The results represent the means±S.E.M. for three or four independent experiments.
Expression and binding competition analyses of wild-type and mutant receptors in recombinant HEK-293 cells
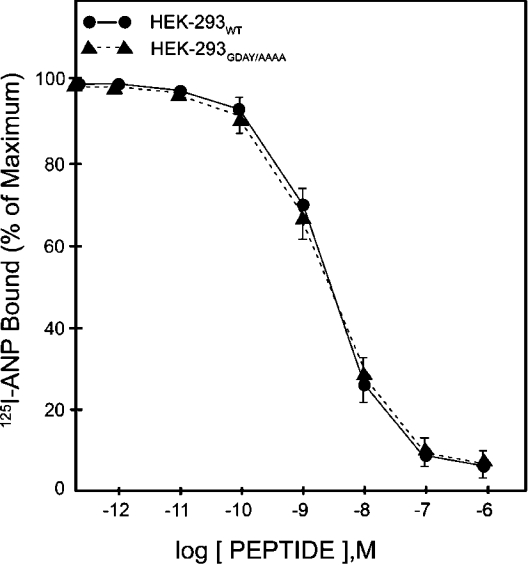
Confluent HEK-293 cells expressing wild-type or mutant NPRA were exposed to 125I-ANP at 4 °C for 1 h. After washing the free ligand, the cell-associated 125I-ANP radioactivity was determined. The results presented in Figure 2 demonstrate the competition binding curves with increasing concentrations of unlabelled ANP. The GDAY/AAAA mutant NPRA displayed a dose–response curve for 125I-ANP binding similar to that seen for the wild-type receptor. As determined by Scatchard plot analysis using the computer program, recombinant HEK-293 cells harbouring wild-type and GDAY/AAAA mutant receptors expressed 1.87×106 and 1.89×106 receptor sites/cell respectively. Receptor-binding affinity was comparable in both cell lines, with Kd∼2.4×10−10 M (Table 1). In the initial studies, two independent HEK-293 recombinant cell lines harbouring wild-type, G920A, D921A, Y923A or GDAY/AAAA receptors were utilized for the functional studies, and each clonal cell line yielded comparable binding results (results not shown).
Figure 2. Competition binding of 125I-ANP in HEK-293 cells expressing wild-type or GDAY/AAAA mutant receptors.
Confluent HEK-293 cells in 6 cm2 dishes were incubated in 2 ml of assay medium with 1 nM 125I-ANP and increasing concentrations of unlabelled ANP at 4 °C for 1 h. Cells were washed four times, each with 2 ml of assay medium to remove the unbound radioligand, and then dissolved in 1 M NaOH as described in the Materials and methods section. Specific 125I-ANP radioactivity was determined in the solubilized cell extract. The binding curves are derived from the specific binding data from at least three separate experiments. The results are expressed as percentage maximum of bound/free hormone. The results represent the means±S.E.M. for three independent experiments with triplicate dishes in each replicate.
Table 1. Competition of 125I-ANP binding and comparative receptor parameters in HEK-293 cells expressing wild-type or GDAY/AAAA mutant NPRA.
Confluent HEK-293 cells in 6 cm2 dishes expressing either wild-type or GDAY/AAAA mutant receptors were washed twice with 2 ml of assay medium (DMEM containing 0.1% BSA) and then treated with 125I-ANP at 4 °C for 1 h in the presence or absence of unlabelled ANP as described in the Materials and methods section. The cells were washed four times with assay medium and cell-bound 125I-ANP radioactivity was determined. The non-specific binding was determined by using 100-fold excess molar concentrations of unlabelled ANP. EC50 or Kd values and receptor densities (Bmax) were determined from the binding competition and by Scatchard analysis of the 125I-ANP binding data. The amino acid sequences encoded by the exon regions for the wild-type and mutant GDAY/AAAA NPRA cDNAs are indicated with single-letter amino acid residues. The results represent the means for three separate determinations with three replicate dishes in each experiment.
| Transfection | Exon sequence | Kd (M) | Bmax (receptors sites/cell) |
|---|---|---|---|
| Wild-type NPRA | VYKVETIGDAYMVSG | 2.4×10−10 | 1.86×106 |
| GDAY/AAAA NPRA | VYKVETIAAAAMVSG | 2.4×10−10 | 1.89×106 |
Kinetics of internalization and intracellular sequestration of wild-type and mutant NPRA in HEK-293 cells
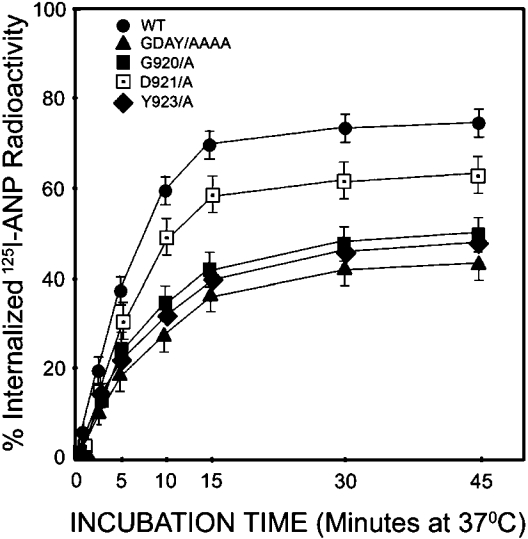
The bound 125I-ANP–NPRA complexes were measured to examine the kinetics of internalization, trafficking and sequestration of wild-type, G920A, D921A, Y923A and triple-mutant GDAY/AAAA receptors in stably expressing HEK-293 cells. The radioligand 125I-ANP was bound to the cell-surface receptors during incubation at 4 °C and then allowed to internalize after warming the cells at 37 °C. The time course of 125I-ANP internalization was determined for wild-type, G920A, D921A, Y923A and GDAY/AAAA recombinant cell lines. The results presented in Figure 3 show that 125I-ANP bound to both wild-type and mutant NPRA was rapidly internalized, and total intracellular radioactivity increased to the maximum levels in 15 min at 37 °C. Results are expressed as a percentage of the total cell-associated radioactivity that was internalized at each time point. The initial rate of ligand internalization was clearly decreased by almost 50% (P<0.001) in recombinant HEK-293 cells expressing G920A, Y923A and triple-mutant GDAY/AAAA receptors when compared with recombinant cells expressing wild-type receptor. At 15 min, an internalization defect of 46±3, 49±4 and 52±4% was observed in cells expressing G920A, Y923A and GDAY/AAAA mutant NPRA respectively. However, the D921A mutant receptor showed only an 18% reduction in internalization when compared with wild-type NPRA.
Figure 3. Internalization of 125I-ANP in HEK-293 cells expressing recombinant wild-type or mutant receptors.
HEK-293 cells expressing wild-type, G920A, D921A, Y923A or GDAY/AAAA mutant receptors were preincubated for 1 h at 4 °C with labelled 125I-ANP. Surface 125I-ANP binding to wild-type and mutant recombinant HEK-293 cells accounted for an average of approx. 28958±750 c.p.m. Cells were subsequently warmed at 37 °C for the indicated time periods to permit the internalization of ligand–receptor complexes into the cell interior. Internalization of 125I-ANP was quantified at each time point as described in the Materials and methods section. The results represent the means±S.E.M. for triplicate experiments.
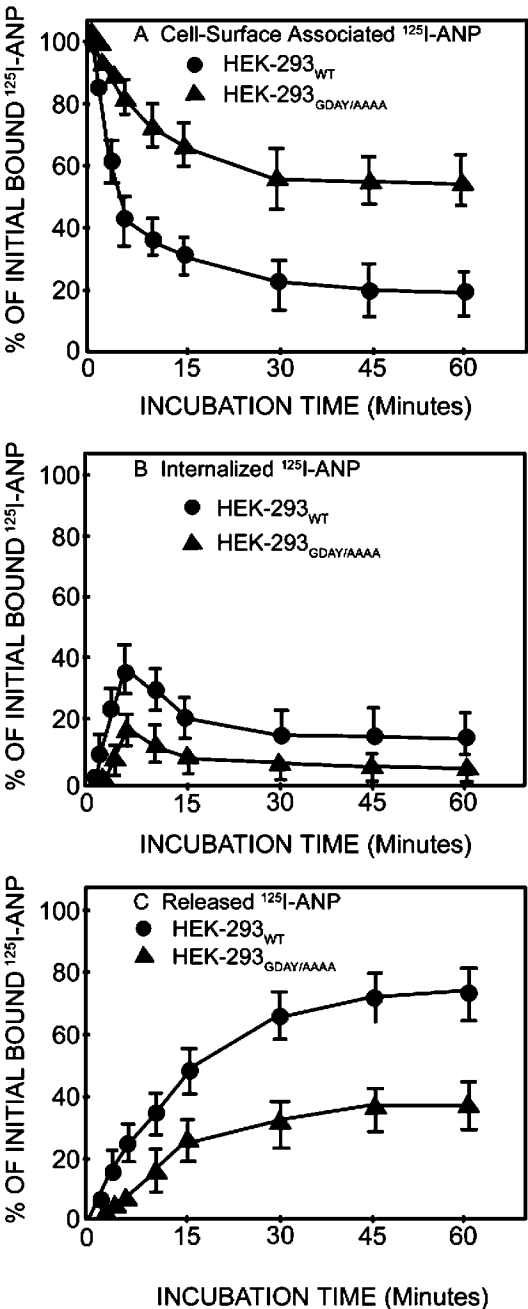
We determined the cell-surface-associated, internalized and released 125I-ANP radioactivity in HEK-293 cells expressing either wild-type or GDAY/AAAA mutant receptor by the acid-wash procedure. During the internalization period, culture dishes in replicates were removed from the medium at 37 °C and placed at 4 °C, and media were collected. Cells were washed once with HBSS and then treated with glycine acidic buffer (pH 3.8) at 4 °C to release the cell-surface-associated 125I-ANP at the indicated time periods. HEK-293 cells expressing wild-type NPRA showed a rapid internalization of ligand–receptor complexes, with almost 60–65% of the receptors internalized inside the cells after a 5 min incubation at 37 °C. On the contrary, only 30–35% of the receptors were internalized in HEK-293 cells expressing GDAY/AAAA mutant receptor for the same incubation time periods (Figure 4A). The amount of internalized receptor decreased rapidly after 5 min of incubation at 37 °C. The population of internalized ligand–receptor complexes in recombinant HEK-293 cells expressing GDAY/AAAA mutant NPRA was decreased by almost 50% after a 5 min incubation at 37 °C when compared with HEK-293 cells expressing wild-type NPRA (Figure 4B). The wild-type recombinant HEK-293 cells accumulated intracellular 125I-ANP radioactivity at much higher levels when compared with HEK-293 cells expressing GDAY/AAAA mutant NPRA. This difference was noted because of the differential rates of internalization of bound 125I-ANP to wild-type and mutant NPRA. Approx. 40% of the 125I-ANP bound to NPRA was in the intracellular compartments and was resistant to removal by acid wash, in the HEK-293 cells expressing wild-type receptors. In contrast, only approx. 20% of the 125I-ANP-bound ligand–receptor complexes were localized in the intracellular compartments in HEK-293 cells expressing GDAY/AAAA mutant NPRA. We observed that a large proportion of ligand–receptor complexes were not internalized, and remained on the plasma membrane of HEK-293 cells expressing GDAY/AAAA mutant receptor.
Figure 4. Quantitative analyses of cell-surface-associated, internalized and released 125I-ANP radioactivity in HEK-293 cells expressing wild-type or GDAY/AAAA mutant receptors.
Confluent HEK-293 cells in 6 cm2 dishes expressing wild-type or mutant receptors were allowed to bind 125I-ANP at 4 °C for 1 h. Cells were washed four times, each with 2 ml of assay medium, to remove the unbound ligand and then re-incubated in fresh medium at 37 °C. At the indicated time points, dishes were transferred at 4 °C and the media were collected. The cell-surface-associated (acid-sensitive) radioactivity was eluted with glycine acidic buffer (pH 3.8) and cells were dissolved in 1 M NaOH to measure the internalized (acid-resistant) radioactivity. Cell-surface-associated (A), internalized (B) and released (C) 125I-ANP radioactivity was determined in the acid eluate, cell extract and culture medium respectively as described in the Materials and methods section. Results represent the means±S.E.M. for three to four independent experiments with triplicate dishes in each replicate.
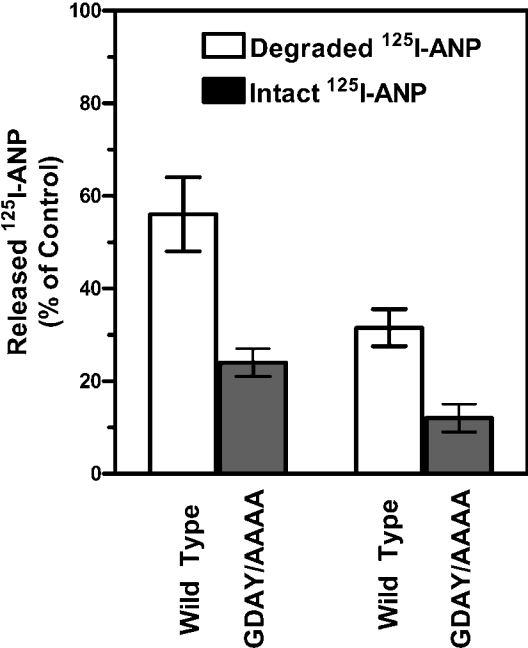
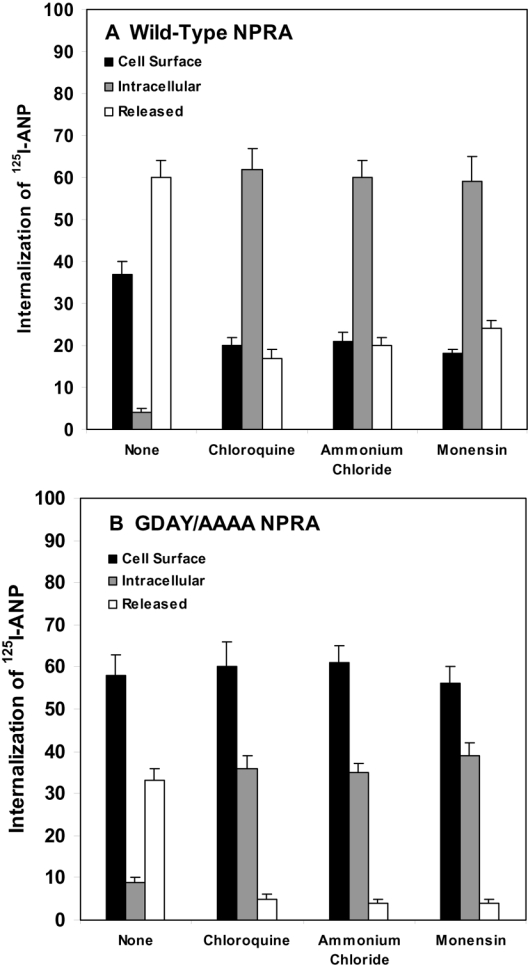
The release of 125I-ANP into the culture medium of HEK-293 cells expressing wild-type NPRA increased progressively, reaching 70–80% with equilibrium after 30 min incubation at 37 °C. However, only 40–45% of 125I-ANP was released into the culture medium of HEK-293 cells expressing GDAY/AAAA mutant NPRA (Figure 4C). Thus HEK-293 cells expressing GDAY/AAAA mutant receptors exhibited defective internalization and degradation of bound ANP–NPRA complexes under physiological and steady-state conditions. A quantitative assessment of the intact and degraded ligand was performed by measuring the solubility of 125I-ANP products in 10% trichloroacetic acid solution. The trichloroacetic acid precipitates (containing intact ligand) and supernatants (containing degraded ligand products) were separated by centrifugation of trichloroacetic acid-soluble extracts. Higher amounts of both intact and degraded ligand products were found in the culture medium of cells expressing wild-type NPRA when compared with the culture medium of cells expressing GDAY/AAAA mutant receptor. The total released 125I-ANP was composed of approx. 50–55% of degraded ligand products and 20–25% of intact ligand in the culture medium of cells expressing wild-type receptor (Figure 5). On the other hand, the total 125I-ANP released into the culture medium of cells expressing the mutant receptor was composed of 30–35% of degraded ligand products and only 10–15% of intact ligand. The degraded and intact ligand released into the culture medium was quantitatively determined on the basis of the amount of total initial 125I-ANP bound on the cell surface. Treatment of the HEK-293 cells expressing wild-type and GDAY/AAAA mutant receptors with the lysosomotropic agents chloroquine, ammonium chloride and monensin significantly blocked the degradation of internalized 125I-ANP–NPRA complexes when compared with untreated control cells (Figures 6A and 6B). It is noteworthy that, after treatment with lysosomotropic agents, cells expressing wild-type NPRA (Figure 6A) contained almost 50% more 125I-ANP-bound receptor levels in the intracellular compartments when compared with HEK-293 cells expressing GDAY/AAAA mutant receptor (Figure 6B).
Figure 5. Quantitative analysis of degraded and intact 125I-ANP released into the culture medium of HEK-293 cells expressing wild-type or GDAY/AAAA mutant receptors.
HEK-293 cells expressing wild-type or GDAY/AAAA mutant receptors were allowed to bind 125I-ANP at 4 °C for 1 h. Then, the cells were washed four times with ice-cold assay medium to remove the unbound ligand and then re-incubated in 2 ml of fresh assay medium at 37 °C for 30 min. After incubation, the culture medium was collected and the intact and degraded ligand products were quantified as described in the Materials and methods section. The results represent the means±S.E.M. for three independent determinations.
Figure 6. Quantitative analyses of cell-surface-associated, internalized and released 125I-ANP radioactivity after treatment with lysosomotropic agents in HEK-293 cells expressing wild-type or GDAY/AAAA mutant NPRA.
Confluent HEK-293 cells expressing either (A) wild-type or (B) mutant receptors were washed with assay medium and preincubated in 2 ml of fresh assay medium with the indicated lysosomotropic agents: chloroquine (200 μM), ammonium chloride (10 mM) and monensin (50 μM), at 37 °C for 60 min. The cells were then washed with assay medium and incubated with 125I-ANP in 2 ml of fresh assay medium for 60 min at 4 °C as described in the Materials and methods section. The treatment with lysosomotropic agents was maintained throughout the ligand-binding and internalization periods of the experiment. After the binding was completed, cells were washed four times with the assay medium and then placed in 2 ml of fresh medium at 37 °C. After 10 min of internalization and incubation, culture dishes were removed from the medium at 37 °C and placed at 4 °C. The medium was collected and each dish was treated with 2 ml of cold glycine acidic buffer (pH 3.8) for 2 min at 4 °C. After collecting the acid eluate, cells were dissolved in 0.5 M NaOH. 125I-ANP radioactivity in the acid eluate, cell extract and culture medium was counted to determine the cell-surface-associated, internalized and released 125I-ANP radioactivity respectively. The results represent the means±S.E.M. for three independent determinations with triplicate dishes in each experiment.
Down-regulation of NPRA in HEK-293 cells expressing wild-type and mutant receptors
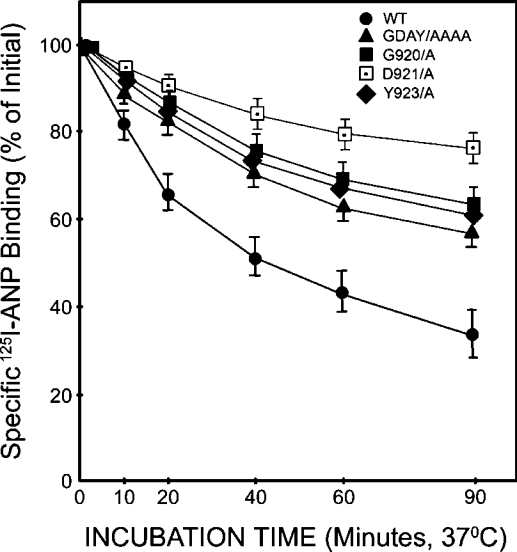
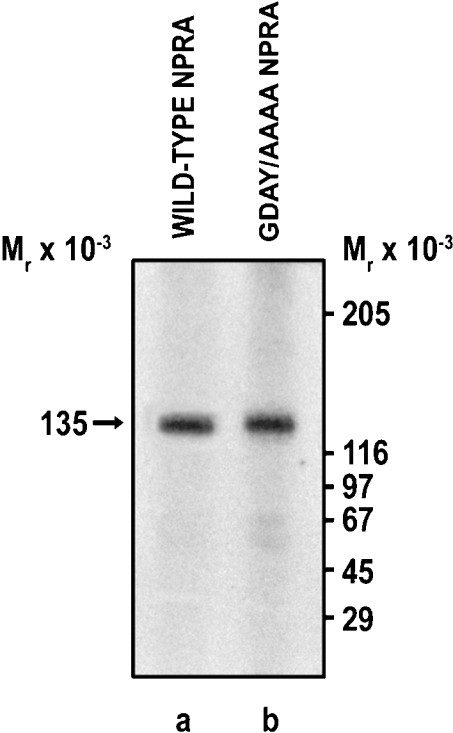
Confluent HEK-293 cells expressing wild-type, G920A, D921A, Y923A or GDAY/AAAA mutant receptors were pretreated with unlabelled ANP (10 nM) at 37 °C for the indicated time periods and then washed with glycine acidic buffer (pH 3.8) to remove any unbound ligand. Cells were then washed three times with the assay medium (DMEM containing 0.1% BSA) and allowed to bind 125I-ANP in 2 ml of fresh assay medium at 4 °C for the indicated time periods. After treating the cells with unlabelled ANP for 60 min, 125I-ANP-binding sites were decreased by almost 68±5% in HEK-293 cells expressing wild-type NPRA and by 28±3, 32±3 and 36±4% in cells expressing G920A, Y923A or GDAY/AAAA mutant receptors respectively (Figure 7). The mutation D921A had only a minimal effect on the down-regulation of the receptor. Thus the treatment of HEK-293 cells with unlabelled ANP (10 nM) caused a substantial decrease in the ANP-binding capacity of wild-type NPRA; however, the mutant receptors were more resistant to ligand-mediated down-regulation. To confirm independently that a decrease in the functional activity of GDAY/AAAA mutant NPRA in terms of receptor internalization is indeed due to the point mutation created in cDNA, the expressions of both wild-type and mutant receptors were monitored immunologically. Cells expressing the wild-type or mutant NPRA were labelled with [35S]methionine, and receptors were immunoprecipitated utilizing the site-directed polyclonal antibodies raised against the extracellular domain of murine NPRA. The results presented in Figure 8 show that both wild-type and GDAY/AAAA mutant receptors are expressed on the cell surface with almost similar densities, and the mutations created in the C-terminal domain of NPRA did not affect the total receptor expression in HEK-293 cells.
Figure 7. ANP-induced receptor down-regulation in HEK-293 cells expressing wild-type or mutant receptors.
Confluent HEK-293 cells expressing wild-type, G920A, D921A, Y923A or GDAY/AAAA mutant receptors in 6 cm2 culture dishes were pretreated with unlabelled ANP (10 nM) for the indicated time periods. Cells were then washed with glycine acidic buffer (pH 3.8) to remove any surface-bound 125I-ANP. After washing the cells four times with assay medium (2 ml each), cells were reincubated in 2 ml of fresh assay medium. The 125I-ANP binding was assayed at the indicated time periods as described in the Materials and methods section. The specific 125I-ANP binding was then determined and expressed as a percentage of the initial radioligand 125I-ANP binding. The results represent the means±S.E.M. for four independent determinations with triplicate dishes in each experiment.
Figure 8. Expression and immunoprecipitation of wild-type or GDAY/AAAA mutant NPRA in recombinant HEK-293 cells.
Confluent recombinant HEK-293 cells expressing either wild-type or mutant receptors were labelled with [35S]methionine. Cell lysates (200 μg of protein) were immunoprecipitated using the site-directed polyclonal antibodies against NPRA. The labelled protein was separated on a 7.5% (w/v) polyacrylamide gel as stated in the Materials and methods section. Lanes a and b represent the expressed recombinant wild-type and GDAY/AAAA mutant receptor bands in HEK-293 cells respectively. The arrow indicates the position of the 135 kDa NPRA protein band. The positions of molecular-mass standards are shown on the right in kDa.
Recycling of internalized wild-type and mutant receptors in HEK-293 cells
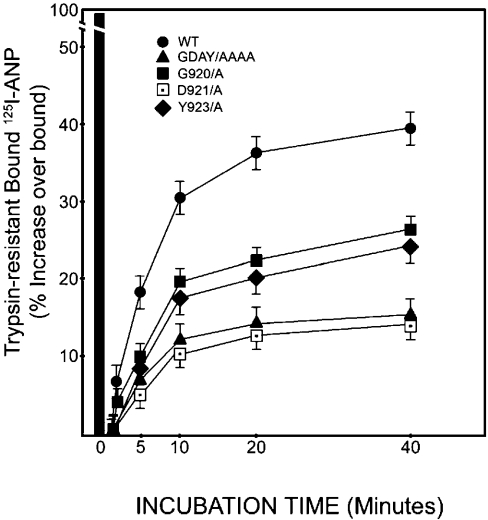
To examine the rate of recycling of wild-type, G920A, D921A, Y923A and GDAY/AAAA mutant NPRA, the recombinant HEK-293 cells expressing the respective receptors were treated with a low concentration of trypsin (0.025%) at 4 °C for 10 min, which abolished cell-surface receptors. To stop the trypsin reaction, cells were treated with soya-bean trypsin inhibitor and washed free of trypsin with serum containing DMEM. Cells were re-incubated in fresh medium and then 125I-ANP binding was determined. Cells expressing G920A and Y923A mutant receptors exhibited almost 35% decreased return in 125I-ANP binding when compared with cells expressing wild-type NPRA (Figure 9). However, cells expressing D921A or triple-mutant GDAY/AAAA receptors showed almost 60% decreased return in 125I-ANP binding activity when compared with wild-type NPRA. Results from these experiments provided evidence that a higher return in 125I-ANP binding in wild-type recombinant HEK-293 cells is due to a greater percentage of internalized receptors when compared with cells harbouring G920A, D921A, Y923A or GDAY/AAAA mutant NPRA.
Figure 9. Quantitative analysis of trypsin-resistant intracellular NPRA in HEK-293 cells expressing wild-type or mutant receptors.
Confluent HEK-293 cells expressing wild-type, G920A, D921A, Y923A or GDAY/AAAA mutant NPRA in 6 cm2 culture dishes were treated with 0.025% trypsin in the assay medium DMEM for 10 min at 4 °C as described in the Materials and methods section. The parallel sets of control culture dishes were incubated at 4 °C in the absence of trypsin treatment. The trypsin reaction was stopped by adding soya-bean trypsin inhibitor (200 μg/ml) and by washing the cells with a medium containing 10% FBS. 125I-ANP binding was determined in recombinant HEK-293 cells expressing either wild-type or different mutant receptors at the indicated time periods. The black bar represents 125I-ANP binding in control cells not treated with trypsin. The results represent the means±S.E.M. for three independent determinations with triplicate dishes in each experiment.
Quantitative analysis of the total and intracellular pools of NPRA in HEK-293 cells expressing wild-type and mutant receptors
To examine the possibility whether NPRA was re-inserted into the plasma membrane from the pre-existing intracellular pool, the cell-surface and total receptors were measured in intact and solubilized preparations of HEK-293 cells expressing either wild-type or GDAY/AAAA mutant NPRA (Table 2). To assess the proportion of both wild-type and GDAY/AAAA mutant receptors in the cell interior, 125I-ANP binding was measured in solubilized extracts of both trypsin-treated and untreated recombinant HEK-293 cells. 125I-ANP binding in trypsin-treated and Triton X-100-solubilized HEK-293 cells expressing the wild-type and GDAY/AAAA mutant receptors indicated that most of the receptors were present on the plasma membrane, and only a small fraction of the receptor population (15–20%) could be assigned to the pre-existing intracellular pool (Table 2).
Table 2. Quantification of the total and intracellular pool of wild-type and GDAY/AAAA mutant NPRA in intact and solubilized recombinant HEK-293 cells treated with and without trypsin.
HEK-293 cells expressing wild-type or GDAY/AAAA mutant receptors were washed with binding assay medium (DMEM containing 0.1% BSA) and cells were allowed to bind 125I-ANP in control and trypsin-treated (0.025%) groups. In parallel experiments, the control and trypsin-treated cells were solubilized in a buffer containing 1% Triton X-100, 15% glycerol and protease inhibitors. The binding of 125I-ANP in intact and solubilized cells was determined as described in the Materials and methods section. The percentage relative binding was determined from the specific 125I-ANP binding parameters. The results represent the means±S.E.M. for four independent measurements.
| Wild-type NPRA | GDAY/AAAA NPRA | |||
|---|---|---|---|---|
| Treatment | Specific 125I-ANP binding (%) | Relative binding (%) | Specific 125I-ANP binding (%) | Relative binding (%) |
| Untreated | 20.64±2.92 | 100 | 21.03±3.01 | 100 |
| Trypsin-treated | 1.48±0.16 | 7.17 | 1.76±0.14 | 5.99 |
| Triton X-100-solubilized | 25.73±2.81 | 100 | 23.92±2.54 | 100 |
| Trypsin plus Triton X-100 | 6.28±0.30 | 24.40 | 4.46±0.28 | 18.68 |
DISCUSSION
Results of the present study provide new insights into the structure and function of NPRA by the demonstration that the GDAY motif plays a dual role in receptor internalization into the cell interior and in subsequent recycling of the internalized receptor back to the plasma membrane. The mutations G920A and Y923A in NPRA cDNA markedly attenuated the internalization of mutant receptors by almost 50% when compared with the wild-type receptor. The mutation D921A showed only a minimal effect; however, Ala922 failed to decrease the internalization of NPRA. Results of the present study suggest that the regulation of receptor internalization relies largely on Gly920 and Tyr923 in the C-terminal domain of NPRA. On the other hand, the mutation D921A significantly attenuated the recycling of the internalized receptor back to the plasma membrane. The role of the NPRA cytoplasmic tail, therefore, is important and comparable with the thyrotropin-stimulating-hormone receptor [22], insofar as its disruption attenuates receptor internalization. Our previous deletion studies provided evidence that the C-terminal region of NPRA is critical for internalization; however, the involvement of a specific sequence motif in its internalization process was not determined [21]. In the present study, we have extended the results of those previous findings by showing that point mutations within the C-terminal region of NPRA have major effects on the internalization and recycling of this receptor protein. The present series of experiments utilized site-directed mutagenesis to replace selected Gly920, Asp921 and Try923 residues with alanines in the tetrameric sequence G920DAY923 located within the cytoplasmic tail of NPRA. The mutant receptors were found to be internalized at a significantly lower rate when compared with wild-type NPRA.
The consensus sequence NPXY in the cytoplasmic domain of an LDL (low-density lipoprotein) receptor has been shown to be necessary for receptor internalization [23]. The sequence NPXY is also present in the cytoplasmic domains of several other membrane proteins, including the insulin receptor [24,25]. Specifically, tyrosine residues have been suggested to play an important role in the internalization of various hormone receptors [26–28]. The internalization of the platelet-activating factor is also regulated by a putative motif DPXXY [29] and that of type 2 vasopressin receptor by the NPXXY sequence [30]. Yet another internalization sequence has been identified in the transferrin receptor, namely YXRF, in which the tyrosine residue is at the first position [31]. Similarly, the YXXL motif has been suggested to play a role in the endocytosis of the LDL receptor-related protein [32]. A common feature of all these internalization motifs, including the currently reported GDAY, is the presence of a tyrosine residue either in the beginning or at the end of the tetrapeptide sequence. Moreover, tyrosine residues in the mannose-6-phosphate receptor [24] and in the haemagglutinin of the influenza virus [29,33] are also involved in endocytosis, even though they do not occur in the context of NPXY or YXRF consensus sequences. Therefore, if a universal internalization signal exists, it may not be based on a universal amino acid sequence. It has been suggested that the critical characteristics of all these sequences might be their specification of a particular conformation, a tight β-turn in protein structure [31]. The role of the NPEY sequence in receptor internalization has been well recognized. The present study provides evidence for a new signal motif GDAY that is required for both internalization and recycling of NPRA. Our results clearly show that the GDAY sequence is necessary for the internalization of NPRA, but not absolutely sufficient, since the mutation of GDAY to AAAA did not completely block the internalization of NPRA.
Gly920 and Tyr923 constitute important elements of the GDAY internalization signal. Similarly, the G950PLY953 motif has been suggested to play a role in the internalization of insulin receptor, and Gly950 and Tyr953 have been shown to be critical in this process [28]. These authors suggested that a common internalization motif consists of four amino acids with an aromatic residue, especially a tyrosine residue, in the fourth position. It has also been suggested that tyrosine recognition signals may form a small surface loop structure [34], but this structure differs from that proposed by others in terms of positioning of the tyrosine residue in the loop [35]. Additional studies provided direct evidence that the NPXY sequence in the LDL receptor forms a β-turn structure and showed that peptides containing the NPXY motif assume a reverse-turn structure conformation with tyrosine in the fourth position of the turn [36]. The substitution of tyrosine with the residues known to be inactive in endocytosis resulted in a disruption of the β-turn conformation. A similar approach was also used to obtain evidence that the PPGY sequence of the acid phosphatase cytoplasmic tail forms a type 1 β-turn with the tyrosine residue at position 4 of the turn [37]. All these studies indicated that the tyrosine in the fourth position of internalization signals is critical for receptor endocytosis. Similarly, seven transmembrane G-protein-linked receptors have a homologous motif, NPXXY, and mutation of this sequence to NPXXA resulted in a complete loss of agonist-induced receptor sequestration [38,39]. The conserved tyrosine residue was required for internalization of the vasopressin receptor, and the NPXXY sequence represented a general sequestration motif for seven transmembrane G-protein-linked receptors [30]. However, there are certain exceptions to this rule, for example, in mannose-6-phosphate/insulin-like growth factor receptors, the critical tyrosine residue is included at the N-terminal first position of the consensus internalization signal YRHV [34].
Intriguing was the finding that the single-mutation D921A did not have a major effect on receptor internalization; however, it significantly attenuated the recycling of the internalized receptor back to the plasma membrane. On the other hand, mutation of Gly920 and Tyr923 to alanine residues inhibited the internalization of NPRA, but these residues did not have any discernible effect on the recycling of this receptor protein. These findings suggested that the tyrosine-based motif of the GDAY sequence seems to modulate the early internalization process of NPRA, whereas the aspartic residue in GDAY mediates recycling or subsequent sorting events of NPRA. Thus two overlapping motifs within GDAY in the C-terminal domain of NPRA exert different but specific effects on endocytosis and subsequent trafficking of this receptor molecule. Previous studies have demonstrated that acidic motifs such as DSLL in G-protein-coupled receptors act as recycling signals [40–42]. However, further studies are needed to clarify the dual role of the GDAY motif in the events involved in internalization, recycling and sequestration of NPRA.
Our earlier studies and reports from other laboratories have demonstrated that endogenous NPRA in PC-12 and MA-10 cells, as well as recombinant NPRA in COS-7 and HEK-293 cells, undergo rapid internalization in a ligand-dependent manner [13,15,20]. However, one report has indicated that ANP–NPRA complexes were not processed intracellularly in renomedullary interstitial cells, and a rapid dissociation of ligand–receptor complexes probably occurred on ANP binding at 37 °C [43]. It has also been suggested that a trace amount of bound 125I-ANP may be released from the receptor by neutral endopeptidases in neuroblastoma cells [44]. Studies in our laboratory using recombinant HEK-293 cells firmly established that ANP–NPRA complexes are rapidly internalized, sequestered into the intracellular compartments, and the degraded products are released into the culture medium [15]. After treatment of HEK-293 cells by ANP for 60 min at 37 °C, the 125I-ANP-binding sites were decreased by almost 68% in cells expressing wild-type NPRA and by 30–38% in cells expressing G920A, Y923A and GDAY/AAAA mutant receptors. Our results provide direct evidence that treatment of cells with unlabelled ANP accelerated the disappearance of surface receptors, indicating that ANP-dependent down-regulation of NPRA involves the internalization of ligand–receptor complexes and the mutant receptors were relatively more resistant to ligand-mediated down-regulation. An invaluable experimental paradigm in the elucidation of ligand-dependent desensitization of NPRA would be the use of ANP-induced receptor down-regulation. Desensitization and/or inactivation of NPRA have been suggested to occur by mechanisms involving ANP-dependent dephosphorylation [45]. However, the exact mechanisms involving dephosphorylation-dependent inactivation/desensitization of NPRA remain to be established. On the other hand, it is anticipated that desensitization of NPRA might occur intracellularly, and blockade of internalization should prevent this process.
Previously, it has been suggested that ligand-dependent endocytosis and sequestration of NPRA may involve a series of sequential sorting steps through which ligand–receptor complexes could eventually be degraded, the receptor recycled back to the plasma membrane and the ligand be released intact into the cell exterior [15]. Recycling of endocytosed receptor to the plasma membrane and the release of intact ANP into the cell exterior occur at the same time as the processes leading to the degradation of most of the ligand–receptor complexes into lysosomes. Results of the present study suggest that a return in 125I-ANP binding in trypsin-treated cells implicate that 125I-ANP binding in trypsin-treated cells is due to recycling of NPRA. However, it should be noted that HEK-293 cells expressing G920A and Y923A mutant receptors exhibited almost 35% decreased 125I-ANP binding after trypsin treatment when compared with cells expressing wild-type NPRA. On the other hand, cells expressing D921A or triple-mutant GDAY/AAAA receptor showed more than 60% decrease in 125I-ANP binding after trypsin treatment when compared with wild-type receptor. The present study provides evidence that, after trypsin treatment, the greater return of 125I-ANP binding in recombinant wild-type HEK-293 cells is due to a large population of internalized receptors and subsequent recycling to the plasma membrane when compared with cells expressing mutant receptors.
In summary, our results demonstrate that internalization and recycling of NPRA require the GDAY sequence and provide evidence that this tetrapeptide sequence functions as a dual signal motif for both rapid internalization and recycling of NPRA. Although the GDAY signal motif seems to be necessary for rapid internalization and trafficking of NPRA, it is probably not sufficient, since the triple mutation of GDAY to AAAA did not completely block the internalization or recycling of ligand–receptor complexes. Nevertheless, the precise mechanism(s) for ligand-dependent endocytosis and intracellular routing of NPRA and involvement of adaptor proteins in this process remain to be determined. Furthermore, the connection between the GDAY motif and other internalization signals and, in particular, its role in the internalization of other receptors, remain to be established.
Acknowledgments
We thank Dr G. D. Sharma and Dr S.-J. Shi for their assistance during the initial ligand–receptor binding studies of this work. We also thank Mrs K. Pandey for her help in preparing this paper. This work was supported by grant no. HL57531 from the National Institutes of Health.
References
- 1.Rosenzweig A., Seidman C. E. Atrial natriuretic factor and related peptide hormones. Annu. Rev. Biochem. 1991;60:229–255. doi: 10.1146/annurev.bi.60.070191.001305. [DOI] [PubMed] [Google Scholar]
- 2.de Bold A. J. Atrial natriuretic factor: a hormone produced by the heart. Science. 1985;230:767–770. doi: 10.1126/science.2932797. [DOI] [PubMed] [Google Scholar]
- 3.Brenner B. M., Ballerman B. J., Gunning M. E., Zeidel M. L. Diverse biological actions of atrial natriuretic peptide. Physiol. Rev. 1990;70:665–699. doi: 10.1152/physrev.1990.70.3.665. [DOI] [PubMed] [Google Scholar]
- 4.Pandey K. N. Vascular action: natriuretic peptide receptors. In: Sowers J. R., editor. Contemporary Endocrinology: Endocrinology of the Vasculature. Totawa, NJ: Humana Press; 1996. pp. 255–267. [Google Scholar]
- 5.Levin E. R., Gardner D. G., Samson W. K. Natriuretic peptides. N. Engl. J. Med. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 6.Pandey K. N., Singh S. Molecular cloning and expression of murine guanylate cyclase/atrial natriuretic factor receptor cDNA. J. Biol. Chem. 1990;265:12342–12348. [PubMed] [Google Scholar]
- 7.Garbers D. L. Guanylyl cyclase receptors and their endocrine, paracrine and autocrine ligands. Cell (Cambridge, Mass.) 1992;71:1–4. doi: 10.1016/0092-8674(92)90258-e. [DOI] [PubMed] [Google Scholar]
- 8.Drewett J. G., Garbers D. L. The family of guanylyl cyclase receptors and their ligands. Endocr. Rev. 1994;15:135–188. doi: 10.1210/edrv-15-2-135. [DOI] [PubMed] [Google Scholar]
- 9.Pandey K. N. Kinetic analysis of internalization, recycling and redistribution of atrial natriuretic factor-receptor complex in cultured vascular smooth muscle cells: ligand-dependent receptor down-regulation. Biochem. J. 1992;288:55–61. doi: 10.1042/bj2880055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anand-Srivastava M. B., Trachte G. J. Atrial natriuretic factor receptor and signal transduction mechanisms. Pharmacol. Rev. 1993;45:455–497. [PubMed] [Google Scholar]
- 11.Lucas K. A., Pitari G. M., Dazerounian S., Ruiz-Stewart I., Park J., Schulz S., Chepenic K. P., Waldman S. A. Guanylyl cyclase and signaling by cyclic-GMP. Pharmacol. Rev. 2000;52:375–413. [PubMed] [Google Scholar]
- 12.Sharma R. K. Evolution of membrane guanylate cyclase transduction system. Mol. Cell. Biochem. 2002;230:3–30. [PubMed] [Google Scholar]
- 13.Pandey K. N. Stoichiometric analysis of internalization, recycling and redistribution of photoaffinity labeled guanylyl cyclase/atrial natriuretic factor receptor-A in cultured murine Leydig tumor cells. J. Biol. Chem. 1993;268:4382–4390. [PubMed] [Google Scholar]
- 14.Cao L., Wu J., Gardner D. G. Atrial natriuretic peptide suppresses the transcription of its guanylyl cyclase-linked receptor. J. Biol. Chem. 1995;270:248291–248297. doi: 10.1074/jbc.270.42.24891. [DOI] [PubMed] [Google Scholar]
- 15.Pandey K. N., Nguyen H. T., Sharma G. D., Shi S.-H., Kriegel A. M. Ligand-regulated internalization, trafficking, and down-regulation of guanylyl cyclase/atrial natriuretic peptide receptor-A in human embryonic kidney 293 cells. J. Biol. Chem. 2002;277:4618–4627. doi: 10.1074/jbc.M106436200. [DOI] [PubMed] [Google Scholar]
- 16.Oliver P. M., Fox J. E., Kim R., Rockman H. A., Reddick R. L., Pandey K. N., Milgram S. L., Smithies O., Maeda N. Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor-A. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14730–14735. doi: 10.1073/pnas.94.26.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi S.-J., Vellaichamy E., Chin S.-Y., Smithies O., Navar L. G., Pandey K. N. Natriuretic peptide receptor A mediates renal sodium excretory responses to blood volume expansion. Am. J. Physiol. Renal Physiol. 2003;285:F694–F702. doi: 10.1152/ajprenal.00097.2003. [DOI] [PubMed] [Google Scholar]
- 18.van den Akker F., Zhang X., Myagi M., Huo X., Misono K. S., Yee V. C. Structure of the dimerized hormone-binding domain of a guanylyl cyclase-coupled receptor. Nature (London) 2000;406:101–104. doi: 10.1038/35017602. [DOI] [PubMed] [Google Scholar]
- 19.Pandey K. N., Inagami T., Misono K. S. Atrial natriuretic factor receptor on cultured Leydig tumor cells: ligand binding and photoaffinity labeling. Biochemistry. 1986;25:8467–8472. doi: 10.1021/bi00374a022. [DOI] [PubMed] [Google Scholar]
- 20.Rathinavelu A., Isom G. E. Differential internalization and processing of atrial natriuretic factor B and C receptors in PC-12 cells. Biochem. J. 1991;276:493–497. doi: 10.1042/bj2760493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandey K. N., Kumar R., Li M., Nguyen H. Functional domains and expression of truncated atrial natriuretic peptide receptor-A: the carboxyl-terminal regions direct the receptor internalization and sequestration in COS-7 cells. Mol. Pharmacol. 2000;57:259–267. [PubMed] [Google Scholar]
- 22.Nussenzveig D. R., Heinflink M., Gershengorn M. C. Agonist-stimulated internalization of the thyrotropin-releasing hormone receptor is dependent on two domains in the receptor carboxyl terminus. J. Biol. Chem. 1993;268:2389–2392. [PubMed] [Google Scholar]
- 23.Chen W. J., Goldstein J. L., Brown M. S. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J. Biol. Chem. 1990;265:3116–3123. [PubMed] [Google Scholar]
- 24.Thies R. S., Webster N. J., McClain D. A. A domain of the insulin receptor required for endocytosis in the rat fibroblasts. J. Biol. Chem. 1990;265:10132–10137. [PubMed] [Google Scholar]
- 25.Backer J. M., Kahn C. R., Cahill D. A., Ullrich A., White M. F. Receptor-mediated internalization of insulin requires a 12-amino acid sequence in the juxtamembrane region of the insulin receptor β subunit. J. Biol. Chem. 1990;265:16450–16454. [PubMed] [Google Scholar]
- 26.Lobel P., Fujimoto K., Ye R. D., Griffiths G., Kornfeld S. Mutations in the cytoplasmic domain of the 275 kd mannose 6-phosphate receptor differentially alter lysosomal enzyme sorting and endocytosis. Cell (Cambridge, Mass.) 1989;57:787–796. doi: 10.1016/0092-8674(89)90793-9. [DOI] [PubMed] [Google Scholar]
- 27.Jing S., Spencer T., Miller K., Hopkins C., Trowbridge I. S. Role of the human transferrin receptor cytoplasmic domain in endocytosis: localization of a specific signal sequence for internalization. J. Cell Biol. 1990;110:283–294. doi: 10.1083/jcb.110.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajagopalan M., Neidigh J. L., McClain D. A. Amino acid sequence Gly-Pro-Leu-Tyr and Asn-Pro-Glu-Tyr in the submembranous domain of the insulin receptor are required for normal endocytosis. J. Biol. Chem. 1991;266:23068–23073. [PubMed] [Google Scholar]
- 29.Chen Z., Dupre D. J., LeGouill C., Rola-Pleszczynski M., Stankova J. Agonist-induced internalization of the platelet-activating factor receptor is dependent on arrestins but independent of G-protein activation. Role of the C-terminus and (D/N) PXXY motif. J. Biol. Chem. 2002;277:7356–7362. doi: 10.1074/jbc.M110058200. [DOI] [PubMed] [Google Scholar]
- 30.Bouley R., Sun T. X., Chenard M., McLaughlin M., McKee M., Lin H. Y., Brown D., Ausiello D. A. Functional role of the NPxxY motif in internalization of the type 2 vasopressin receptor in LLC-PK1 cells. Am. J. Physiol. 2003;285:C750–C762. doi: 10.1152/ajpcell.00477.2002. [DOI] [PubMed] [Google Scholar]
- 31.Collawn J. F., Stangel M., Kuhn L. A., Esekogwu V., Jing S., Trowbridge I. S., Tainer J. A. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell (Cambridge, Mass.) 1990;63:1061–1072. doi: 10.1016/0092-8674(90)90509-d. [DOI] [PubMed] [Google Scholar]
- 32.Li Y., Marzolo M. P., van Kerkhof P., Strous G. J., Bu G. The YxxL motif, but not the two NPXY motifs, serve as the dominant endocytosis signal for low density lipoprotein receptor-related protein. J. Biol. Chem. 2000;275:17187–17194. doi: 10.1074/jbc.M000490200. [DOI] [PubMed] [Google Scholar]
- 33.Lazarovits J., Roth M. A single amino acid change in the cytoplasmic domain allows the influenza virus hemagglutinin to be endocytosed through coated pits. Cell (Cambridge, Mass.) 1988;53:743–752. doi: 10.1016/0092-8674(88)90092-x. [DOI] [PubMed] [Google Scholar]
- 34.Ktistakis N. T., Thomas D., Roth M. G. Characteristics of the tyrosine recognition signal for internalization of transmembrane surface glycoproteins. J. Cell Biol. 1990;111:1393–1407. doi: 10.1083/jcb.111.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collawn J. F., Kuhn L. A., Liu L.-F. S., Tainer J. A., Trowbridge I. S. Transplanted LDL and mannose-6-phosphate receptor internalization signals promote high-efficiency endocytosis of the transferrin receptor. EMBO J. 1991;10:3247–3253. doi: 10.1002/j.1460-2075.1991.tb04888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bansal A., Gierasch L. M. The NPXY internalization signal of the LDL receptor adopts a reverse-turn conformation. Cell (Cambridge, Mass.) 1991;67:1195–1201. doi: 10.1016/0092-8674(91)90295-a. [DOI] [PubMed] [Google Scholar]
- 37.Eberle W., Sander C., Klaus W., Schmidt B., von Figura K., Peters C. The essential tyrosine of the internalization signal in lysosomal acid phosphatase is part of a beta turn. Cell (Cambridge, Mass.) 1991;67:1203–1209. doi: 10.1016/0092-8674(91)90296-b. [DOI] [PubMed] [Google Scholar]
- 38.Barak L. S., Tiberi M., Freeman N. J., Kwatra M. M., Lefkowitz R. J., Caron M. G. A highly conserved tyrosine residue in G protein-coupled receptors is required for agonist-mediated beta 2-adrenergic receptor sequestration. J. Biol. Chem. 1994;269:2790–2795. [PubMed] [Google Scholar]
- 39.Gobilondo A. M., Krasel C., Lohse M. J. Mutations of Tyr326 in the β2-adrenoreceptor disrupt multiple receptor functions. Eur. J. Pharmacol. 1996;307:243–250. doi: 10.1016/0014-2999(96)00247-6. [DOI] [PubMed] [Google Scholar]
- 40.Gage R. M., Kim K. A., Cao T. T., von Zastrow M. A transplantable sorting signal that is sufficient to mediate rapid recycling of G-protein-coupled receptors. J. Biol. Chem. 2001;276:44712–44720. doi: 10.1074/jbc.M107417200. [DOI] [PubMed] [Google Scholar]
- 41.Kishi M., Liu X., Hirakawa T., Reczek D., Bretscher A., Ascoli M. Identification of two distinct structural motifs that when added to the C-terminal tail of the rat LH receptor, redirect the internalized hormone-receptor complex from a degradation to a recycling pathway. Mol. Endocrinol. 2001;15:1624–1635. doi: 10.1210/mend.15.9.0698. [DOI] [PubMed] [Google Scholar]
- 42.Sorkin A., von Zastrow M. Signal transduction and endocytosis: close encounters of many kinds. Mol. Cell. Biol. 2002;3:600–614. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- 43.Koh G. Y., Nussenzweig D. R., Okolicany J., Price D. A., Maack T. Dynamics of atrial natriuretic factor-guanylate cyclase receptors and receptor-ligand complexes in cultured glomerular mesangial and renomedullary interstitial cells. J. Biol. Chem. 1992;267:11987–11994. [PubMed] [Google Scholar]
- 44.Delporte C., Poloczek P., Tastenoy M., Winard J., Christopher J. Atrial natriuretic peptide binds to ANP-R1 receptors in neuroblastoma cells is degraded extracellularly at the Ser-Phe bond. Eur. J. Pharmacol. 1992;227:247–256. doi: 10.1016/0922-4106(92)90002-d. [DOI] [PubMed] [Google Scholar]
- 45.Potter L. R., Garbers D. L. Protein kinase C-dependent desensitization of the atrial natriuretic peptide receptor is mediated by dephosphorylation. J. Biol. Chem. 1994;269:14636–14642. [PubMed] [Google Scholar]