Abstract
SMCT (sodium-coupled monocarboxylate transporter; slc5a8) is a Na+-coupled transporter for lactate, pyruvate and short-chain fatty acids. Similar to these already known substrates of SMCT, the water-soluble B-complex vitamin nicotinic acid also exists as a monocarboxylate anion (nicotinate) under physiological conditions. Therefore we evaluated the ability of SMCT to mediate the uptake of nicotinate. In mammalian cells, the cloned mouse SMCT (slc5a8) induced the uptake of nicotinate. The SMCT-induced uptake was Na+-dependent. The Michaelis constant for the uptake process was 296±88 μM. The Na+-activation kinetics indicated that at least two Na+ ions are involved in the process. Among the various structural analogues tested, nicotinate was the most effective substrate. Nicotinamide and methylnicotinate were not recognized by the transporter. 2-Pyrazine carboxylate and isonicotinate interacted with the transporter to a moderate extent. SMCT-mediated uptake of nicotinate was inhibited by lactate and pyruvate. In the Xenopus laevis oocyte expression system, SMCT-mediated nicotinate transport was electrogenic, as evident from the nicotinate-induced inward currents under voltage-clamp conditions. Substrate-induced currents in this expression system corroborated the substrate specificity determined in the mammalian cell expression system. The kinetic parameters with regard to the affinity of the transporter for nicotinate and the Hill coefficient for Na+ activation, determined by using the oocyte expression system, were also similar to those obtained from the mammalian cell expression system. We conclude that SMCT functions not only as a Na+-coupled transporter for short-chain fatty acids and lactate but also as a Na+-coupled transporter for the water-soluble vitamin nicotinic acid.
Keywords: electrogenicity, monocarboxylate transport, nicotinate transport, slc5a8, sodium-coupled monocarboxylate transporter (SMCT), sodium dependence
Abbreviations: HRPE cells, human retinal pigment epithelial cells; NMDG, N-methyl-D-glucamine; SMCT, sodium-coupled monocarboxylate transporter
INTRODUCTION
Nicotinic acid (niacin, vitamin B3) is a water-soluble vitamin and a constituent of the coenzymes NAD+ and NADP+ [1]. NAD+ and NADP+ and their respective reduced forms (NADH and NADPH) are essential for a multitude of enzymatic reactions involved in the catabolic as well as anabolic processes. Nicotinic acid is also a component of nicotinic acid–adenine dinucleotide phosphate, a novel intracellular calcium-mobilizing agent [2–4]. Deficiency of this vitamin leads to a variety of clinical symptoms including diarrhoea, dermatitis and dementia, collectively known as pellagra. Nicotinic acid is also an important therapeutic agent widely used as a lipid-lowering drug and in the treatment of atherosclerotic cardiovascular disease [5,6]. The therapeutic efficacy of this vitamin in modulating lipid metabolism stems mostly from its ability to serve as a ligand for a recently identified G-protein-coupled receptor [7–9].
Given the wide spectrum of biological roles of nicotinic acid, it is important to understand the mechanisms by which mammalian cells acquire this vitamin. It is also important to identify the mechanisms by which intestinal epithelial cells absorb this vitamin from dietary sources, and kidney epithelial cells reabsorb this vitamin from the glomerular filtrate. Nicotinic acid is a weak electrolyte with a pKa of 4.9 and therefore any transport system that handles this vitamin probably mediates its uptake in the form of nicotinate anion under physiological conditions. There is compelling evidence that MCT1, one of the members of the monocarboxylate transporter gene family SLC16, is capable of transporting nicotinic acid [10,11]. This mechanism may be at least partially responsible for the intestinal absorption of this vitamin [10,11]. Transport of nicotinic acid by MCT1 involves electroneutral co-transport of nicotinate and H+ (H+/nicotinate stoichiometry, 1:1). Bilitranslocase, a membrane protein responsible for the extraction of unconjugated bilirubin from the blood into hepatocytes, has also been shown to transport nicotinate by an electrogenic mechanism, but there is no co-transport of any other ion in this process [12]. However, since the expression of bilitranslocase is restricted to liver and stomach, whereas the expression of MCT1 is ubiquitous, it is currently believed that the latter is the primary transporter for the uptake of nicotinate into most types of cells. But MCT1 and bilitranslocase may not be the only transporters responsible for uptake of nicotinate in mammalian cells. Studies with kidney brush border membrane vesicles have provided evidence for the presence of a Na+-coupled transport system for nicotinate and the structurally related compound pyrazinoate [13–16]. The molecular identity of the transporter mediating the Na+-coupled nicotinate uptake in the kidney has not been established. On the basis of the known functional features of MCT1 and bilitranslocase, neither of these transporters is probably responsible for Na+-coupled transport of nicotinate.
Recently, a member of the Na+/glucose co-transporter gene family has been shown to transport short-chain fatty acids as well as lactate and pyruvate in a Na+-coupled and electrogenic manner [17–19]. This transporter, known as SMCT (sodium-coupled monocarboxylate transporter), is expressed in the colon and kidney [17,18,20]. The transporter is also expressed in the thyroid gland where it has been suggested to function as an anion exchanger for iodide [21]. Even though there is no structural similarity between MCT1 and SMCT, and the transport mechanism in terms of ion coupling and electrogenicity differs between the two transporters, both transporters exhibit surprisingly similar substrate specificity [10,11,17–19]. Since MCT1 can transport nicotinate, we initiated the present study to determine whether SMCT is capable of recognizing this vitamin as a transportable substrate. SMCT is a Na+-coupled transporter and is expressed in the kidney. Therefore this transporter is a potential candidate for the Na+-coupled transport of nicotinate observed in renal brush border membrane vesicles. In the present study, we show using two different heterologous expression systems that mouse SMCT (slc5a8) is indeed capable of mediating Na+-coupled and electrogenic transport of nicotinate.
MATERIALS AND METHODS
Materials
[14C]Nicotinic acid (specific radioactivity, 55 mCi/mmol) was purchased from American Radiolabeled Chemicals (St. Louis, MO, U.S.A.). L-Lactate, pyruvate, succinate, short-chain fatty acids and structural analogues of nicotinic acid were all purchased from Sigma. The cloning of the full-length SMCT from a mouse kidney cDNA library has been described previously [18].
Functional expression of mouse SMCT cDNA in HRPE cells (human retinal pigment epithelial cells)
The full-length mouse SMCT cDNA with its 5′- and 3′-untranslated regions, present in pSPORT1 vector under the control of T7 promoter, was expressed functionally in HRPE cells using the vaccinia virus expression technique [22]. The vaccinia virus expression technique is suitable for heterologous expression of cloned transporters using a variety of mammalian cell lines; however, we routinely use the HRPE cell line for this purpose because this particular cell line withstands the lytic action of the virus much better than other cell lines (e.g. HeLa, a human cervical cancer cell line). HRPE cells stay attached to the impermeable support in culture dishes even 12–20 h after infection with the virus. This makes it easy for measurement of transport activity. In contrast, HeLa cells start detaching from the impermeable support following virus infection and therefore it is difficult to measure transport activity using these cells. HRPE cells transfected with vector alone served as control for the determination of endogenous transport activity. The transport of [14C]nicotinate was measured simultaneously in control cells and in cDNA-transfected cells. The transport buffer was 25 mM Hepes/Tris (pH 7.5), containing 140 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4 and 5 mM glucose. When the transport was measured in the absence of Na+, the composition of the transport buffer was modified by replacing NaCl with an equimolar concentration of NMDG (N-methyl-D-glucamine) chloride, KCl or LiCl. To study the involvement of chloride in the transport process, transport was measured in some experiments in the absence of chloride. The transport buffer in these experiments was 25 mM Hepes/Tris (pH 7.5), containing 140 mM sodium gluconate, 5.4 mM potassium gluconate, 1.8 mM calcium gluconate, 0.8 mM MgSO4 and 5 mM glucose. Interaction of various monocarboxylates and structural analogues of nicotinic acid with the transporter was assessed by monitoring the ability of these compounds to compete with [14C]nicotinate for SMCT-mediated transport. The SMCT-specific transport was determined by subtracting the transport values measured in vector-transfected cells from the transport values measured in cDNA-transfected cells. Substrate saturation kinetics was analysed by fitting the SMCT-specific transport data to the Michaelis–Menten equation. The kinetic constants (Michaelis constant for transport, Kt, and maximal velocity, Vmax) were calculated using non-linear as well as linear regression methods. The dependence of SMCT-mediated nicotinate transport on Na+ was determined by monitoring the transport in the presence of increasing concentrations of Na+ where NaCl was iso-osmotically replaced by NMDG chloride in the transport buffer. Na+-activation kinetics was analysed by fitting the SMCT-specific transport data to the Hill equation. The Hill coefficient (h, the number of Na+ involved in the activation process) was determined using non-linear as well as linear regression methods. Experiments were repeated three times with three independent transfections and transport measurements were made in duplicate in each experiment.
Functional expression of mouse SMCT in Xenopus laevis oocytes
Capped cRNA from the full-length mouse SMCT cDNA (present in pSPORT1 vector) was synthesized using the mMESSAGE mMACHINE kit (Ambion, Austin, TX, U.S.A.). Mature oocytes from X. laevis were isolated by treatment with collagenase A (1.6 mg/ml), manually defolliculated and maintained at 18 °C in modified Barth's medium supplemented with 10 mg/ml gentamicin as described previously [17,18]. On the following day, oocytes were injected with 50 ng of cRNA. Water-injected oocytes served as controls. The oocytes were used for electrophysiological studies 4–6 days after cRNA injection. Electrophysiological studies were performed by the two-microelectrode voltage-clamp method [17,18]. Oocytes were perfused with a NaCl-containing buffer (100 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 3 mM Hepes, 3 mM Mes and 3 mM Tris, pH 7.5), followed by the same buffer containing nicotinate or its structural analogues, lactate, pyruvate and various short-chain fatty acids. The membrane potential was clamped at −50 mV. Saturation kinetics of nicotinate-induced currents was analysed by determining the currents in the presence of increasing concentrations of nicotinate in the perfusion buffer. Na+-activation kinetics of nicotinate-induced currents was analysed by determining the currents in the presence of increasing concentrations of Na+. In these experiments, the osmolality was maintained by the addition of appropriate concentrations of NMDG chloride. To study the current–membrane-potential (I–V) relationship, step changes in membrane potential were applied, each for a duration of 100 ms in 20 mV increments.
In the analysis of saturation kinetics, the kinetic parameter K0.5 (i.e. the substrate concentration necessary for the induction of half-maximal current) was calculated by fitting the values for the nicotinate-induced currents to Michaelis–Menten equation. This experiment was repeated with four different oocytes. Since the magnitudes of nicotinate-induced currents varied from oocyte to oocyte due to differences in the levels of the expression of the injected cRNA, the values for each oocyte were normalized by taking the maximal current induced by the highest concentration of nicotinate as 1. In the analysis of Na+-activation kinetics, the Hill coefficient (h) and K0.5 for Na+ were determined by fitting the values for the nicotinate-induced currents to the Hill equation. This experiment was repeated with three different oocytes and again the currents in each oocyte were normalized by taking the currents measured at 100 mM Na+ as 1. The kinetic parameters were determined using the commercially available computer program Sigma Plot, version 6.0 (SPSS, Chicago, IL, U.S.A.). Data were analysed by non-linear regression and confirmed by linear regression.
RESULTS
Analysis of nicotinate transport by mouse SMCT in the mammalian cell expression system
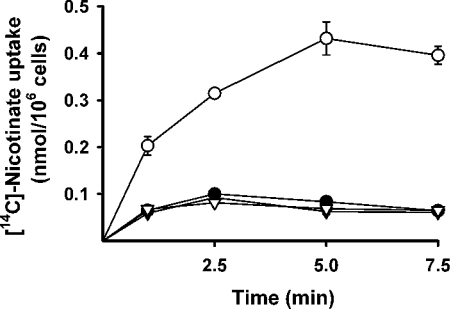
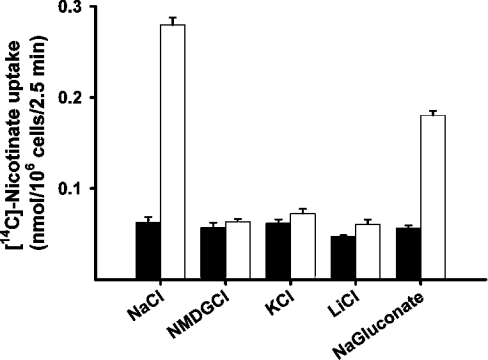
We compared the uptake of nicotinate (40 μM) in HRPE cells transfected with pSPORT1 vector alone and in cells transfected with pSPORT1-mSMCT cDNA construct (Figure 1). When the uptake was measured in the presence of NaCl in the transport buffer, nicotinate uptake was 3–4-fold higher in cDNA-transfected cells than in control cells transfected with empty vector. However, when uptake was measured in the absence of Na+, the cDNA-induced uptake was not detected. These results show that heterologous expression of mouse SMCT in HRPE cells leads to an increase in nicotinate uptake activity and that the uptake process is Na+-dependent. The results in Figure 1 also show that there is no Na+-dependent nicotinate uptake in HRPE cells transfected with empty vector, indicating that these cells do not express SMCT uptake activity constitutively. The cDNA-induced nicotinate uptake was absolutely dependent on the presence of Na+, as substitution of this ion with one among NMDG, K+ or Li+ completely abolished the uptake (Figure 2). cDNA-induced uptake is, however, not absolutely dependent on chloride. Uptake measured in the absence of chloride but in the presence of Na+ was only slightly less (35% decrease) than that measured in the presence of Na+ and Cl−.
Figure 1. Na+-dependent uptake of nicotinate by mouse SMCT.
HRPE cells were transfected with either vector alone (pSPORT1, ●, ▼) or mouse SMCT cDNA (mSMCT, ○, ▽). Uptake of [14C]nicotinate (40 μM) was measured at pH 7.5 for various time periods in the presence of NaCl (●, ○) or NMDG chloride (▼, ▽).
Figure 2. Ion-dependence of SMCT-mediated nicotinate uptake.
HRPE cells were transfected with either vector alone (black bars) or mouse SMCT cDNA (open bars). Uptake of [14C]nicotinate (40 μM) was measured for 2.5 min in the presence of NaCl or in the presence of equimolar concentration of NMDG chloride, KCl, LiCl or sodium gluconate in place of NaCl.
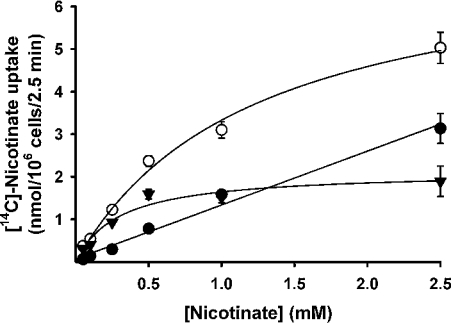
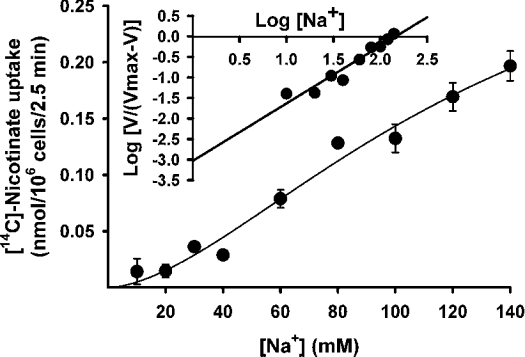
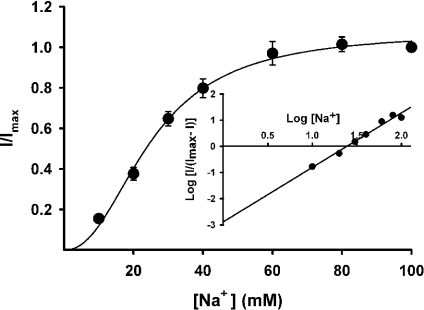
We then analysed the saturation kinetics of nicotinate uptake mediated by mouse SMCT (Figure 3). This was done by measuring nicotinate uptake over a concentration range of 25–2500 μM in control cells and in cells transfected with cDNA. The measured uptake was linear in control cells over this concentration range, indicating absence of saturable uptake for nicotinate in HRPE cells. However, the uptake was saturable in cells expressing mouse SMCT. When the cDNA-specific uptake was calculated by subtracting the uptake in control cells from the uptake in cDNA-transfected cells and analysed according to the Michaelis–Menten equation, the data fit well for a model describing a single saturable transport system. The kinetic parameters for the cDNA-specific uptake were then calculated. The values of Kt and the Vmax for the uptake process were 296±88 μM and 2.1±0.2 nmol·(106 cells)−1·(2.5 min)−1 respectively. The Na+-activation kinetics of cDNA-specific nicotinate uptake was studied by measuring the uptake in the presence of different concentrations of Na+ (10–140 mM). The concentration of Cl− was kept constant at 140 mM in these experiments. Uptake measurements were made in parallel in control cells transfected with vector alone and in cells expressing mouse SMCT. The cDNA-specific uptake was then calculated and used for analysis. The results for cDNA-specific uptake, shown in Figure 4, indicate that the relationship between uptake and Na+ concentration was sigmoidal. However, the activation did not reach its maximum at a Na+ concentration of 140 mM. We did not perform the experiment with concentrations higher than 140 mM because of the consequent changes in osmolality. Nevertheless, the data fit reasonably well to the Hill equation (correlation coefficient, 0.98). The value for the Hill coefficient (h) was 1.7±0.4, suggesting that at least two Na+ ions are involved in the uptake process mediated by mouse SMCT. Thus the Na+/nicotinate stoichiometry for the process is 2:1.
Figure 3. Saturation kinetics of SMCT-mediated nicotinate uptake.
HRPE cells were transfected with either vector alone (●) or mouse SMCT cDNA (○). Uptake of nicotinate was measured for 2.5 min in the presence of NaCl over a nicotinate concentration range of 25–2500 μM. cDNA-specific uptake (▼) was calculated by subtracting the uptake in vector-transfected cells from the uptake in cDNA-transfected cells.
Figure 4. Na+-activation kinetics for SMCT-mediated nicotinate uptake.
HRPE cells were transfected with mouse SMCT cDNA and uptake of nicotinate (40 μM) was measured for 2.5 min in the presence of different concentrations of Na+ (10–140 mM). Concentration of Cl− was kept constant at 140 mM. Na+-dependent uptake was calculated by subtracting the uptake in the absence of Na+ from the uptake in the presence of Na+. Data for the Na+-dependent uptake were used in the analysis. Inset: Hill plot.
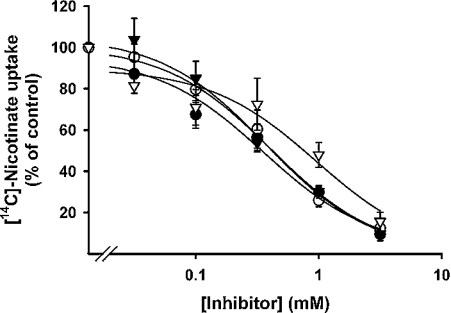
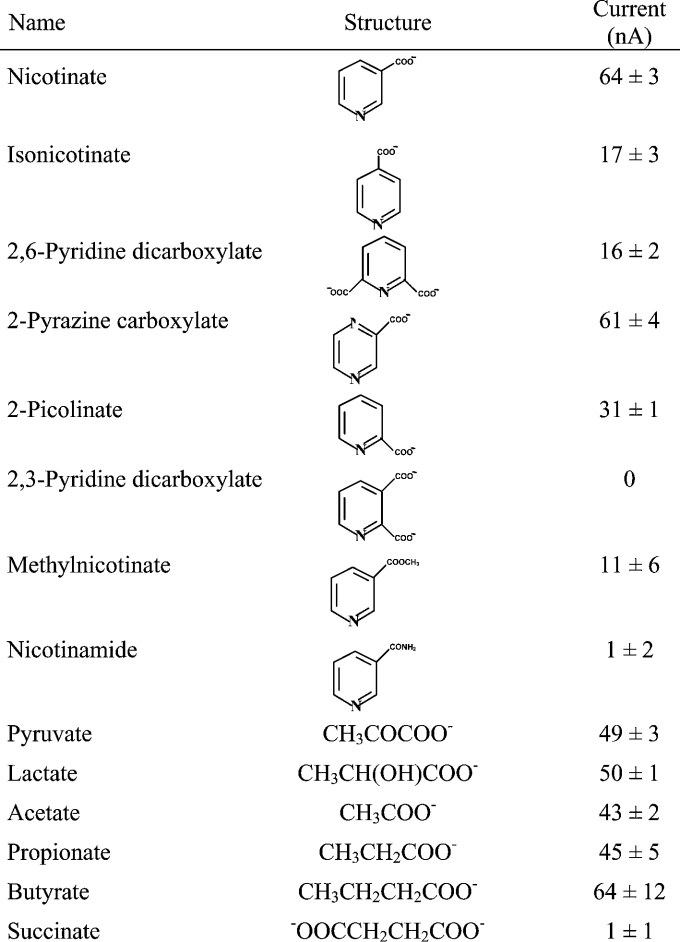
The substrate specificity of the uptake process mediated by mouse SMCT was studied by assessing the ability of various structural analogues of nicotinate (5 mM) to inhibit the cDNA-specific uptake of [14C]nicotinate (40 μM). Again, these experiments were performed in parallel in control cells transfected with vector alone and in cells expressing mouse SMCT. This allowed us to calculate the cDNA-specific uptake. The data are shown in Table 1. Unlabelled nicotinate inhibited the uptake of [14C]nicotinate potently as expected. On the other hand, nicotinamide showed negligible inhibition, indicating that the free carboxy group is essential for the recognition by the transporter as a substrate. Among the various structural analogues tested, 2-pyrazine carboxylate was the most potent, followed by isonicotinate, 2-picolinate, 2,6-pyridine dicarboxylate, 2,3-pyridine dicarboxylate and methylnicotinate. Since we have shown earlier that mouse SMCT functions as a Na+-coupled transporter for lactate and pyruvate, we also tested these monocarboxylates for their ability to compete with [14C]nicotinate for an SMCT-mediated uptake process. These two compounds were found to be as potent as nicotinate in inhibiting the cDNA-specific uptake of radiolabelled nicotinate. We then analysed the dose–response relationship for the inhibition of cDNA-specific uptake of [14C]nicotinate by nicotinate, 2-pyrazine carboxylate, lactate and pyruvate (Figure 5). The IC50 values (concentration necessary to cause 50% inhibition) calculated from these studies for these four compounds were 0.36±0.13, 0.95±0.31, 0.41±0.04 and 0.38±0.06 mM respectively.
Table 1. Substrate specificity of mouse SMCT in the mammalian cell expression system.
HRPE cells were transfected with either vector alone (pSPORT1) or pSPORT1-mSMCT cDNA. Uptake of [14C]nicotinate (40 μM) was measured for 2.5 min in a NaCl-containing buffer in the absence or presence of inhibitors (5 mM). Data (means±S.E.M.) are for three independent transfections. % refers to the uptake in the presence of the various inhibitors as a percentage of the control.
| [14C]Nicotinate uptake | ||||
|---|---|---|---|---|
| Inhibitor | pSPORT1 | pSPORT1-SMCT cDNA | cDNA-specific | % |
| None | 0.08±0.005 | 0.29±0.008 | 0.21±0.007 | 100 |
| Nicotinate | 0.04±0.004 | 0.07±0.007 | 0.02±0.006 | 10 |
| Isonicotinate | 0.04±0.004 | 0.14±0.006 | 0.09±0.005 | 44 |
| Methylnicotinate | 0.06±0.003 | 0.24±0.017 | 0.18±0.009 | 84 |
| Nicotinamide | 0.07±0.007 | 0.26±0.003 | 0.19±0.005 | 91 |
| 2,6-Pyridine dicarboxylate | 0.06±0.002 | 0.21±0.019 | 0.14±0.011 | 63 |
| 2-Picolinate | 0.04±0.002 | 0.14±0.005 | 0.11±0.003 | 48 |
| 2,3-Pyridine dicarboxylate | 0.07±0.002 | 0.24±0.016 | 0.16±0.009 | 75 |
| 2-Pyrazine carboxylate | 0.04±0.003 | 0.08±0.005 | 0.04±0.004 | 20 |
| Lactate | 0.04±0.001 | 0.06±0.005 | 0.02±0.003 | 9 |
| Pyruvate | 0.04±0.004 | 0.05±0.006 | 0.01±0.005 | 5 |
Figure 5. Dose–response relationship for the inhibition of SMCT-mediated [14C]nicotinate uptake by unlabelled nicotinate, lactate, pyruvate and 2-pyrazine carboxylate.
HRPE cells were transfected with either vector alone or mouse SMCT cDNA. Uptake of [14C]nicotinate (40 μM) was measured for 2.5 min in the presence of NaCl and in the presence of increasing concentrations of various monocarboxylates. cDNA-specific uptake was calculated by subtracting the uptake in vector-transfected cells from the corresponding uptake in cDNA-transfected cells. Results shown are for cDNA-specific uptake. ●, Unlabelled nicotinate; ○, lactate; ▼, pyruvate; and ▽, 2-pyrazine carboxylate.
Analysis of nicotinate transport by mouse SMCT in the X. laevis oocyte expression system
Studies with the mammalian cell expression system have clearly shown that mouse SMCT functions as a Na+-coupled transporter for nicotinate. Since nicotinic acid exists predominantly as an anion under the experimental conditions (pH 7.5) and the Na+/nicotinate stoichiometry is 2:1, the transport process must be electrogenic with the transfer of one positive charge into the cells for each transport cycle. Such a transport process is expected to be associated with membrane depolarization. To study the electrogenic features of SMCT-mediated nicotinate transport, we expressed mouse SMCT in X. laevis oocytes and studied its transport function by electrophysiological means using the two-microelectrode voltage-clamp method.
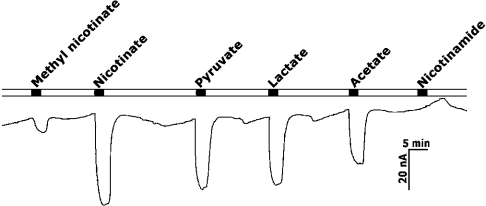
When SMCT-expressing oocytes were perfused under voltage-clamp conditions with nicotinate (5 mM) in the presence of NaCl, there was induction of inward currents (∼60 nA) (Figure 6). Under identical conditions, methylnicotinate induced less than 10 nA current and nicotinamide did not induce any detectable current. But pyruvate, lactate and acetate induced currents comparable in magnitude with nicotinate-induced currents. Table 2 provides data for the relative magnitude of inward currents induced by various structural analogues of nicotinate, lactate, pyruvate and short-chain fatty acids. The data are from four different oocytes. Nicotinate, 2-pyrazine carboxylate, short-chain fatty acids (acetate, propionate and butyrate), lactate and pyruvate induced comparable inward currents (40–65 nA). This group of compounds were followed by 2-picolinate, isonicotinate, 2,6-pyridine dicarboxylate and methylnicotinate with significantly lesser inward currents (10–30 nA). Nicotinamide and 2,3-pyridine dicarboxylate failed to induce detectable currents. Succinate, a dicarboxylate, also failed to induce any current. The current–voltage (I–V) relationship for nicotinate-induced currents revealed that the induced current was dependent on membrane potential (results not shown). The magnitude of currents induced by a fixed concentration of nicotinate (5 mM) increased when the membrane potential was hyperpolarized and decreased when the membrane potential was depolarized.
Figure 6. Inward currents induced by various monocarboxylates in oocytes expressing mouse SMCT.
Mouse SMCT was expressed in X. laevis oocytes by cRNA injection. Substrate-induced currents were recorded using the two-microelectrode voltage-clamp technique by perfusing the oocyte with various substrates (5 mM) in the presence of NaCl.
Table 2. Substrate specificity of mouse SMCT in the X. laevis oocyte expression system.
Mouse SMCT was expressed in X. laevis oocytes by cRNA injection. Substrate-induced currents were monitored by perfusing the oocytes with different substrates (5 mM) in the presence of NaCl. Data (means±S.E.M.) are from four different oocytes.
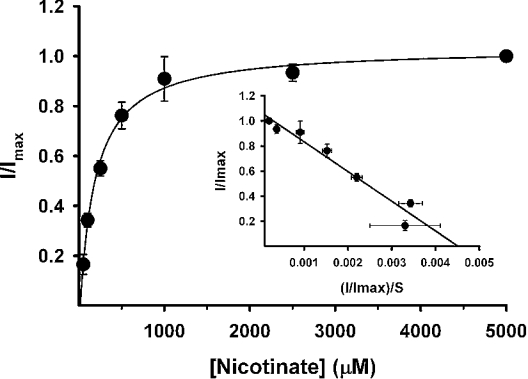
The inward currents inducible by nicotinate were saturable (Figure 7) with a K0.5 of 170±30 μM. The nicotinate-induced currents were dependent on the presence of Na+, as no detectable current was seen when NaCl was substituted with NMDG chloride in the perfusion buffer. This allowed us to analyse the Na+-activation kinetics for nicotinate-induced currents (Figure 8). The relationship between nicotinate (5 mM)-induced currents and Na+ concentration was sigmoidal. When the data were analysed according to the Hill equation, a value of 2.1±0.1 was obtained for the Hill coefficient (h). The value of K0.5 for Na+ was 25±1 mM.
Figure 7. Saturation kinetics for nicotinate-induced currents in oocytes expressing mouse SMCT.
Nicotinate-induced currents were measured in SMCT-expressing oocytes in the presence of increasing concentrations of nicotinate (50–5000 μM). NaCl was present in the perfusion buffer. The experiment was repeated with four different oocytes. Since the expression levels of injected cRNA varied significantly among the oocytes, data were normalized by taking the current induced by 5 mM nicotinate as 1 in each oocyte. Inset: Eadie–Hofstee plot.
Figure 8. Na+-activation kinetics for nicotinate-induced currents in oocytes expressing mouse SMCT.
Nicotinate (5 mM)-induced currents were measured in SMCT-expressing oocytes in the presence of increasing concentrations of Na+ (10–100 mM). Osmolality of the perfusion buffer was maintained by the addition of appropriate concentrations of NMDG chloride as a substitute for NaCl. Na+-dependent currents were calculated by subtracting the currents in the absence of Na+ from the currents measured in the presence of different concentrations of Na+. The experiment was repeated with three different oocytes. Since the expression levels of injected cRNA varied to a significant extent in different oocytes, data were normalized by taking the current induced in the presence of 100 mM Na+ as 1 in each oocyte. Inset: Hill plot.
DISCUSSION
Transporters specific for the cellular uptake of various water-soluble vitamins have been identified. This includes RFT-1 (reduced-folate transporter-1) for folic acid [23–26], ThT1 (thiamine transporter 1) and ThT2 for thiamine [26–29], SVCT1 (sodium-dependent vitamin C transporter 1) and SVCT2 for ascorbic acid [30–32] and SMVT (sodium-coupled multivitamin transporter) for biotin, pantothenate and lipoate [33–35]. The expression of a sodium-coupled transporter for the water-soluble vitamin nicotinic acid has been demonstrated in the kidney [13–16], but the molecular identity of the transporter responsible for this process remained elusive. In the present study, we report on the molecular identity of the first transporter that is capable of mediating the uptake of nicotinic acid in mammalian cells by a mechanism energized by a transmembrane electrochemical Na+ gradient. The transporter is SMCT, which we have previously identified as a Na+-coupled transporter for lactate, pyruvate and short-chain fatty acids such as acetate, propionate and butyrate [17,18]. All of the previously known substrates of this transporter exist as monocarboxylate anions under physiological conditions. Similarly, the newly identified substrate nicotinic acid also exists predominantly as a monocarboxylate anion in vivo.
The functional features of SMCT-mediated nicotinic acid transport include the following: (a) the transport process is obligatorily dependent on the presence of Na+; (b) the transporter recognizes the anionic form of nicotinic acid (nicotinate) as the substrate, since the anionic form predominates under the experimental conditions employed in these studies; (c) lactate, pyruvate and short-chain fatty acids, which are known substrates for SMCT, compete with nicotinate for the transport process; (d) the transport process is electrogenic with a Na+/nicotinate stoichiometry of 2:1; and (e) Kt for the interaction of the transporter with nicotinate is 200–300 μM, depending on the experimental technique used for the determination of the kinetic constant. When the ability of SMCT to transport various structural analogues of nicotinic acid is analysed, an interesting pattern emerges. The substrate for the transporter must be present in the anionic form as evident from the inability of the transporter to recognize nicotinamide and methylnicotinate as substrates. The relative position of the carboxylate group with regard to the nitrogen atom in the pyridine ring seems to be important for recognition by the transporter. Nicotinate, in which the carboxylate group and the nitrogen atom are separated by a single carbon atom, is the best substrate. If the carboxylate group is adjacent to the nitrogen atom as in 2-picolinate, the transport efficiency decreases. Similarly, if the carboxylate group lies farther from the nitrogen atom as in isonicotinate, the transport efficiency decreases even further. The presence of more than one anionic group in the molecule also compromises to a significant extent the ability of the transporter to recognize the compound as a substrate (e.g. 2,3-pyridine dicarboxylate and 2,6-pyridine dicarboxylate).
SMCT is expressed primarily in the kidney, colon and thyroid gland [17,18,20,21]. In the kidney, the transporter is probably responsible for the reabsorption of lactate, pyruvate, as well as nicotinate. The presence of a Na+-coupled transport process for nicotinate has been demonstrated in renal brush border membrane vesicles by other studies [13–16]. Some studies have also shown that the nicotinate transport system in these membranes interacts with lactate [13,15]. With our findings that SMCT recognizes not only lactate but also nicotinate as substrates, it is very probable that the renal reabsorption of lactate and nicotinate is mediated by a single transporter, namely SMCT. However, there are some differences in terms of the transport mechanism for SMCT-mediated nicotinate/lactate transport and for nicotinate/lactate transport in renal brush border membrane vesicles. The present study and also our previous studies [17,18] have shown unequivocally that SMCT functions as an electrogenic transporter irrespective of whether the substrate is lactate or nicotinate. In contrast, some studies have indicated that the Na+-coupled uptake of nicotinate in renal brush border membrane vesicles may be an electroneutral process with a Na+/nicotinate stoichiometry of 1:1 [15]. But there is significant controversy regarding the electrogenic nature of the transport process studied with renal brush border membrane vesicles. With lactate as the substrate for the transport system, some studies have concluded that the transport process is electroneutral [36], whereas other studies have shown that the process is electrogenic [37–39]. It has to be pointed out that most of these studies employed the Na+-activation kinetics of nicotinate or lactate uptake in membrane vesicles as the sole means of determining the electrogenic nature of the transport process. This method is error-prone, because the manner in which the various concentrations of Na+ are selected for the experiment would influence the outcome of the study. If only a few concentrations are selected at the lower end of the concentration range, the relationship between Na+ concentration and uptake may seem like hyperbolic rather than sigmoidal. This would lead to wrong conclusions regarding the Na+/substrate stoichiometry. The present study and also our previous studies [17,18] using the cloned SMCT employed the electrophysiological means to determine the electrogenic nature of the transport process. The results from these studies have provided strong and unequivocal evidence for the electrogenic nature of Na+-coupled nicotinate transport (present study) or Na+-coupled lactate transport [17,18] by SMCT. These findings have been confirmed independently by other studies [19]. Therefore we conclude that the renal reabsorption of lactate as well as nicotinate occurs by the same transporter, namely SMCT, energized by the transmembrane electrochemical gradient for Na+.
Ullrich et al. [40–42] have performed a detailed analysis in terms of substrate specificity for the transport system that is responsible for the Na+-coupled reabsorption of lactate across the brush border membrane of renal proximal tubular cells. These studies were done by assessing the ability of various structurally related compounds to compete with D-lactate for the reabsorption process. We have shown earlier that D-lactate is a substrate for SMCT and that SMCT is responsible for the Na+-coupled transport of lactate in renal brush border membrane vesicles [18]. Therefore the substrate specificity studies reported by Ullrich et al. [40–42] are relevant to the substrate specificity of SMCT. These studies have shown that the Na+-coupled D-lactate transfer across the renal brush border membrane is inhibitable by short-chain fatty acids such as acetate, propionate and butyrate [41]. This agrees with our findings that SMCT is capable of mediating Na+-coupled transport of short-chain fatty acids [17,18]. D-Lactate transfer across the renal brush border membranes is also inhibitable by various aromatic or heterocyclic monocarboxylic acids such as benzoic acid and nicotinic acid [42]. In terms of different substitutions in nicotinic acid and the resultant changes in the interaction of these compounds with the transport system, there is an excellent correlation between the data of Ullrich et al. [42] and those from the present study. The influence of various substitutions in the aromatic or heterocyclic ring in the ortho-, meta- and para-positions on the ability of either benzoic acid or nicotinic acid to interact with the transport system seems to depend on the effects of these substitutions on the ionization of the carboxylic group. If the substitutions interfere with the ionization of the carboxylic group, such substitutions decrease the ability of benzoic acid or nicotinic acid to interact with the transport system. On the other hand, if the substitutions enhance the ionization of the carboxylic group, such substitutions facilitate the interaction with the transport system. Interestingly, the presence of an ionizable group alone is not sufficient for interaction with the transport system because benzoic acid is an excellent substrate for the transport system, whereas benzenesulphonic acid, which has an SO3H group in place of CO2H group attached to a benzene ring, does not interact with the transport system [42].
In the colon, the primary substrates for SMCT are most probably the short-chain fatty acids that are generated by bacterial fermentation of unabsorbed carbohydrates and dietary fibre. Whether SMCT plays any significant role in the intestinal absorption of dietary nicotinic acid is not clear at present. We have preliminary results showing that SMCT is expressed predominantly in the colon and in the ileum but not in the jejunum (E. Gopal, S. Miyauchi, P. M. Martin and V. Ganapathy, unpublished work). Therefore it is possible that the Na+-coupled absorption of dietary nicotinic acid may occur in the terminal part of the small intestine and also in the colon by SMCT. Nicotinic acid (niacin) is also used widely as an active oral drug for the treatment of hypercholesterolaemia and hyperlipidaemia. SMCT expressed in the intestinal tract may be at least partially responsible for the high oral bioavailability of this drug.
Nicotinic acid is an essential vitamin for the normal function of all cells. SMCT is not expressed ubiquitously in mammalian tissues, suggesting that this transporter cannot be responsible for the entry of nicotinic acid into every cell. The monocarboxylate transporter MCT1 can transport nicotinic acid and this transporter is expressed ubiquitously. Therefore it seems reasonable to conclude that MCT1 plays a significant role in the delivery of nicotinic acid into most mammalian cells. However, transport of nicotinic acid by MCT1 is probably not efficient based on the known energetic aspects of this transporter. MCT1 mediates the cellular uptake of nicotinate by a H+-coupled mechanism with a H+/nicotinate stoichiometry of 1:1 [10,11]. This coupling ratio makes the transport process electroneutral. Since there is no significant transmembrane H+ gradient across the plasma membrane of most cells in the body, the uptake of nicotinate by MCT1 cannot be active. In contrast, SMCT mediates the transport of nicotinate by a Na+-coupled, electrogenic mechanism. The Na+/nicotinate coupling ratio of 2 and the electrogenic nature render the transport process highly active. This makes SMCT an appropriate transporter for the active reabsorption of nicotinic acid in the kidney. In fact, SMCT may be the only transporter in the kidney that is responsible for the reabsorption of nicotinic acid across the brush border membrane because there is no evidence for the expression of MCT1 in this membrane [37]. The highly active, energy-coupled transport process, mediated by SMCT, is essential for efficient reclamation of nicotinic acid from the glomerular filtrate because of the low levels of this vitamin in circulation (0.1–3 μM) [43,44]. The situation is probably different in the intestinal tract. MCT1 is expressed in the brush border membrane of the small intestine and in the colon [10,11]. It has been shown clearly that MCT1 does play a role in the absorption of nicotinic acid in the small intestine [10,11]. Furthermore, there is a significant transmembrane H+ gradient across the intestinal brush border membrane due to the acid microclimate pH that exists on the luminal surface of the mucosal epithelial layer [45,46]. Therefore MCT1 may contribute to a significant extent to the intestinal absorption of this vitamin. Since SMCT is also expressed in the intestinal tract, we conclude that both SMCT and MCT1 may participate in the active absorption of nicotinic acid from dietary sources in the intestinal tract. The relative contribution of the two transporters to the overall absorption of nicotinic acid through the entire intestinal tract remains to be determined because of the differences in the driving forces necessary for the energization of the two transport processes and in the expression pattern of the two transporters along the longitudinal axis of the intestinal tract.
References
- 1.Henderson L. M. Niacin. Annu. Rev. Nutr. 1983;3:289–307. doi: 10.1146/annurev.nu.03.070183.001445. [DOI] [PubMed] [Google Scholar]
- 2.Lee H. C. Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Annu. Rev. Pharmacol. Toxicol. 2001;41:317–345. doi: 10.1146/annurev.pharmtox.41.1.317. [DOI] [PubMed] [Google Scholar]
- 3.Patel S., Churchill G. C., Galione A. Coordination of Ca2+ signaling by NAADP. Trends Biochem. Sci. 2001;26:482–489. doi: 10.1016/s0968-0004(01)01896-5. [DOI] [PubMed] [Google Scholar]
- 4.Genazzani A. A., Billington R. A. NAADP: an atypical Ca2+-release messenger? Trends Pharmacol. Sci. 2002;23:165–167. doi: 10.1016/s0165-6147(00)01990-8. [DOI] [PubMed] [Google Scholar]
- 5.Ganji S. H., Kamanna V. S., Kashyap M. L. Niacin and cholesterol: role in cardiovascular disease. J. Nutr. Biochem. 2003;14:298–305. doi: 10.1016/s0955-2863(02)00284-x. [DOI] [PubMed] [Google Scholar]
- 6.McKenney J. New perspectives on the use of niacin in the treatment of lipid disorders. Arch. Intern. Med. 2004;164:697–705. doi: 10.1001/archinte.164.7.697. [DOI] [PubMed] [Google Scholar]
- 7.Soga T., Kamohara M., Takasaki J., Matsumoto S., Saito T., Ohishi T., Hiyama H., Matsuo A., Matsushime H., Furuichi K. Molecular identification of nicotinic acid receptor. Biochem. Biophys. Res. Commun. 2003;303:364–369. doi: 10.1016/s0006-291x(03)00342-5. [DOI] [PubMed] [Google Scholar]
- 8.Wise A., Foord S. M., Fraser N. J., Barnes A. A., Elshourbagy N., Eilert M., Ignar D. M., Murdock P. R., Steplewski K., Green A., et al. Molecular identification of high and low affinity receptors for nicotinic acid. J. Biol. Chem. 2003;278:9869–9874. doi: 10.1074/jbc.M210695200. [DOI] [PubMed] [Google Scholar]
- 9.Tunaru S., Kero J., Schaub A., Wufka C., Blaukat A., Pfeffer K., Offermanns S. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat. Med. 2003;9:352–355. doi: 10.1038/nm824. [DOI] [PubMed] [Google Scholar]
- 10.Simanjuntak M. T., Tamai I., Terasaki T., Tsuji A. Carrier-mediated uptake of nicotinic acid by rat intestinal brush-border membrane vesicles and relation to monocarboxylic acid transport. J. Pharmacobiodyn. 1990;13:301–309. doi: 10.1248/bpb1978.13.301. [DOI] [PubMed] [Google Scholar]
- 11.Takanaga H., Maeda H., Yabuuchi H., Tamai I., Higashida H., Tsuji A. Nicotinic acid transport mediated by pH-dependent anion antiporter and proton cotransporter in rabbit intestinal brush-border membrane. J. Pharm. Pharmacol. 1996;48:1073–1077. doi: 10.1111/j.2042-7158.1996.tb05902.x. [DOI] [PubMed] [Google Scholar]
- 12.Passamonti S., Battiston L., Sottocasa G. L. Gastric uptake of nicotinic acid by bilitranslocase. FEBS Lett. 2000;482:167–168. doi: 10.1016/s0014-5793(00)02041-x. [DOI] [PubMed] [Google Scholar]
- 13.Besseghir K., Roch-Ramel F. Pyrazinoate transport in the isolated perfused rabbit proximal tubule. Pflugers Arch. 1986;407:643–648. doi: 10.1007/BF00582646. [DOI] [PubMed] [Google Scholar]
- 14.Guggino S. E., Aronson P. S. Paradoxical effects of pyrazinoate and nicotinate on urate transport in dog renal microvillus membranes. J. Clin. Invest. 1985;76:543–547. doi: 10.1172/JCI112004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manganel M., Roch-Ramel F., Murer H. Sodium-pyrazinoate cotransport in rabbit renal brush border membrane vesicles. Am. J. Physiol. 1985;249:F400–F408. doi: 10.1152/ajprenal.1985.249.3.F400. [DOI] [PubMed] [Google Scholar]
- 16.Schuette S., Rose R. C. Renal transport and metabolism of nicotinic acid. Am. J. Physiol. 1986;250:C694–C703. doi: 10.1152/ajpcell.1986.250.5.C694. [DOI] [PubMed] [Google Scholar]
- 17.Miyauchi S., Gopal E., Fei Y. J., Ganapathy V. Functional identification of SLC5A8, a tumor suppressor down-regulated in colon cancer, as a Na+-coupled transporter for short-chain fatty acids. J. Biol. Chem. 2004;279:13293–13296. doi: 10.1074/jbc.C400059200. [DOI] [PubMed] [Google Scholar]
- 18.Gopal E., Fei Y. J., Sugawara M., Miyauchi S., Zhuang L., Martin P., Smith S. B., Prasad P. D., Ganapathy V. Expression of slc5a8 in kidney and its role in Na+-coupled transport of lactate. J. Biol. Chem. 2004;279:44522–44532. doi: 10.1074/jbc.M405365200. [DOI] [PubMed] [Google Scholar]
- 19.Coady M. J., Chang M. H., Charron F. M., Plata C., Wallendorff B., Sah J. F., Markowitz S. D., Romero M. F., Lapointe J. Y. The human tumour suppressor gene SLC5A8 expresses a Na+-monocarboxylate cotransporter. J. Physiol. (Cambridge, U.K.) 2004;557:719–731. doi: 10.1113/jphysiol.2004.063859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H., Myeroff L., Smiraglia D., Romero M. F., Pretlow T. P., Kasturi L., Lutterbaugh J., Rerko R. M., Casey G., Issa J. P., et al. SLC5A8, a sodium transporter, is a tumor suppressor gene silenced by methylation in human colon aberrant crypt foci and cancers. Proc. Natl. Acad. Sci. U.S.A. 2003;100:8412–8417. doi: 10.1073/pnas.1430846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez A. M., Perron B., Lacroix L., Caillou B., Leblanc G., Schlumberger M., Bidart J. M., Pourcher T. Identification and characterization of a putative human iodide transporter located at the apical membrane of thyrocytes. J. Clin. Endocrinol. Metab. 2002;87:3500–3503. doi: 10.1210/jcem.87.7.8797. [DOI] [PubMed] [Google Scholar]
- 22.Blakely R. D., Clark J. A., Rudnick G., Amara S. G. Vaccinia-T7 RNA polymerase expression system: evaluation for the expression cloning of plasma membrane transporters. Anal. Biochem. 1991;194:302–308. doi: 10.1016/0003-2697(91)90233-j. [DOI] [PubMed] [Google Scholar]
- 23.Dixon K. H., Lanpher B. C., Chiu J., Kelley K., Cowan K. H. A novel cDNA restores reduced folate carrier activity and methotrexate sensitivity to transport deficient cells. J. Biol. Chem. 1994;269:17–20. [PubMed] [Google Scholar]
- 24.Williams F. M., Murray R. C., Underhill T. M., Flintoff W. F. Isolation of a hamster cDNA clone coding for a function involved in methotrexate uptake. J. Biol. Chem. 1994;269:5810–5816. [PubMed] [Google Scholar]
- 25.Prasad P. D., Ramamoorthy S., Leibach F. H., Ganapathy V. Molecular cloning of the human placental folate transporter. Biochem. Biophys. Res. Commun. 1995;206:681–687. doi: 10.1006/bbrc.1995.1096. [DOI] [PubMed] [Google Scholar]
- 26.Ganapathy V., Smith S. B., Prasad P. D. SLC19: the folate/thiamine transporter family. Pflugers Arch. 2004;447:641–646. doi: 10.1007/s00424-003-1068-1. [DOI] [PubMed] [Google Scholar]
- 27.Fleming J. C., Tartaglini E., Steinkamp M. P., Schorderet D. F., Cohen N., Neufeld E. J. The gene mutated in thiamine-responsive anaemia with diabetes and deafness (TRMA) encodes a functional thiamine transporter. Nat. Genet. 1999;22:305–308. doi: 10.1038/10379. [DOI] [PubMed] [Google Scholar]
- 28.Dutta B., Huang W., Molero M., Kekuda R., Leibach F. H., Devoe L. D., Ganapathy V., Prasad P. D. Cloning of the human thiamine transporter, a member of the folate transporter family. J. Biol. Chem. 1999;274:31925–31929. doi: 10.1074/jbc.274.45.31925. [DOI] [PubMed] [Google Scholar]
- 29.Rajgopal A., Edmondnson A., Goldman I. D., Zhao R. SLC19A3 encodes a second thiamine transporter ThTr2. Biochim. Biophys. Acta. 2001;1537:175–178. doi: 10.1016/s0925-4439(01)00073-4. [DOI] [PubMed] [Google Scholar]
- 30.Tsukaguchi H., Tokui T., Mackenzie B., Berger U. V., Chen X. Z., Wang Y., Brubaker R. F., Hediger M. A. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature (London) 1999;399:70–75. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- 31.Wang H., Dutta B., Huang W., Devoe L. D., Leibach F. H., Ganapathy V., Prasad P. D. Human Na+-dependent vitamin C transporter 1 (hSVCT1): primary structure, functional characteristics and evidence for a non-functional splice variant. Biochim. Biophys. Acta. 1999;1461:1–9. doi: 10.1016/s0005-2736(99)00182-0. [DOI] [PubMed] [Google Scholar]
- 32.Rajan D. P., Huang W., Dutta B., Devoe L. D., Leibach F. H., Ganapathy V., Prasad P. D. Human placental sodium-dependent vitamin C transporter (SVCT2): molecular cloning and transport function. Biochem. Biophys. Res. Commun. 1999;262:762–768. doi: 10.1006/bbrc.1999.1272. [DOI] [PubMed] [Google Scholar]
- 33.Prasad P. D., Wang H., Kekuda R., Fujita T., Fei Y. J., Devoe L. D., Leibach F. H., Ganapathy V. Cloning and functional expression of a cDNA encoding a mammalian sodium-dependent vitamin transporter mediating the uptake of pantothenate, biotin, and lipoate. J. Biol. Chem. 1998;273:7501–7506. doi: 10.1074/jbc.273.13.7501. [DOI] [PubMed] [Google Scholar]
- 34.Wang H., Huang W., Fei Y. J., Xia H., Yang-Feng T. L., Leibach F. H., Devoe L. D., Ganapathy V., Prasad P. D. Human placental Na+-dependent multivitamin transporter. Cloning, functional expression, gene structure, and chromosomal localization. J. Biol. Chem. 1999;274:14875–14883. doi: 10.1074/jbc.274.21.14875. [DOI] [PubMed] [Google Scholar]
- 35.Prasad P. D., Wang H., Huang W., Fei Y. J., Leibach F. H., Devoe L. D., Ganapathy V. Molecular and functional characterization of the intestinal Na+-dependent multivitamin transporter. Arch. Biochem. Biophys. 1999;366:95–106. doi: 10.1006/abbi.1999.1213. [DOI] [PubMed] [Google Scholar]
- 36.Barbarat B., Podevin R. A. Stoichiometry of the renal sodium-L-lactate cotransporter. J. Biol. Chem. 1988;263:12190–12193. [PubMed] [Google Scholar]
- 37.Barac-Nieto M., Murer H., Kinne R. Lactate-sodium cotransport in rat renal brush border membranes. Am. J. Physiol. 1980;239:F496–F506. doi: 10.1152/ajprenal.1980.239.5.F496. [DOI] [PubMed] [Google Scholar]
- 38.Mengual R., Claude-Schlageter M. H., Poiree J. C., Yagello M., Sudaka P. Characterization of sodium and pyruvate interactions of the two carrier systems specific of mono- and di- or tricarboxylic acids by renal brush-border membrane vesicles. J. Membr. Biol. 1989;108:197–205. doi: 10.1007/BF01871734. [DOI] [PubMed] [Google Scholar]
- 39.Mengual R., Schlageter M. H., Sudaka P. Kinetic asymmetry of renal Na+-L-lactate cotransport. Characteristic parameters and evidence for a ping pong mechanism of the trans-stimulating exchange by pyruvate. J. Biol. Chem. 1990;265:292–299. [PubMed] [Google Scholar]
- 40.Ullrich K. J., Rumrich G., Kloss S. Reabsorption of monocarboxylic acids in the proximal tubule of the rat kidney. I. Transport kinetics of D-lactate, Na+-dependence, pH-dependence and effect of inhibitors. Pflugers Arch. 1982;395:212–219. doi: 10.1007/BF00584812. [DOI] [PubMed] [Google Scholar]
- 41.Ullrich K. J., Rumrich G., Kloss S. Reabsorption of monocarboxylic acids in the proximal tubule of the rat kidney. II. Specificity for aliphatic compounds. Pflugers Arch. 1982;395:220–226. doi: 10.1007/BF00584813. [DOI] [PubMed] [Google Scholar]
- 42.Ullrich K. J., Rumrich G., Kloss S., Fasold H. Reabsorption of monocarboxylic acids in the proximal tubule of the rat kidney. III. Specificity for aromatic compounds. Pflugers Arch. 1982;395:227–231. doi: 10.1007/BF00584814. [DOI] [PubMed] [Google Scholar]
- 43.Takikawa K., Miyazaki K., Arita T. High performance liquid chromatographic determination of nicotinic acid and its metabolites, nicotinuric acid and nicotinamide, in plasma. J. Chromatogr. 1982;233:343–348. doi: 10.1016/s0378-4347(00)81765-x. [DOI] [PubMed] [Google Scholar]
- 44.Guilarte T. R., Pravlik K. Radiometric-microbiologic assay of niacin using Kloeckera brevis: analysis of human blood and food. J. Nutr. 1983;113:2587–2594. doi: 10.1093/jn/113.12.2587. [DOI] [PubMed] [Google Scholar]
- 45.Ganapathy V., Leibach F. H. Is intestinal peptide transport energized by a proton gradient? Am. J. Physiol. 1985;249:G153–G160. doi: 10.1152/ajpgi.1985.249.2.G153. [DOI] [PubMed] [Google Scholar]
- 46.Ganapathy V., Leibach F. H. Proton-coupled solute transport in the animal cell plasma membrane. Curr. Opin. Cell Biol. 1991;3:695–701. doi: 10.1016/0955-0674(91)90043-x. [DOI] [PubMed] [Google Scholar]