Abstract
Transient tachypnea of newborn (TTN) results from failure of the newborn to effectively clear the fetal lung fluid soon after birth. TTN represents the most common etiology of respiratory distress in term gestation newborns and sometimes requires admission to the neonatal intensive care unit. TTN can lead to maternal-infant separation, the need for respiratory support, extended unnecessary exposure to antibiotics and prolonged hospital stays. Recent evidence also suggests that TTN may be associated with wheezing syndromes later in childhood. New imaging modalities such as lung ultrasound can help in the diagnosis of TTN and early management with distending pressure using continuous positive airway pressure may prevent exacerbation of respiratory distress.
Introduction
The immediate survival of a newly born infant following separation from the placenta depends on the lungs for gas exchange. In preparation for birth, the newborn must undergo various dynamic and complex changes to ensure a smooth transition to extrauterine life. For the lungs to allow for adequate ventilation and oxygenation, several steps must first occur: (1) establishment of continuous breathing, (2) alveolar distension, (3) clearance of lung fluid, (4) secretion of surfactant, (5) fall in pulmonary vascular resistance and an increase in pulmonary blood flow, and (6) cessation of right to left shunting at the atrial and ductal levels followed by closure of the ductus arteriosus [1, 2].
Failure of the newborn to effectively clear the fetal lung fluid soon after birth can lead to respiratory distress from retained fetal lung fluid, also referred to as Transient Tachypnea of Newborn (TTN)—to reflect that tachypnea is the most common clinical feature. In most cases, TTN is self-limited and resolves without the need for medical intervention. Nevertheless, TTN represents the most common etiology of respiratory distress in newborns at term gestation admitted to a neonatal intensive care unit (NICU) [3] and, rarely, can lead to hypoxic respiratory failure as a consequence of persistent pulmonary hypertension of the newborn (PPHN) [4]. Therefore, TTN may lead to increased monitoring, maternal–infant separation, the need for respiratory support, potentially unnecessary interventions, including antibiotic therapy and prolonged hospital stays [2]. At hospitals that do not provide IV fluid or gavage feeding support of newborns, the inability to feed orally due to respiratory distress itself may necessitate transfer and resultant separation of mother and newborn. An in-depth understanding of the wide spectrum of clinical presentation of TTN and its associated morbidities will allow clinicians to better identify newborns who may be at greatest risk to require respiratory support. This review focuses on the pathophysiology of TTN, current management strategies to prevent and treat TTN and the long-term outcomes of newborns diagnosed with TTN.
Fetal lung fluid and the first breaths
Fetal lung fluid is actively produced by the lungs rather than from amniotic fluid [5]. The ionic composition of lung fluid is higher in chloride (Cl−) and lower in sodium (Na+) and bicarbonate concentration compared to amniotic fluid. Lung fluid arises from active Cl− secretion (by type 2 pneumocytes) by the developing lung epithelium with concomitant passive flow of Na+ and water into the fetal alveolar space (Fig. 1) [6, 7]. Fetal lung fluid is critical for normal lung growth and function [8]. Studies in fetal lambs have shown that the amount of fetal lung fluid is directly proportional to lung growth and development. Drainage of lung fluid in fetal lambs resulted in significant pulmonary hypoplasia while obstruction of outflow of lung fluid resulted in pulmonary hyperplasia and hyperexpansion [9].
Fig. 1. Illustration detailing mechanisms of lung fluid secretion and clearance during fetal gestation and after birth.
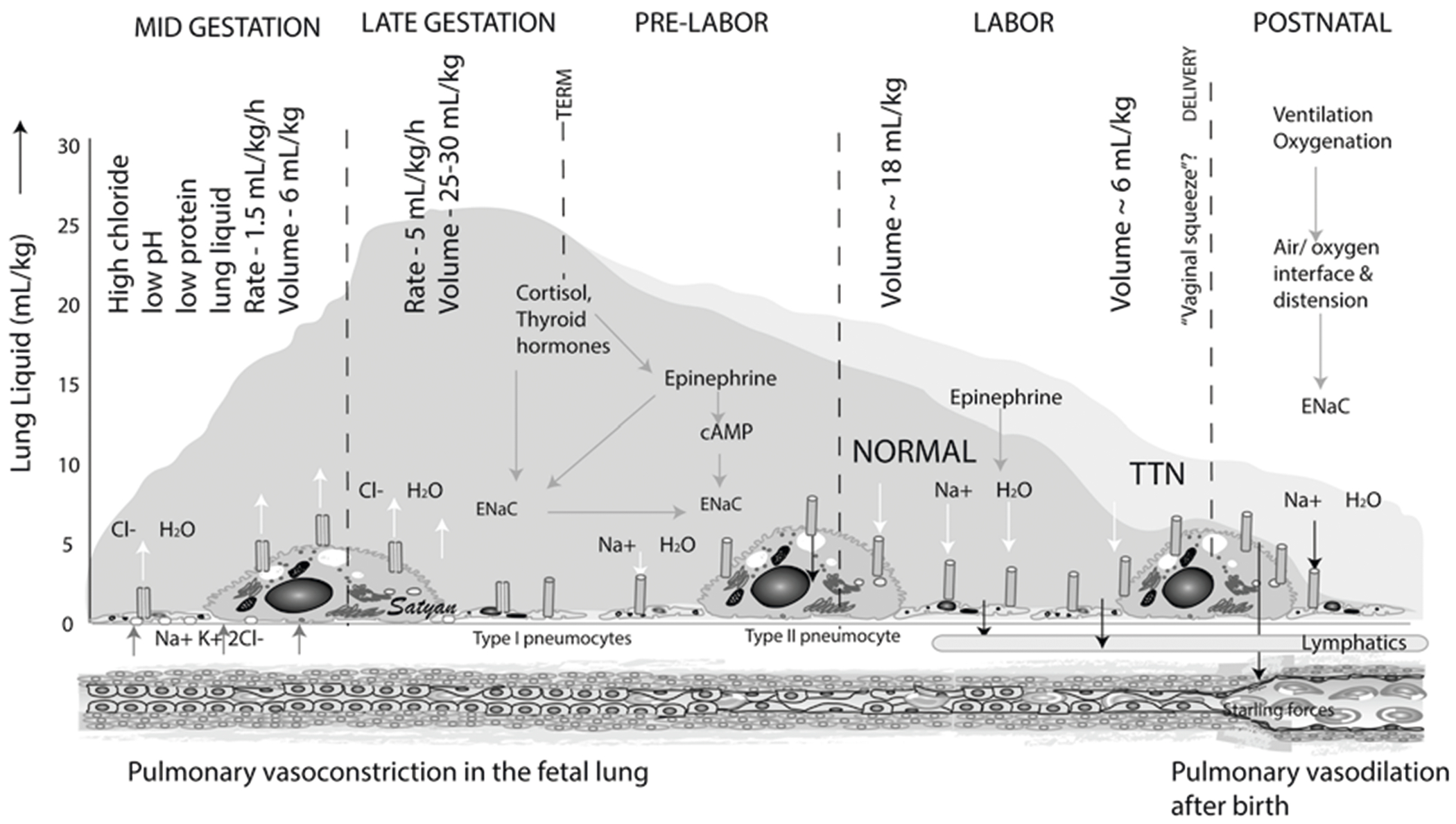
During fetal gestation, type 2 alveolar pneumocytes actively secrete chloride (Cl−) into the alveolar space. Sodium (Na+) and water passively accompany Cl−. The fluid secretion peaks at 5 ml/kg/h at a maximum volume of 25–30 ml/kg in late gestation. During labor, epithelial sodium channels (ENaC) become activated by adrenergic stimulation. Basolateral Na+/K+ ATPase helps move Na+ into the interstitium along with Cl− and water. Most interstitial lung liquid moves into the pulmonary circulation; some drains via the lung lymphatics. The darker blue hue represents normal vaginal delivery and the lighter hue represents delayed fluid resorption in TTN. Modified from Pathophysiology of fetal lung development by Mathew et al. in Essentials of Neonatal Edition (Elsevier); Copyright Satyan Lakshminrusimha.
Fetal lung fluid production increases from about 1.5 ml/kg/h in mid-gestation to 5 ml/kg/h near term to reach fetal lung volumes of 25–30 ml/kg, approximating the functional residual capacity (FRC) of a term newborn (Figs. 1 and 2) [10, 11]. The distending pressure provided by the fetal lung fluid, which is 1 to 2 mmHg greater relative to the amniotic fluid, is essential for normal lung development [12]. This pressure differential also results in lung fluid to be passively expelled from the trachea into the oropharynx, where it is either swallowed or expelled, thus contributing to the amniotic fluid [13].
Fig. 2. Airway liquid retention and role of respiration.
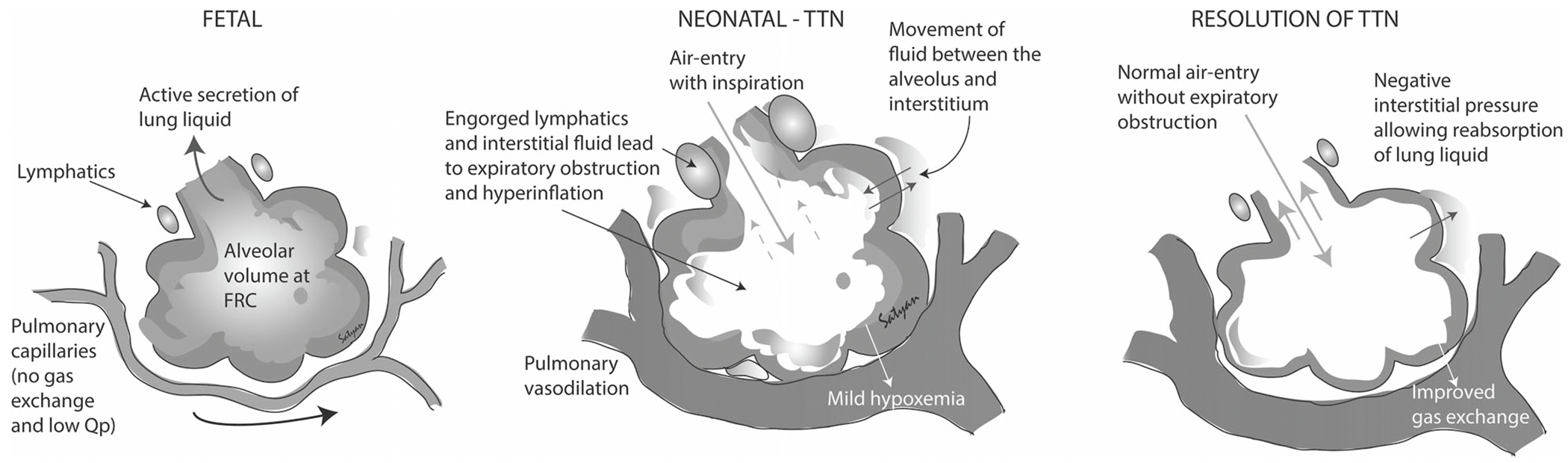
The fetal fluid-filled lungs do not participate in gas exchange but the lung volume approximates the functional residual capacity (FRC) of the airfilled lung after birth. Following delivery of the head and air breathing, the pressure from inspiration creates a pressure differential that promotes airway liquid to move into the lung tissue, which can raise interstitial pressure. A high interstitial pressure at end-expiration may shift fluid back into the alveoli. Copyright Satyan Lakshminrusimha.
From the onset of labor to the delivery of the newborn, ~100 ml of lung liquid needs to be cleared in a term newborn infant. The primary mechanism responsible for airway liquid clearance at birth is believed to result from Na+ uptake across the airway epithelium, which reverses the osmotic gradient leading to airway liquid reabsorption [14]. Toward the end of gestation, there is a surge of fetal glucocorticoids and thyroid hormones that activates the Na+ absorptive channels [15]. The stress of labor and birth results in the production of fetal epinephrine, which activates epithelial Na+ channels (ENaC) and reverses the process of lung fluid secretion to fluid absorption [16]. Aquaporin 4 and 5 (AQP4 and AQP5) water channels are expressed in type 1 alveolar pneumocytes and mediate the bulk of water transport across the apical membrane of alveolar epithelia [17]. AQP5 is the predominant water channel on type 1 alveolar cells. AQP1 is mainly located in pulmonary capillary endothelium (Fig. 1) [18]. AQP5 expression has been found to be higher in TTN patients when compared to controls. It is unclear whether this upregulation of APQ5 is a contributing factor to the development of TTN or a compensatory mechanism to aid in clearing alveolar lung fluid [19]. The mechanical squeeze during vaginal delivery also contributes to the expulsion of the fetal lung fluid, albeit to a lesser extent [20]. Most interstitial liquid moves into the pulmonary circulation and some drains via the lung lymphatics [21]. Following birth, the ongoing epinephrine surge and the abrupt rise in oxygen tension accelerate lung fluid absorption, and most of the lung liquid is cleared from the airways within 2–6 h [11, 22]. Nitric oxide (NO) which is produced from l-Arginine via the enzyme NO synthase (NOS) plays a vital role in regulating pulmonary vasomotor tone and pulmonary blood flow [23]. In fetal lambs, NO instillation into the fetal lung decreases lung liquid production [24]. Asymmetrical dimethylarginine (ADMA) is an endogenous analog of l-arginine, which directly inhibits NOS (Fig. 1). ADMA levels were found to be elevated in patients with TTN compared to control newborns. While the exact mechanism is unknown, the role of ADMA in the pathogenesis of TTN may be due to a combination of increased synthesis of ADMA via protein arginine methyltransferases and reduced breakdown of ADMA via dimethylarginine dimethylaminohydrolase [25].
TTN is thought to be influenced by the absence of the stress or hormonal influences of labor, which decreases Na+ reabsorption, resulting in fetal lung fluid retention [26]. As air can only enter the lungs once the newborn’s head is delivered and breathing starts, respiratory activity may play the final and possibly more significant role in airway liquid clearance [27, 28]. Recent evidence suggests that the pathophysiology associated with TTN may not be exclusively due to lung fluid retention but also as a result from the presence of higher lung fluid volumes at the onset of respiration after birth (Fig. 1) [29]. Greater volumes mean that more fluid must be contained within lung tissue following lung aeration, resulting in higher interstitial tissue pressures and a possibility of liquid pooling back into the airways when the lungs are at FRC [30]. Airway liquid clearance results from transepithelial pressure gradients generated during inspiration, which provides the pressure gradient for liquid to move across the epithelium into the surrounding lung tissue (Fig. 2). This has been shown in term newborn rabbits where pulmonary interstitial pressures increased initially from birth to 2 h, and became sub-atmospheric at 5 h favoring movement of the interstitial fluid into the pulmonary capillaries [31]. Premature rabbits also showed the same increase in interstitial pressures at 2 h, but their subsequent drop in interstitial pressures was significantly slowed in atelectatic regions, thereby promoting fluid movement from the circulation into interstitial space [32].
Additional studies in spontaneously breathing newborn rabbits using phase-contrast X-ray imaging have shown that lung aeration occurs almost entirely during inspiration with no liquid clearance occurring between breaths [27, 28]. At birth, a term infant can generate mean inspiratory pressures of approximately −50 cm H2O (range −28 to −105 cm H2O) to achieve an inspired volume of around 40 ml. The first breaths generate even higher expiratory pressures (mean 71 cm H2O; range 18–115 cm H2O) to facilitate air distribution within the lung and promote lung fluid clearance [33]. Furthermore, the newborn’s first breaths are characterized by short deep inspirations followed by prolonged expiratory phases through a partially closed larynx, known as expiratory braking, often observed during crying immediately after birth [34, 35]. Accordingly, newborns who have decreased respiratory effort at birth are at increased risk to develop TTN.
Epidemiology and risk factors
TTN was originally described in 1966 as the clinical manifestation of delayed clearance of fetal lung fluid [36]. TTN is the most common cause of respiratory distress in term and late-preterm infants with an estimated incidence of 4.0–5.7 per 1000 term births [37, 38]. TTN is estimated to occur in ~10% of 33–34 weeks gestation and 5% of 35–36 weeks gestation newborns [39]. The prevalence in premature infants is likely higher, although an accurate estimation is difficult because respiratory distress syndrome (RDS) and TTN can coexist in premature newborns [40, 41]. The Antenatal Late Preterm Steroids (ALPS) trial, a large multicenter, double-blind randomized controlled trial, examined the effects of antenatal betamethasone injection at 340/7 through 366/7 weeks gestation on pulmonary morbidity [42]. The primary outcome was a neonatal composite of need for respiratory support or neonatal death within 72 h of birth. There was a significant reduction in the primary outcome observed in the betamethasone (beta) treated group compared to the placebo group (165 of 1427 infants [11.6%] compared to 202 of 1400 [14.4%], respectively). In addition, severe respiratory complications, TTN, surfactant use, and bronchopulmonary dysplasia also occurred significantly less frequently in the beta group. In a similar study published before the ALPS, Stutchfield et al. randomized mothers undergoing elective Cesarian section (C-section) at 37–39 weeks to beta vs. placebo (no treatment) with a primary outcome of admission to a special care nursery for respiratory morbidity [43]. The overall rates of respiratory problems would be expected to be lower in this early term population, but were halved for those pretreated with betamethasone compared to control babies: the incidence of TTN was 4% in the control group and 2.1% in offspring from mothers treated with betamethasone prior to elective C-section delivery [43]. There is clear evidence that antenatal corticosteroids decrease the incidence of TTN, however, any potential long-term adverse effects of this intervention remain to be seen. In fact, this study suggested that if one waited until 39 weeks there was little need for the steroid pre-treatment and provides some evidence for targeting 39 weeks as a minimum for elective induction and delivery.
Cesarean deliveries are now well known to be associated with an increased occurrence of TTN. In addition, the absence of labor in infants born via elective C-section has a significant impact on respiratory morbidity. Infants born via elective C-section prior to the onset of labor have an incidence of respiratory morbidity of 3.55% compared to 1.22% among infants born via C-section following onset of labor. In contrast, infants born via vaginal delivery have the lowest incidence of respiratory morbidity at 0.53% [37]. Delaying elective C-section until after 39 weeks gestation has been shown to reduce respiratory morbidity, which led to the current recommendation by the American College of Obstetrics and Gynecology in 2013 to avoid nonmedically indicated vaginal or cesarean deliveries at less than 39 weeks gestation [3, 44, 45]. A study that looked at the cost analysis of an elective repeat cesarean delivery at 37–39 weeks gestation and neonatal outcomes found a fivefold increase in the mean cost at discharge due to the increased risk of developing TTN via elective C-section compared to vaginal births [46].
Maternal history of asthma, hypertension, and gestational diabetes have shown to be associated with an increased risk of developing TTN [37, 47]. Fetal risk factors include perinatal asphyxia, male sex, lower gestational age, small for gestational age, and macrosomia (Fig. 3) [41].
Fig. 3. Risk factors, symptoms and signs, management of transient tachypnea of the newborn and associated childhood respiratory complications.
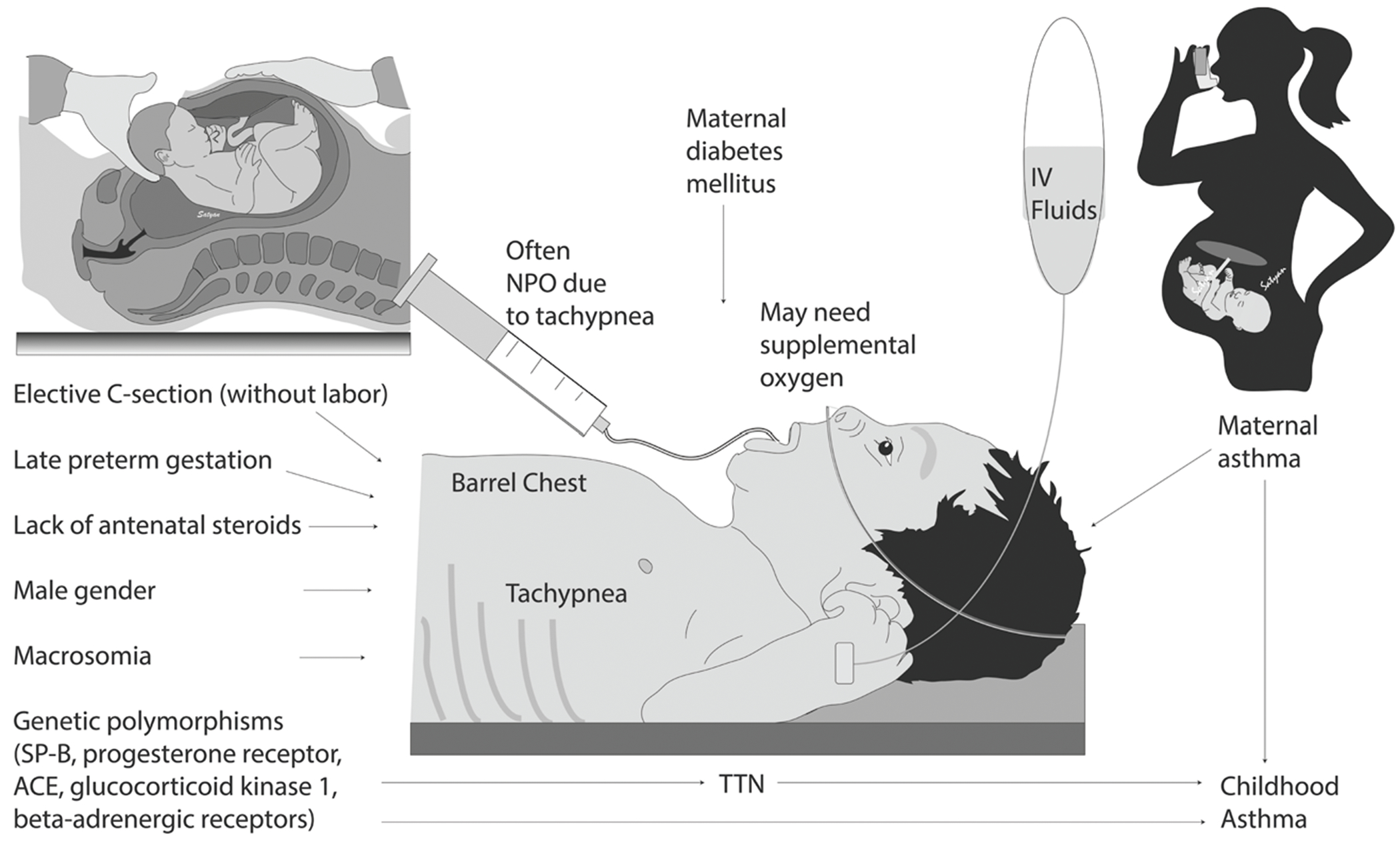
List of prenatal, maternal and infant risk factors of transient tachypnea of the newborn (TTN). Genetic polymorphisms and maternal asthma may increase risk of childhood asthma in newborns who have TTN.
Diagnosis and clinical features
TTN is a diagnosis of exclusion and cannot be made until other causes of respiratory distress have been ruled out. The infant’s clinical presentation, physical examination and chest X-ray are helpful in making a diagnosis of TTN. TTN tends to present shortly after birth with tachypnea, grunting, nasal flaring, retractions, and occasionally cyanosis. Typically, the tachypnea is between 60 and 80 breaths per minute, however, the degree of tachypnea can sometimes be substantial and exceed 80–100 breaths per minute. TTN can be associated with hyperinflation of the lungs, which may give the infant a barrel-shaped chest on physical exam (Fig. 3) [48].
Due to the nonspecific symptoms of TTN, if the newborn shows worsening respiratory distress, an alternative diagnosis needs to be considered. There are several causes of newborn respiratory distress other than TTN that can present at birth: (1) Aspiration (as a consequence of meconium stained amniotic fluid [or amniotic fluid by itself] or blood after a placental abruption), (2) Congential anomalies (e.g., congenital diaphragmatic hernia, congenital lobar emphysema, or cystic adenomatoid malformations), (3) HYaline membrane disease (also known as RDS or surfactant deficiency), (4) PNeumonia, Primary Ciliary Dyskinesia (5) Effusion, and (6) Air-leak syndromes (e.g., pneumothorax or pneumomediastium). The common causes of tachypnea can be remembered by using the mnemonic “TACHYPNEA” (Fig. 4). Primary ciliary dyskinesia (PCD) is rare but requires consideration because 75–85% of babies with PCD have a history of neonatal respiratory distress [49].
Fig. 4. Differential diagnosis of transient tachypnea of the newborn with associated roentgenograms.
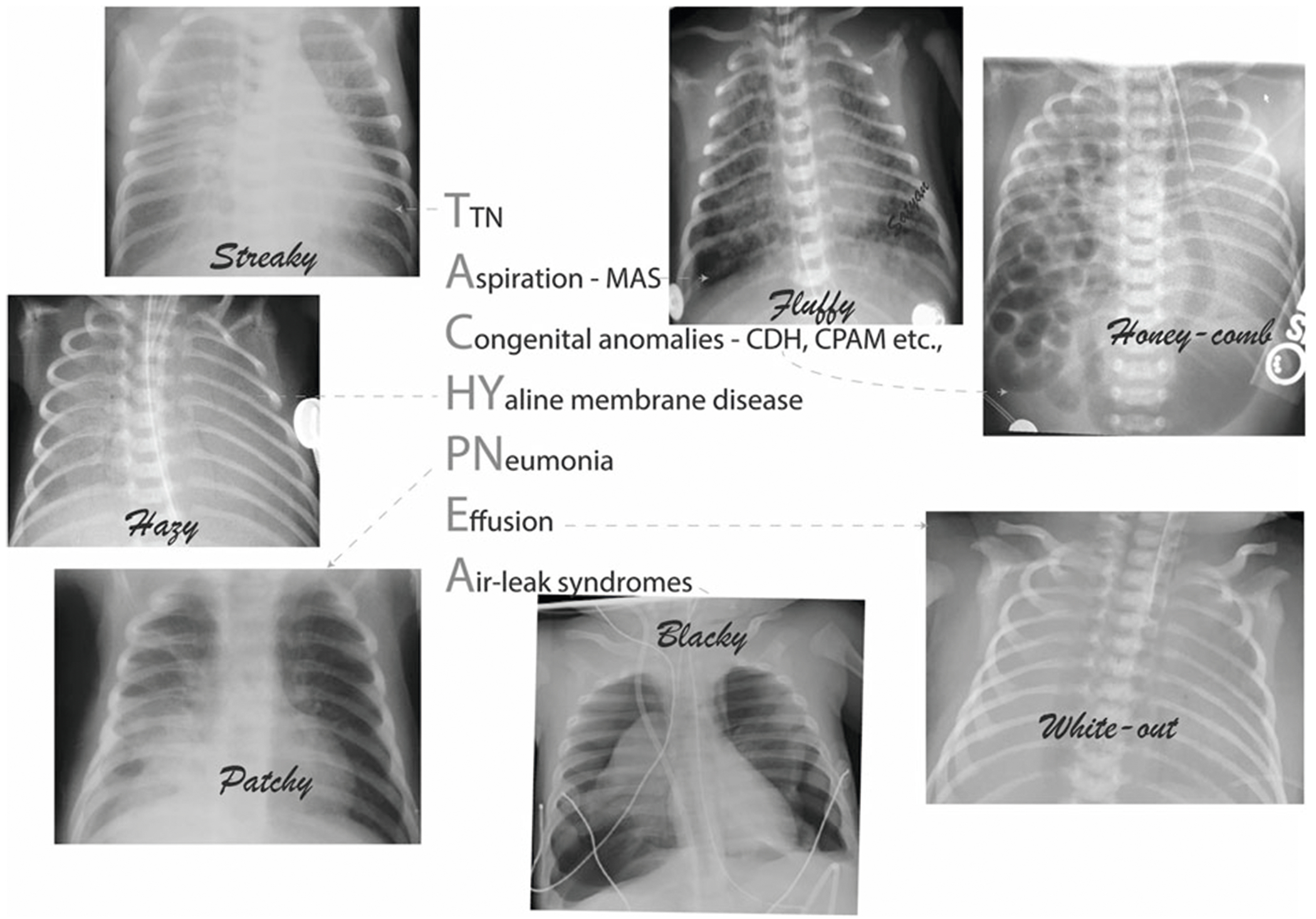
Differential diagnosis of tachypnea in the newborn can be remembered by using the mnemonic “TACHYPNEA” T: transient tachypnea of the newborn, A: Aspiration C: Congential anomalies, HY: Hyaline membrane disease, P: Pneumonia, Primary Ciliary Dyskinesia, E: Effusion, and A: Air-leak syndromes.
Imaging features
Retained lung fluid or TTN will cause engorgement of the lymphatics and capillaries. Chest radiography (X-ray) can reveal retained lung fluid by showing diffuse streaky pulmonary interstitial opacities, edema of the interlobar septae and fluid in the fissures (Fig. 5). The prominent perihilar pulmonary vascular markings observed on chest X-ray are sometimes referred to as a “sunburst” pattern. There may be some degree of hyperinflation and fluid may be seen at the costophrenic angles with widening of the intercostal spaces. One key feature of TTN is interval improvement of the radiographic imaging in 48–72 h, however, complete disappearance of radiographic findings may take up to 7 days [1, 48].
Fig. 5. X-ray findings of transient tachypnea of the newborn.
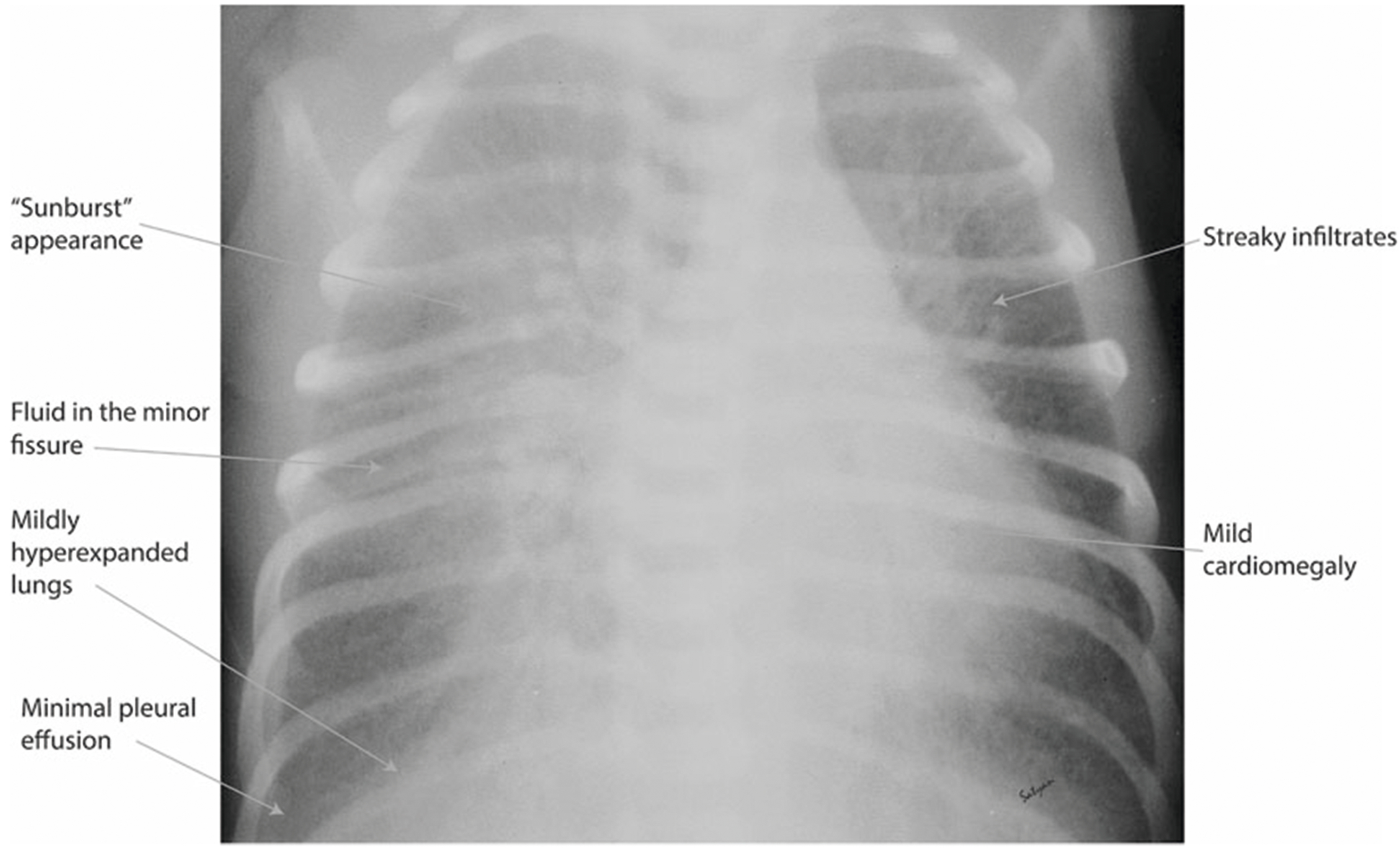
Retained lung fluid may result in diffuse streaky pulmonary interstitial opacities, and fluid in the minor fissures. Prominent perihilar pulmonary vascular markings observed are sometimes referred to as a “sunburst” pattern. There may be a degree of hyperinflation and pleural effusions that are usually small. Occasionally mild cardiomegaly can be seen.
Ultrasound of the lung has become an emerging method of diagnosing TTN (vs. other lung problems) and studies have shown lung ultrasound to be a reliable imaging modality (Fig. 6) [50–52]. A normal lung ultrasound characteristically shows hypoechogenic tissue with the presence of smooth pleural lines and A-lines in a normal-term infant without respiratory distress (Fig. 6a). The pleural line is a regular echogenic line under the skin and superficial layers of the thorax and moves continuously with respiration (lung-sliding). The A-lines are a series of echogenic lines parallel to the pleural line equidistant from one another below the pleural line. In patients with TTN, the following features are observed: thickening or fuzziness of the pleural line, partial or complete disappearance of A-lines, and appearance of B-lines (also known as lung comets—hyperechoic narrow-based artifacts spreading from the pleural line to the edge of the screen). Interstitial syndrome is the presence of more than 3 B-lines in every examined area and is observed in TTN (Fig. 6b). In addition, there is a difference in lung echogenicity between the superior fields and inferior fields on a longitudinal scan in approximately three-fourths of patients with TTN. This sharp cut-off point between the upper and lower lung fields is known as the double lung point (DLP) [52]. Neonates with RDS have “compact” or coalesced B-lines without horizontal reverberations resulting in “white lung,” consolidation and air bronchograms (Fig. 6c). DLP is usually not a feature of RDS. Potential limitations of lung ultrasonography include variance in sonographer skill and only being able to view lesions closest to the pleural surface [53]. Experienced neonatal lung ultrasonographers can distinguish between RDS and TTN and this could be a useful clinical tool as we gain experience with this modality.
Fig. 6. Lung ultrasound as a diagnostic tool for newborn lung pathology.
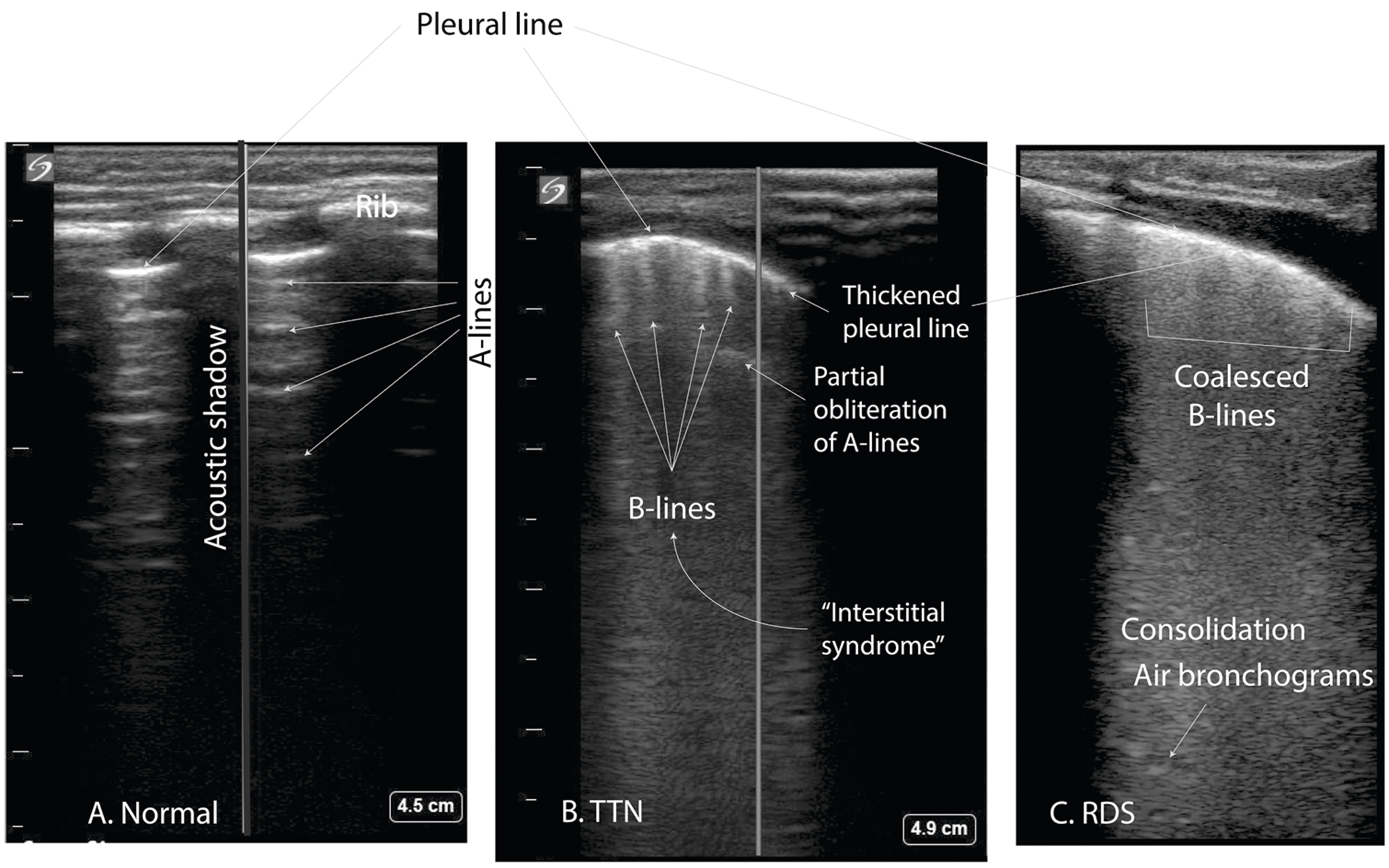
a Normal lung showing horizontal A-lines (reverberation artifact from pleural line). b In TTN, A-lines are obscured and B-lines are separated giving the appearance of comet tails. c Patient with respiratory distress syndrome (RDS) showing an irregular and thickened pleural line, consolidation, and so-called “coalesced B-lines:” referring to the inability to distinguish B-lines, absence of A-lines, making a “white lung” appearance. Ultrasound pictures provided by J. Lauren Ruoss, MD and colleagues I. Prelipcean and D. Rajderkar, University of Florida.
Management
Since the prenatal risk factors (C-section, maternal diabetes, macrosomia, family history of asthma) are widespread, most newborns at risk of developing TTN are delivered at hospitals without a higher level of NICU care available. Given the limited resources, newborns with TTN who require respiratory support often need to be transferred to a higher level of care, which leads to separation of the mother from her newborn. The availability of a therapy to improve the natural course of TTN, reduce the need for respiratory support and need for intensive care would be advantageous.
Diuretic therapy has been studied as a potential medication to aid in lung fluid clearance. A systematic review including two randomized trials [54, 55] involving 100 patients comparing furosemide with placebo in newborns with TTN did not show a change in duration of hospital stay or severity of symptoms [56]. Inhaled β-agonists have been hypothesized to stimulate lung fluid absorption by up regulating the ion channels involved in lung fluid resorption. Initially, some small studies showed improvement of respiratory symptoms and reduction in the duration of supplemental oxygen with the use of short acting inhaled β-agonists [57–59]. However, a recent systematic review concluded that there is insufficient evidence to determine the efficacy and safety of short acting β-agonists in the management of TTN [60]. To test the hypothesis that a relative deficiency in catecholamines may play a role in the etiology of TTN, a small randomized trial of 20 patients compared the effect of inhaled epinephrine vs. placebo on TTN resolution [61]. Inhaled epinephrine did not reduce the duration of treatment with oxygen or the need for respiratory support.
Finally, fluid restriction in patients with TTN has been shown to reduce the duration of symptoms and decrease the duration of respiratory support [62, 63]. Clinicians should be aware, however, that commonly practiced intravascular neonatal fluid administration may represent fluid overload relative to the enteral intake of the healthy breastfed infant (term newborns receive ~15 ml/kg of breast milk in the first couple of days) [64]. Larger scale clinical trials are needed before fluid restriction becomes fully implemented into practice. We are continuing to learn more about the fundamental mechanisms involved in control of lung fluid absorption; for example, it is possible that the natriuretic peptide system could play a role, particularly in interfacing with the β-adrenergic system [65–67]. These and other investigations may provide the therapeutic means to regulate the pathophysiology contributing to the clinical symptomatology of TTN.
The diagnosis of TTN is one of exclusion, which is based on a constellation of clinical symptoms and radiographic findings. The question of which infants will require diagnostic testing and when to transfer the newborn from a community hospital or newborn nursery to a level II or III NICU for further evaluation and management remains a difficult one to answer. Hein et al. [68] recommended the “rule of 2 h.” If the newborn continues to show symptoms of respiratory distress with no improvement in symptoms 2 h after birth, a chest radiograph should be obtained. Newborns need to be transferred to a higher level of care if symptoms worsen, respiratory support or oxygen administration is required to maintain oxyhemoglobin saturations between 90 and 95% or if the chest X-ray confirms a diagnosis other than TTN as the cause of respiratory distress.
Newborns with continued respiratory distress require continuous oxyhemoglobin saturation monitoring to assess need for supplemental oxygen, which should be provided if the SpO2 is <90% [69]. Neonates who show increased work of breathing and persistent tachypnea may require continuous positive airway pressure (CPAP) to maintain FRC and normal oxyhemoglobin saturations. Blood gas analysis will help determine ventilation status and guide escalation or weaning of respiratory support depending on the partial pressure of carbon dioxide. If the patient has tachypnea with respiratory rates (RR) over 65–80 breaths per minute, the newborn may be placed nil per os due to the risk of aspiration with oral feeds. During this time, gavage feedings, IV fluids or a combination of both may be started. Starting a term infant at 10% dextrose fluid via an IV line or oral feedings via a gavage tube at 60 ml/kg/day should be sufficient to maintain euglycemia. Many clinicians feel comfortable giving gavage feedings as long as there is not significant increased work of breathing and the RR remains <80. If the infant remains on IV hydration after the first 24 h of life, then electrolytes will need to be added to the IV fluids. Most cases of TTN will resolve within 48 h but if tachypnea persists, echocardiography should be considered to rule out congenital heart disease.
If infection is suspected due to clinical symptoms and risk factors, with signs of systemic illness rather than simple tachypnea, then a complete blood count, C-reactive protein, and blood culture should be drawn. Routine use of empiric antibiotics in TTN may be detrimental, resulting in alterations in gut microflora and an increase in antibiotic resistant organisms [70]. Empiric antibiotics may be considered in patients with prolonged tachypnea and systemic signs of illness. If the newborn has signs of hypotension and/or encephalopathy, a lactate and ammonia level may be done to help rule out inborn errors of metabolism. Central lines may be placed if the patient is mechanically ventilated, has an oxygen requirement >40%, or is hypotensive and requires vasoactive medications.
There have been cases in which TTN resulted in respiratory failure and death. Keszler et al. found that babies with TTN are overrepresented in the extracorporeal membrane oxygenation (ECMO) population [71]. “Malignant TTN” has been used to describe severe respiratory morbidity and subsequent mortality in newborns delivered by elective cesarean delivery who developed PPHN [71]. This is thought to develop due to a combination of two factors, one of which is absorption atelectasis. When delivering high oxygen concentrations, nitrogen is replaced by oxygen. In contrast to nitrogen, oxygen is extremely soluble in blood and diffuses very rapidly into the pulmonary vasculature, which leads to insufficient amounts of gas inside the alveoli to maintain patency and leads to alveolar collapse [72]. The second contributing factor is the formation of reactive oxygen species as a result of high alveolar oxygen exposure, which can lead to increased pulmonary vascular reactivity and PPHN [73].
One possible strategy when managing these newborns may be early use of distending pressure (CPAP) [4]. Osman et al. [74] performed a randomized control trial to examine the efficacy of delivering early CPAP via Neopuff in reducing the severity and duration of respiratory distress in TTN. The trial was performed in late-preterm/term infants who were delivered by C-section and who presented with respiratory distress shortly after birth. In the group that received CPAP, the duration of tachypnea was shorter and treatment resulted in fewer admissions to the NICU. The use of early prophylactic CPAP in late-preterm/term newborns delivered by C-section has also been shown to decrease NICU admissions: 259 eligible newborns (340/7–386/7 gestation) born via cesarean delivery were randomized to receive 20 min of prophylactic CPAP (n = 134) or no treatment (n = 125) [75]. Thereafter, newborns were observed for respiratory distress over a 2-h period. By the end of the observation period, 20/134 (14.9%) of the newborns in the intervention group required ongoing respiratory support compared to 31/125 (24.8%; p = 0.046) in the control group. Four of 134 (3%) compared to 11/125 (8.8%; p = 0.045) required admission to the NICU, respectively. Of the 15 infants admitted, 7/259 (2.7%) were diagnosed with TTN: 1 infant (0.7%) with TTN was in the intervention group, 6 (4.8%) in the control group (p = 0.059) and the remaining 8 infants were diagnosed with delayed transition [75].
Long-term outcomes and the association with asthma
As the name implies, the transient nature of the symptomatic phase of TTN usually lasts for a few days in the immediate newborn period. There are some studies that have evaluated the long-term outcomes in children who had history of TTN in the newborn period, which may suggest that the mechanisms involved in the pathogenesis of TTN may have long-term implications beyond the newborn period. While there are no studies that have delved into the mechanism of these long-term respiratory morbidities, there have been several studies reporting an association between TTN and respiratory symptoms in later childhood. Shohat et al. [76] reported home follow-up data for 67 children between the ages of 4 and 5 years of age who had a history of TTN and compared them to a similar number of age-matched controls that were born at the same time at a single center in Israel. They found a higher incidence of recurrent wheezing (p < 0.02) and asthma (p < 0.03) with atopy in the TTN group. Using the health claims database for Manitoba, Schaubel et al. [77] evaluated the impact of neonatal history on asthma-related hospitalizations in the 0–4 year age group. In addition to other risk factors such as low birth weight (<1500 g), male gender and prematurity, they also noted TTN in the newborn period to be associated with development of asthma (odds ratio =;1.36). While these odds were not higher than some of the other risk factors, other studies reported in different populations have found a similar association. A study of hospital discharge records from Scotland [78] assessed the diagnosis of asthma at the time of discharge and, after tracing back their neonatal records, found a higher cumulative incidence of asthma in children with history of respiratory morbidity in the neonatal period (hazard ratio = 1.7, 95% CI 1.4–2.2). Two large population database studies reported the association of TTN with childhood asthma. A study by Birnkrant et al. [79] that looked at a directory of 18,379 term infants from which 2137 patients with a known diagnosis of asthma were compared to matched controls demonstrated that TTN was associated with childhood asthma [adjusted odds ratio (OR) = 1.50, 95% confidence interval (CI): 1.13–1.99; P = 0.0052]. This association of asthma and TTN was found to be more pronounced among male infants, non-white males, males whose mothers lived in an urban area and males whose mothers did not have asthma [79]. The authors proposed that TTN may be a marker of deficient pulmonary function reflecting inherited susceptibility to asthma. Another retrospective review identified 12,763 babies born at term in 1995 in Manitoba and specifically looked at children diagnosed with a wheezing syndrome (defined as bronchiolitis, acute bronchitis, chronic bronchitis, asthma, or prescription for asthma medication) up to age 7 years in this cohort [80]. Three hundred and eight of these infants (2.4%) had a diagnosis of TTN at birth. Risk factors that were identified for the development of TTN included male sex, urban location, birth weight ≥4500 g and maternal history of asthma. In addition, infants with the diagnosis of TTN at birth had a statistically significant risk of developing a wheezing disorder in childhood (adjusted hazard ratio [HR] = 1.17, 95% CI 1.02–1.34) [80]. A retrospective analysis by Cakan et al. [81] of all the births at a Turkish hospital over a 1-year period identified 103 children with a history of TTN, and 33 had onset of wheezing between 1 month and 1 year of age. Only ten among this group had three or more wheezing attacks reported and no additional follow-up beyond 1 year of age was available in this study. A historical cohort study from Iran [82] followed 70 infants with history of TTN 4 years later with a telephone follow-up to determine if they had any long-term respiratory symptoms. In comparison to a similar number of controls, they found higher incidence of wheezing in children with a history of TTN (RR = 2.8, p = 0.014) even though the incidence of asthma was similar in both groups. Interestingly, there are some studies that have shown a higher incidence of TTN in infants born to mothers with asthma [83–85]. Schatz et al. [84] first suggested that babies born to mothers with asthma are at higher risk for development of TTN, but the mechanism for this association was uncertain. Another retrospective review of mothers with asthma complicating their pregnancy (n = 2289) in comparison to a fourfold larger randomly selected control group of non-asthmatic mothers (n = 9156) showed that infants of asthmatic mothers were more likely [OR, 1.79; 95% CI, 1.35–2.37] than infants of control mothers to exhibit TTN [83]. Furthermore, stratified analysis by sex and gestational age showed significant associations in male infants (OR, 1.91; 95% CI, 1.35–2.71) as opposed to female infants (OR, 1.51; 95% CI, 0.92–2.47) and in term infants (OR, 2.02; 95% CI, 1.42–2.87) as opposed to preterm infants (OR, 1.51; 95% CI, 0.94–2.43) [83]. This could suggest that the association between the development of asthma later in life and TTN may not arise from TTN itself, but because from other genetic determinants that are common to the pathogenesis of TTN and asthma. In other words, maternal asthma may be associated with these infants having a higher risk of TTN as well as a higher risk of asthma later in life. The genetic factors that determine this risk remain unidentified but there are several potential gene polymorphisms [86–90] that have been reported (Fig. 3) in association with TTN. Of these, the polymorphisms in the beta adrenergic receptor (ADRB) might be one of the most promising connections. This is because genetic polymorphisms in ADRB genes have been linked to asthma [91], but there are a lot of variations between different populations [92]. Further mechanisms involved in this complex interplay among maternal asthma, risk of TTN and the subsequent development of asthma later in life in children needs to be explored further.
Conclusion
TTN is a respiratory disorder thought to be due to inadequate or delayed clearance of fetal lung fluid after birth. Failure to clear this fluid results in a clinical syndrome of respiratory distress with a broad differential diagnosis. The volume of lung liquid present in the airways at birth may exacerbate symptoms as some of the liquid in the interstitial lung tissue may re-enter the alveoli at the end of expiration. Therefore, targeting mechanisms to prevent re-entry of fluid into the lung, such as CPAP, are likely to improve respiratory function and prevent further worsening of TTN. Although TTN may seem to some as a “nuisance” disease, separation from mothers, need for NICU admission, and in some cases deterioration with hypoxemia and/or PPHN requiring ECMO can have significant consequences. Finally, there are epidemiological factors that lead us to associate TTN with later wheezing and asthma-related symptoms.
Acknowledgements
The authors would like to thank J.L. Ruoss, MD, I. Prelipcean, MD and D. Rajdekar from the University of Florida for their contribution of Fig. 6.
Conflict of interest
PV is supported by NIH grant (1R03HD09299-01). SL is supported by NIH grant (5R01HD072929-08). ZA, LG, and RMR have no financial relationships to disclose relevant to this article. This commentary does not contain a discussion of an unapproved/investigative use of a commercial product/device. The use of antenatal betamethasone, diuretics and beta-agonists are not approved by the FDA in the prevention or treatment of TTN.
Footnotes
Content specifications Understand the pathophysiology that underlines Transient Tachypnea of Newborn (TTN). Recognize the clinical manifestations of TTN and the imaging modalities that may help in the diagnosis of TTN. Understand the benefits in providing Continuous Positive Airway Pressure (CPAP) for TTN. Recognize the possible long-term association of TTN with wheezing-related syndromes.
Education gap It can be challenging to diagnose TTN and identify patients who may be at greatest risk of respiratory deterioration. Optimal management has not been established, and caring for patients with TTN, particularly at community hospitals, can be difficult. Increasing knowledge of the pathophysiology leads to improved therapeutic options.
Objectives Define the molecular mechanisms involved in lung fluid clearance. Identify the clinical symptoms and signs, radiologic findings and risk factors associated with TTN. List the differential diagnosis of TTN. Explain the different treatment options for TTN.
References
- 1.Guglani L, Lakshminrusimha S, Ryan RM. Transient tachypnea of the newborn. Pediatr Rev. 2008;29:e59–65. [DOI] [PubMed] [Google Scholar]
- 2.Jain L. Alveolar fluid clearance in developing lungs and its role in neonatal transition. Clin Perinatol. 1999;26:585–99. [PubMed] [Google Scholar]
- 3.Zanardo V, Simbi AK, Franzoi M, Solda G, Salvadori A, Trevisanuto D. Neonatal respiratory morbidity risk and mode of delivery at term: influence of timing of elective caesarean delivery. Acta Paediatr. 2004;93:643–7. [DOI] [PubMed] [Google Scholar]
- 4.Lakshminrusimha S, Keszler M. Persistent pulmonary hypertension of the newborn. Neoreviews. 2015;16:e680–e692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jost A, Policard A. Contribution experimentale à l’étude du développment prenatal du poumon chez le lapin. Arch Anat Micr. 1948;37:327–32. [Google Scholar]
- 6.Olver RE, Strang LB. Ion fluxes across the pulmonary epithelium and the secretion of lung liquid in the foetal lamb. J Physiol. 1974;241:327–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams FH, Fujiwara T, Rowshan G. The nature and origin of the fluid in the fetal lamb lung. J Pediatr. 1963;63:881–8. [DOI] [PubMed] [Google Scholar]
- 8.Joshi S, Kotecha S. Lung growth and development. Early Hum Dev. 2007;83:789–94. [DOI] [PubMed] [Google Scholar]
- 9.Moessinger AC, Harding R, Adamson TM, Singh M, Kiu GT. Role of lung fluid volume in growth and maturation of the fetal sheep lung. J Clin Investig. 1990;86:1270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klaus M, Tooley WH, Weaver KH, Clements JA. Lung volume in the newborn infant. Pediatrics. 1962;30:111–6. [PubMed] [Google Scholar]
- 11.Brown MJ, Olver RE, Ramsden CA, Strang LB, Walters DV. Effects of adrenaline and of spontaneous labour on the secretion and absorption of lung liquid in the fetal lamb. J Physiol. 1983;344:137–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vilos GA, Liggins GC. Intrathoracic pressures in fetal sheep. J Dev Physiol. 1982;4:247–56. [PubMed] [Google Scholar]
- 13.Harding R, Bocking AD, Sigger JN. Influence of upper respiratory tract on liquid flow to and from fetal lungs. J Appl Physiol. 1986;61:68–74. [DOI] [PubMed] [Google Scholar]
- 14.Olver RE, Walters DV, MW S. Developmental regulation of lung liquid transport. Annu Rev Physiol. 2004;66:77–101. [DOI] [PubMed] [Google Scholar]
- 15.Carlton D. Regulation of liquid secretion and absorption by the fetal and neonatal lung. In: Polin R, Fox W, Abman S, editors. Fetal and neonatal physiology, 4th edn, vol. 1. Philadelphia, PA, USA: Elsevier; 2011, p. 907–19. [Google Scholar]
- 16.Olver RE, Ramsden CA, Strang LB, Walters DV. The role of amiloride-blockable sodium transport in adrenaline-induced lung liquid reabsorption in the fetal lamb. J Physiol. 1986;376:321–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borgnia M, Nielsen S, Engel A, Agre P. Cellular and molecular biology of the aquaporin water channels. Annu Rev Biochem. 1999;68:425–58. [DOI] [PubMed] [Google Scholar]
- 18.Wittekindt OH, Dietl P. Aquaporins in the lung. Pflug Arch. 2019;471:519–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Marcoux MO, Gineste M, Vanpee M, Zelenina M, Casper C. Expression of water and ion transporters in tracheal aspirates from neonates with respiratory distress. Acta Paediatr. 2009;98:1729–37. [DOI] [PubMed] [Google Scholar]
- 20.te Pas AB, Davis PG, Hooper SB, Morley CJ. From liquid to air: breathing after birth. J Pediatr. 2008;152:607–11. [DOI] [PubMed] [Google Scholar]
- 21.Bland RD, Hansen TA, Hazinski TA, Haberkern CM, Bressack MA. Studies of lung fluid balance in newborn lambs. Ann N. Y Acad Sci. 1982;384:126–45. [DOI] [PubMed] [Google Scholar]
- 22.Barker PM, Gatzy JT. Effect of gas composition on liquid secretion by explants of distal lung of fetal rat in submersion culture. Am J Physiol. 1993;265:L512–517. [DOI] [PubMed] [Google Scholar]
- 23.Weinberger B, Heck DE, Laskin DL, Laskin JD. Nitric oxide in the lung: therapeutic and cellular mechanisms of action. Pharm Ther. 1999;84:401–11. [DOI] [PubMed] [Google Scholar]
- 24.Cummings JJ. Nitric oxide decreases lung liquid production in fetal lambs. J Appl Physiol. 1997;83:1538–44. [DOI] [PubMed] [Google Scholar]
- 25.Isik DU, Bas AY, Demirel N, Kavurt S, Aydemir O, Kavurt AV, et al. Increased asymmetric dimethylarginine levels in severe transient tachypnea of the newborn. J Perinatol. 2016;36:459–62. [DOI] [PubMed] [Google Scholar]
- 26.Jain L, Eaton DC. Physiology of fetal lung fluid clearance and the effect of labor. Semin Perinatol. 2006;30:34–43. [DOI] [PubMed] [Google Scholar]
- 27.Siew ML, Wallace MJ, Kitchen MJ, Lewis RA, Fouras A, Te Pas AB, et al. Inspiration regulates the rate and temporal pattern of lung liquid clearance and lung aeration at birth. J Appl Physiol. 2009;106:1888–95. [DOI] [PubMed] [Google Scholar]
- 28.Hooper SB, Kitchen MJ, Wallace MJ, Yagi N, Uesugi K, Morgan MJ, et al. Imaging lung aeration and lung liquid clearance at birth. FASEB J. 2007;21:3329–37. [DOI] [PubMed] [Google Scholar]
- 29.Hooper SB, Te Pas AB, Kitchen MJ. Respiratory transition in the newborn: a three-phase process. Arch Dis Child Fetal Neonatal Ed. 2016;101:F266–271. [DOI] [PubMed] [Google Scholar]
- 30.McGillick EV, Lee K, Yamaoka S, Te Pas AB, Crossley KJ, Wallace MJ, et al. Elevated airway liquid volumes at birth: a potential cause of transient tachypnea of the newborn. J Appl Physiol. 2017;123:1204–13. [DOI] [PubMed] [Google Scholar]
- 31.Miserocchi G, Poskurica BH, Del Fabbro M. Pulmonary interstitial pressure in anesthetized paralyzed newborn rabbits. J Appl Physiol. 1994;77:2260–8. [DOI] [PubMed] [Google Scholar]
- 32.Miserocchi G, Poskurica BH, del Fabbro M, Crisafulli B. Pulmonary interstitial pressure in premature rabbits. Respir Physiol. 1995;102:239–49. [DOI] [PubMed] [Google Scholar]
- 33.Vyas H, Field D, Milner AD, Hopkin IE. Determinants of the first inspiratory volume and functional residual capacity at birth. Pediatr Pulmonol. 1986;2:189–93. [DOI] [PubMed] [Google Scholar]
- 34.te Pas AB, Wong C, Kamlin CO, Dawson JA, Morley CJ, Davis PG. Breathing patterns in preterm and term infants immediately after birth. Pediatr Res. 2009;65:352–6. [DOI] [PubMed] [Google Scholar]
- 35.Karlberg P, Koch G. Respiratory studies in newborn infants. III. Development of mechanics of breathing during the first week of life. A longitud study . Acta Paediatr. 1962;135:121–9. [DOI] [PubMed] [Google Scholar]
- 36.Avery ME, Gatewood OB, Brumley G. Transient tachypnea of newborn. Possible delayed resorption of fluid at birth. Am J Dis Child. 1966;111:380–5. [DOI] [PubMed] [Google Scholar]
- 37.Ryan CA, Hughes P. Neonatal respiratory morbidity and mode of delivery at term: influence of timing of elective caesarean section. Br J Obstet Gynaecol. 1995;102:843–4. [DOI] [PubMed] [Google Scholar]
- 38.Kumar A, Bhat BV. Epidemiology of respiratory distress of newborns. Indian J Pediatr. 1996;63:93–98. [DOI] [PubMed] [Google Scholar]
- 39.Raju TN, Higgins RD, Stark AR, Leveno KJ. Optimizing care and outcome for late-preterm (near-term) infants: a summary of the workshop sponsored by the National Institute of Child Health and Human Development. Pediatrics. 2006;118:1207–14. [DOI] [PubMed] [Google Scholar]
- 40.Hillman NH, Kallapur SG, Jobe AH. Physiology of transition from intrauterine to extrauterine life. Clin Perinatol. 2012;39:769–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dani C, Reali MF, Bertini G, Wiechmann L, Spagnolo A, Tangucci M, et al. Risk factors for the development of respiratory distress syndrome and transient tachypnoea in newborn infants. Italian Group of Neonatal Pneumology. Eur Respir J. 1999;14:155–9. [DOI] [PubMed] [Google Scholar]
- 42.Gyamfi-Bannerman C, Thom EA, Blackwell SC, Tita AT, Reddy UM, Saade GR, et al. Antenatal betamethasone for women at risk for late preterm delivery. N. Engl J Med. 2016;374:1311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stutchfield P, Whitaker R, Russell I. Antenatal Steroids for Term Elective Caesarean Section Research T. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. BMJ. 2005;331:662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.American College of O, Gynecologists. ACOG committee opinion no. 559: cesarean delivery on maternal request. Obstet Gynecol. 2013;121:904–7. [DOI] [PubMed] [Google Scholar]
- 45.Tita AT, Landon MB, Spong CY, Lai Y, Leveno KJ, Varner MW, et al. Timing of elective repeat cesarean delivery at term and neonatal outcomes. N Engl J Med. 2009;360:111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson CJ, Villers MS, Johnson DD, Simpson KN. Timing of elective repeat cesarean delivery at term and neonatal outcomes: a cost analysis. Am J Obstet Gynecol. 2010;202:632 e631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Badran EF, Abdalgani MM, Al-Lawama MA, Al-Ammouri IA, Basha AS, Al Kazaleh FA, et al. Effects of perinatal risk factors on common neonatal respiratory morbidities beyond 36 weeks of gestation. Saudi Med J. 2012;33:1317–23. [PubMed] [Google Scholar]
- 48.Cleveland RH. A radiologic update on medical diseases of the newborn chest. Pediatr Radio. 1995;25:631–7. [DOI] [PubMed] [Google Scholar]
- 49.Mullowney T, Manson D, Kim R, Stephens D, Shah V, Dell S. Primary ciliary dyskinesia and neonatal respiratory distress. Pediatrics. 2014;134:1160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu J, Wang Y, Fu W, Yang CS, Huang JJ. Diagnosis of neonatal transient tachypnea and its differentiation from respiratory distress syndrome using lung ultrasound. Medicine. 2014;93:e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ibrahim M, Omran A, AbdAllah NB, El-Sharkawy S. Lung ultrasound in early diagnosis of neonatal transient tachypnea and its differentiation from other causes of neonatal respiratory distress. J Neonatal Perinat Med. 2018;11:281–7. [DOI] [PubMed] [Google Scholar]
- 52.Copetti R, Cattarossi L. The ‘double lung point’: an ultrasound sign diagnostic of transient tachypnea of the newborn. Neonatology. 2007;91:203–9. [DOI] [PubMed] [Google Scholar]
- 53.Sperandeo M, Rea G, Santantonio A, Carnevale V. Lung ultrasonography in diagnosis of transient tachypnea of the newborn: limitations and pitfalls. Chest. 2016;150:977–8. [DOI] [PubMed] [Google Scholar]
- 54.Karabayir N, Kavuncuoglu S. Intravenous frusemide for transient tachypnoea of the newborn: a randomised controlled trial. J Paediatr Child Health. 2006;42:640–2. [DOI] [PubMed] [Google Scholar]
- 55.Wiswell TE, Rawlings JS, Smith FR, Goo ED. Effect of furosemide on the clinical course of transient tachypnea of the newborn. Pediatrics. 1985;75:908–10. [PubMed] [Google Scholar]
- 56.Kassab M, Khriesat WM, Anabrees J. Diuretics for transient tachypnoea of the newborn. Cochrane Database Syst Rev. 2015;11:CD003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Armangil D, Yurdakök M, Korkmaz A, Yiğit S, Tekinalp G. Inhaled beta-2 agonist salbutamol for the treatment of transient tachypnea of the newborn. J Pediatr. 2011;159:398–403.e391. [DOI] [PubMed] [Google Scholar]
- 58.Keles E, Gebesce A, Demirdoven M, Yazgan H, Basturk B, Tonbul A. The effects of inhaled beta-adrenergic agonists in transient tachypnea of the newborn. Glob Pediatr Health. 2016;3:2333794X16645258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim MJ, Yoo JH, Jung JA, Byun SY. The effects of inhaled albuterol in transient tachypnea of the newborn. Allergy Asthma Immunol Res. 2014;6:126–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moresco L, Bruschettini M, Cohen A, Gaiero A, Calevo MG. Salbutamol for transient tachypnea of the newborn. Cochrane Database Syst Rev. 2016;5:CD011878. [DOI] [PubMed] [Google Scholar]
- 61.Kao B, Stewart de Ramirez SA, Belfort MB, Hansen A. Inhaled epinephrine for the treatment of transient tachypnea of the newborn. J Perinatol. 2008;28:205–10. [DOI] [PubMed] [Google Scholar]
- 62.Stroustrup A, Trasande L, Holzman IR. Randomized controlled trial of restrictive fluid management in transient tachypnea of the newborn. J Pediatr. 2012;160:38–43.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dehdashtian M, Aramesh MR, Melekian A, Aletayeb MH, Ghaemmaghami A. Restricted versus standard maintenance fluid volume in management of transient tachypnea of newborn: a clinical trial. Iran J Pediatr. 2014;24:575–80. [PMC free article] [PubMed] [Google Scholar]
- 64.Santoro W Jr., Martinez FE, Ricco RG, Jorge SM. Colostrum ingested during the first day of life by exclusively breastfed healthy newborn infants. J Pediatr. 2010;156:29–32. [DOI] [PubMed] [Google Scholar]
- 65.Mathew B, D’Angelis CA, Lakshminrusimha S, Nickerson PA, Sokolowski JJ, Kumar VHS, et al. Natriuretic peptide C receptor in the developing sheep lung: role in perinatal transition. Pediatr Res. 2017;82:349–55. [DOI] [PubMed] [Google Scholar]
- 66.Olivera W, Ridge K, Wood LD, Sznajder JI. ANF decreases active sodium transport and increases alveolar epithelial permeability in rats. J Appl Physiol. 1993;75:1581–6. [DOI] [PubMed] [Google Scholar]
- 67.Tharaux PL, Dussaule JC, Couette S, Clerici C. Evidence for functional ANP receptors in cultured alveolar type II cells. Am J Physiol. 1998;274:L244–251. [DOI] [PubMed] [Google Scholar]
- 68.Hein HA, Ely JW, Lofgren MA. Neonatal respiratory distress in the community hospital: when to transport, when to keep. J Fam Pract. 1998;46:284–9. [PubMed] [Google Scholar]
- 69.Morgan MC, Maina B, Waiyego M, Mutinda C, Aluvaala J, Maina M, et al. Oxygen saturation ranges for healthy newborns within 24 h at 1800 m. Arch Dis Child Fetal Neonatal Ed. 2017;102:F266–F268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cotten CM. Adverse consequences of neonatal antibiotic exposure. Curr Opin Pediatr. 2016;28:141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Keszler M, Carbone MT, Cox C, Schumacher RE. Severe respiratory failure after elective repeat cesarean delivery: a potentially preventable condition leading to extracorporeal membrane oxygenation. Pediatrics. 1992;89:670–2. [PubMed] [Google Scholar]
- 72.O’Brien J. Absorption atelectasis: incidence and clinical implications. AANA J. 2013;81:205–8. [PubMed] [Google Scholar]
- 73.Lakshminrusimha S, Russell JA, Steinhorn RH, Ryan RM, Gugino SF, Morin FC III, et al. Pulmonary arterial contractility in neonatal lambs increases with 100% oxygen resuscitation. Pediatr Res. 2006;59:137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Osman AM, El-Farrash RA, Mohammed EH. Early rescue Neopuff for infants with transient tachypnea of newborn: a randomized controlled trial. J Matern Fetal Neonatal Med. 2019;32:597–603. [DOI] [PubMed] [Google Scholar]
- 75.Celebi MY, Alan S, Kahvecioglu D, Cakir U, Yildiz D, Erdeve O, et al. Impact of prophylactic continuous positive airway pressure on transient tachypnea of the newborn and neonatal intensive care admission in newborns delivered by elective cesarean section. Am J Perinatol. 2016;33:99–106. [DOI] [PubMed] [Google Scholar]
- 76.Shohat M, Levy G, Levy I, Schonfeld T, Merlob P. Transient tachypnoea of the newborn and asthma. Arch Dis Child. 1989;64:277–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schaubel D, Johansen H, Dutta M, Desmeules M, Becker A, Mao Y. Neonatal characteristics as risk factors for preschool asthma. J Asthma. 1996;33:255–64. [DOI] [PubMed] [Google Scholar]
- 78.Smith GC, Wood AM, White IR, Pell JP, Cameron AD, Dobbie R. Neonatal respiratory morbidity at term and the risk of childhood asthma. Arch Dis Child. 2004;89:956–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Birnkrant DJ, Picone C, Markowitz W, El Khwad M, Shen WH, Tafari N. Association of transient tachypnea of the newborn and childhood asthma. Pediatr Pulmonol. 2006;41:978–84. [DOI] [PubMed] [Google Scholar]
- 80.Liem JJ, Huq SI, Ekuma O, Becker AB, Kozyrskyj AL. Transient tachypnea of the newborn may be an early clinical manifestation of wheezing symptoms. J Pediatr. 2007;151:29–33. [DOI] [PubMed] [Google Scholar]
- 81.Cakan M, Nalbantoglu B, Nalbantoglu A, Demirsoy U, Say A. Correlation between transient tachypnea of the newborn and wheezing attack. Pediatr Int. 2011;53:1045–50. [DOI] [PubMed] [Google Scholar]
- 82.Golshantafti M, Yavari T, Afrand M. Risk of wheezing attacks in infants with transient tachypnea newborns. Iran J Pediatr. 2016;26:e2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Demissie K, Marcella SW, Breckenridge MB, Rhoads GG. Maternal asthma and transient tachypnea of the newborn. Pediatrics. 1998;102:84–90. [DOI] [PubMed] [Google Scholar]
- 84.Schatz M, Zeiger RS, Hoffman CP, Saunders BS, Harden KM, Forsythe AB. Increased transient tachypnea of the newborn in infants of asthmatic mothers. Am J Dis Child. 1991;145:156–8. [DOI] [PubMed] [Google Scholar]
- 85.Mendola P, Mannisto TI, Leishear K, Reddy UM, Chen Z, Laughon SK. Neonatal health of infants born to mothers with asthma. J Allergy Clin Immunol. 2014;133:85–90 e81-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tutdibi E, Hospes B, Landmann E, Gortner L, Satar M, Yurdakok M, et al. Transient tachypnea of the newborn (TTN): a role for polymorphisms of surfactant protein B (SP-B) encoding gene? Klin Padiatr. 2003;215:248–52. [DOI] [PubMed] [Google Scholar]
- 87.Aslan E, Tutdibi E, Martens S, Han Y, Monz D, Gortner L. Transient tachypnea of the newborn (TTN): a role for polymorphisms in the beta-adrenergic receptor (ADRB) encoding genes? Acta Paediatr. 2008;97:1346–50. [DOI] [PubMed] [Google Scholar]
- 88.Alter M, Pfab T, Guthmann F, Burdack A, Kempiners N, Kalk P, et al. Maternal and fetal PROGINS progesterone receptor polymorphism reduces the risk for transient tachypnea of the newborn. Clin Lab. 2010;56:559–67. [PubMed] [Google Scholar]
- 89.Satar M, Taskin E, Ozlu F, Tuli A, Ozcan K, Yildizdas HY. Polymorphism of the angiotensin-converting enzyme gene and angiotensin-converting enzyme activity in transient tachypnea of neonate and respiratory distress syndrome. J Matern Fetal Neonatal Med. 2012;25:1712–5. [DOI] [PubMed] [Google Scholar]
- 90.Oztekin O, Akyol M, Kalay S, Tezel G, Akcakus M, Oygur N. Investigation of the serum glucocorticoid kinase 1 gene in patients with transient tachypnea of the newborn. J Matern Fetal Neonatal Med. 2013;26:990–4. [DOI] [PubMed] [Google Scholar]
- 91.Toraih EA, Hussein MH, Ibrahim A, AbdAllah NB, Mohammad E, Kishk AM, et al. Beta2-adrenergic receptor variants in children and adolescents with bronchial asthma. Front Biosci (Elite Ed). 2019;11:61–78. [DOI] [PubMed] [Google Scholar]
- 92.Cagliani R, Fumagalli M, Pozzoli U, Riva S, Comi GP, Torri F, et al. Diverse evolutionary histories for beta-adrenoreceptor genes in humans. Am J Hum Genet. 2009;85:64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]


