Abstract
We report a sensitive LC (liquid chromatography)/MS/MS assay using selected reaction monitoring to quantify RA (retinoic acid), which is applicable to biological samples of limited size (10–20 mg of tissue wet weight), requires no sample derivatization, provides mass identification and resolves atRA (all-trans-RA) from its geometric isomers. The assay quantifies over a linear range of 20 fmol to 10 pmol, and has a 10 fmol limit of detection at a signal/noise ratio of 3. Coefficients of variation are: instrumental, 0.5–2.9%; intra-assay, 5.4±0.4%; inter-assay 8.9±1.0%. An internal standard (all-trans-4,4-dimethyl-RA) improves accuracy by confirming extraction efficiency and revealing handling-induced isomerization. Tissues of 2–4-month-old C57BL/6 male mice had atRA concentrations of 7–9.6 pmol/g and serum atRA of 1.9±0.6 pmol/ml (±S.E.M.). Tissue 13-cis-RA ranged from 2.9 to 4.2 pmol/g, and serum 13-cis-RA was 1.2±0.3 pmol/ml. CRBP (cellular retinol-binding protein)-null mouse liver had atRA ∼30% lower than wild-type (P<0.05), but kidney, testis, brain and serum atRA were similar to wild-type. atRA in brain areas of 12-month-old female C57BL/6 mice were (±S.E.M.): whole brain, 5.4±0.4 pmol/g; cerebellum, 10.7±0.3 pmol/g; cortex, 2.6±0.4 pmol/g; hippocampus, 8.4±1.2 pmol/g; striatum, 15.3±4.7 pmol/g. These data provide the first analytically robust quantification of atRA in animal brain and in CRBP-null mice. Direct measurements of endogenous RA should have a substantial impact on investigating target tissues of RA, mechanisms of RA action, and the relationship between RA and chronic disease.
Keywords: liquid chromatography/MS/MS (LC/MS/MS), selective reaction monitoring, retinoic acid, retinoid, vitamin A
Abbreviations: APCI, atmospheric pressure chemical ionization; atRA, all-trans-retinoic acid; CRBP, cellular retinol-binding protein; CV, coefficient of variance; ESI, electrospray ionization; GC, gas chromatography; LC, liquid chromatography; LOD, limit of detection; LOQ, limit of quantification; RA, retinoic acid; RAR, RA receptor; RXR, retinoid X receptor; SRM, selected reaction monitoring
INTRODUCTION
Vitamin A (retinol) supports a wide range of biological actions that are essential for vision, development, normal cell proliferation, differentiation, reproduction, the immune response, and nervous system development and maturation. Vitamin A does not function directly, rather it serves as precursor for the biologically active retinoids 11-cis-retinal and atRA (all-trans-retinoic acid) [1–12]. Vision requires adequate generation of 11-cis-retinal; the systemic (non-visual) functions require atRA. Vision consumes most dietary vitamin A, but all vertebrates require the quantitatively minor production of atRA. Abnormal atRA, for example, may cause and/or permit epithelial degeneration, such as xerophthalmia, and neurological disorders, such as schizophrenia, Parkinson's disease, Huntington's disease and Alzheimer's disease [13–15]. Biosynthesis and catabolism seem to carefully control concentrations of atRA in vivo, in temporally/spatially precise patterns, to effect the pleiotropic systemic actions of vitamin A [16]. RA (retinoic acid) mediates its biological functions through binding to and activating ligand-activated transcription factors that regulate gene expression [17,18]. Two families of nuclear receptors [RAR (RA receptor) and RXR (retinoid X receptor)] respond to different geometric RA isomers. atRA activates only RAR; 9-cis-RA activates both RAR and RXR; 13-cis-RA activates neither. atRA and 13-cis-RA occur in vivo, whereas the in vivo occurrence of 9-cis-RA remains controversial [19–22].
Many studies have identified putative sites of RA biosynthesis and/or function by determining expression loci of retinoid-specific binding proteins, enzymes and receptors that contribute to RA generation, signalling and catabolism [23–27]. Quantification of atRA and its biologically occurring geometric isomers would aid our understanding of vitamin A functions and mechanisms of action, at these and other sites. Serum and limited tissue levels of atRA have been reported in the adult, but endogenous levels of atRA and its isomers in many tissues and/or localized regions of tissue have not been established, because analytically robust assays have not been available with the requisite sensitivity. Substitutes for direct RA measurement and analytical robust assays, such as in vitro reporter assays or transgenic RA reporter mouse strains, lack specificity, lack means of quantification or have produced contradictory results [28–30].
In the present paper, we report a direct, specific and sensitive LC (liquid chromatography)/MS/MS assay to quantify RA in small biological samples, and which distinguishes atRA and 13-cis-RA from other retinoids.
EXPERIMENTAL
Materials
atRA, 9-cis-RA and 13-cis-RA were purchased from Sigma–Aldrich. 4,4-Dimethyl RA was a gift from Marcia Dawson (Burnhan Institute, La Jolla, CA, U.S.A.) and Peter Hobbs (SRI International, Menlo Park, CA, U.S.A.) [31]. Retinoid standards were prepared freshly on the day of use and the concentrations were verified spectrophotometrically using published ε values [31,32]. CRBP (cellular retinol-binding protein)-null mice were a gift from Pierre Chambon and Norbert Ghyselinck (both at Institut de Génétique et de Biologie Moléculaire et Cellulaire, CNRS/INSERM/ULP, College de France, Illkirch, France) [33].
Sample preparation
C57BL/6 mice were fed with an 18% protein rodent diet from Harlan Teklad Global (15.4 units of vitamin A acetate/g). Measurements were made in tissues of 2–4-month-old, male wild-type or CRBP-null mice (liver, kidney, testis, whole brain and serum) and in 12-month-old female wild-type mice (whole brain and brain sections). Tissues were placed in ice-cold saline immediately after dissection. Tissues were patted dry, weighed and homogenized on ice with ice-cold 0.9% saline to make a 25% homogenate using a Heidolph homogenizer (five strokes at 1500 rev./min), or, in the case of very small samples, a Kontes ground-glass hand homogenizer. Serum was recovered by centrifuging blood at 7800 g for 10 min in an Eppendorf 5415D centrifuge. Dissections were performed under a Nikon SMZ-10A dissection microscope equipped with a Volpi (Auburn, NY, U.S.A.) NCL 150 light source with a red or yellow filter.
Extraction
Up to 500 μl of tissue homogenate or 200 μl of serum was added to a disposable glass culture tube (16 mm×150 mm), followed by 15 μl of internal standard (50 nM 4,4-dimethyl RA in acetonitrile). A volume of 1 ml of 0.025 M KOH in ethanol was added, and the sample was vortex-mixed. A 10 ml volume of hexane was then added. The samples were vortex-mixed and then centrifuged for 1–3 min at 60 rev./min in a Dynac centrifuge (Becton Dickinson) to facilitate phase separation. The hexane layer, containing neutral lipids, including retinol and retinyl esters, was removed. To recover RA, 60 μl of 4 M HCl was added to the aqueous phase, and the sample was vortex-mixed. A 10 ml volume of hexane was then added, and the sample was vortex-mixed and centrifuged as described above. The phases were separated, and the solvent from the second hexane phase, which contained the RA, was removed under a gentle stream of nitrogen with heating at 25–30 °C. The residue was dissolved in 60–100 μl of acetonitrile and was placed in a deactivated glass autoinjector insert for analysis. Only glass containers, pipettes and calibrated syringes were used to handle samples, because retinoids adhere to plastic, which can cause losses as great as 40%. Resuspended samples were usually analysed immediately; however, they remain stable at room temperature for up to 2 days in acetonitrile.
LC/MS/MS
Resolution of atRA and its isomers was effected with a Supelco ABZ+C-16 alkylamide column (100 mm×2.1 mm, 3 μm) using an Agilent 1100 series HPLC, consisting of a vacuum degasser, binary pump, thermostatically controlled column compartment, thermostatically controlled autosampler and a diode array detector. Mobile-phase A consisted of acetonitrile/methanol/water/methanoic (formic) acid (40:30:30:0.1, by vol.); mobile-phase B consisted of acetonitrile/methanol/water/methanoic acid (55:30:15:0.1, by vol.). A linear gradient was generated at 200 μl/min: 0–5 min, 100% A to 100% B; 5–19 min, 100% B; 19–20 min, 100% B to 100% A; 20–30 min, re-equilibrate with 100% A. The injection volume was 20 μl. The column was controlled at 25 °C, and the autosampler compartment was set to 10 °C.
An Applied Biosystems API-3000 LC/MS/MS triple Q (quadrupole) mass spectrometer with ESI (electrospray ionization) and APCI (atmospheric pressure chemical ionization) sources, controlled by Analyst version 1.3.2 software, was operated in SRM (selected reaction monitoring) mode. The dwell time for RA and the internal standard, 4,4-dimethyl-RA, was 150 ms. Optimum positive APCI conditions included: nebulizer gas, 4; curtain gas, 6; collision gas, 6; nebulizer current, 3; and temperature, 400 °C. Compound-dependent parameters were: declustering potential, 30; focusing potential, 250; entrance potential, 4; collision energy, 20; and collision cell exit potential, 4.
RESULTS AND DISCUSSION
MS conditions
Both ESI and APCI sources were tested with positive- and negative-ion detection. Positive-ion APCI was chosen as the most sensitive for RA, and was used for all development and applications herein. These results agree with previous results, obtained with single quadrupole instruments, in which APCI gave greater signal intensity and had a greater linear dynamic range than ESI [34–36]. Ionization of the analyte in APCI occurs through gas-phase ion–molecule reactions at atmospheric pressure, and thus can be very efficient, because of high collision frequency. The evaporated mobile phase acts as ionizing gas to yield (M+H)+ or (M−H)− respectively. Rapid desolvation and vaporization of the sample in APCI reduces thermal decomposition and preserves the pseudomolecular species [37]. Ionization efficiency was probably enhanced further by the conjugated structure and carboxylic acid of RA. In addition, we found that negative-ion APCI was 4–15-fold less sensitive than positive-ion APCI, and had higher background with biological samples (results not shown).
SRM uses tandem MS, also known as MS/MS, and characteristic analyte decomposition reactions to increase specificity and sensitivity. The first mass analyser (in our case, Q1) selects for throughput of a precursor ion (usually the molecular or pseudomolecular ion). A non-reactive gas (e.g. N2) in the second mass analyser, Q2, fragments the selected ion by collisionally activated decomposition. The third mass analyser, Q3, selects for a characteristic product of the precursor ion. Enhanced specificity results because the analyte must meet two m/z requirements and because monitoring of a specific precursor-to-product ion transition typically reduces background noise 100–1000-fold [37]. For RA, we selected m/z 301.1 (pseudoparent ion) in Q1 and m/z 205.0 (prototypical fragment) in Q3. For the internal standard, 4,4-dimethyl-RA, we selected m/z 329.4 in Q1 and m/z 151.3 in Q3. We based the selection of both product ions on their signal intensities relative to others.
Mobile-phase selection
This assay required co-ordinate optimization of chromatographic and mass spectrometric conditions to balance sensitivity with resolution requirements. LC had to resolve RA and its isomers, and allow ionization with sensitivity. An LC mobile phase of 0.1% methanoic acid in methanol/water allowed the greatest sensitivity with APCI, but could not effect sufficient resolution of RA isomers. In contrast, 0.1% methanoic acid in acetonitrile/water achieved excellent resolution of RA isomers, but reduced sensitivity ∼10-fold compared with methanol/water. Using 0.1% methanoic acid in acetonitrile/methanol/water resolved RA isomers with only slightly less sensitivity than methanol/water alone. A gradient varying acetonitrile and water against a constant proportion of methanol gave the best signal/noise ratio with the sharpest baseline resolution of RA isomers. Methanoic acid was added to prevent peak distortion from partially dissociated carboxylic acid groups and to facilitate protonation for positive-ion APCI.
Sample handling
The susceptibility of retinoids to isomerization and oxidation necessitates care during sample collection, handling and storage [32,38–41]. Described below are details essential to successful execution of this assay. All procedures were carried out under yellow light, starting with tissue harvest and concluding with LC/MS/MS.
Tissue collection
Tissue samples should be handled only under yellow light, including during harvest, because even brief exposure to full spectrum light can isomerize RA extensively before homogenization and extraction. We found that tissues harvested under full spectrum light had decreased atRA, increased RA isomers, and contained isomers that normally do not occur in vivo, compared with tissues harvested under yellow light. For tissue dissection, the light source of the microscope must be filtered. Either red or yellow filters prevented isomerization.
Homogenization
Tissue homogenization should be performed on ice, and the samples should be extracted immediately. The time between homogenization and extraction must be minimized to reduce destruction of retinoids by auto-oxidizing lipid systems [41]. We found, in agreement with the report of Schmidt et al. [41], that homogenates stored >2 h at 4 °C had reduced RA levels. Homogenates stored at −80 °C had ∼50% loss of RA after 1 month.
Extraction
Care must be taken during extraction to avoid contamination of the hexane layer containing the RA with the acidic aqueous layer. Acid contamination catalyses RA isomerization.
Resuspension
Samples resuspended in acetonitrile were more stable than suspensions in other solvents, including mixtures of methanol/acetonitrile/water, methanol/acetonitrile and methanol. Samples should not be resuspended or stored in mobile phases that contain acid.
Storage
Storage of resuspended samples in acetonitrile is possible for 2 days at room temperature in the autosampler. Cooling the autosampler helps preserve sample quality. Resuspended samples stored at −20 °C remain unchanged for ∼4–5 days. RA standards and 4,4-dimethyl-RA remain stable in acetonitrile for several months at 20 °C (protected from white light), although we recommend periodic spectrophotometric confirmation of concentrations.
Internal standard
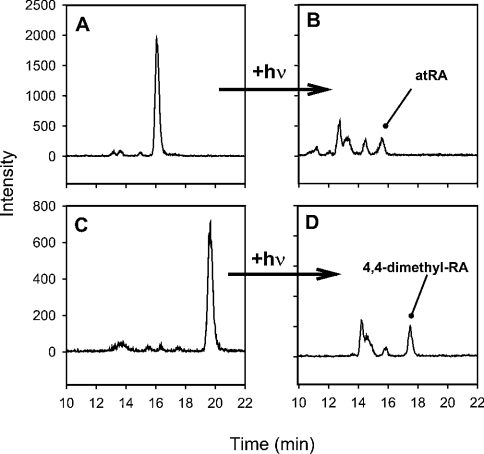
An internal standard improves accuracy by establishing extraction efficiency and revealing handling-induced isomerization. The internal standard we chose, 4,4-dimethyl-RA, has a structure similar to atRA (Figure 1), has similar chromatographic behaviour (Figure 2) and has similar extraction efficiency (results not shown). To test 4,4-dimethyl-RA as an indicator of isomerization, we exposed solutions of atRA and 4,4-dimethyl-RA to mild and severe conditions of isomerization (Figure 3). The extent of 4,4-dimethyl-RA isomerization mirrored closely that of atRA under both mild and severe conditions – a valuable characteristic of the internal standard, because it helps distinguish endogenous RA isomers from those formed during handling. Samples that have been handled properly have no isomers in the 4,4-dimethyl-RA chromatogram, indicating that isomers in the RA chromatogram are endogenous (Figure 4A). Samples with artifactual isomers also show isomers in the 4,4-dimethyl-RA chromatogram (Figure 4B). Note the difference in the decrease in atRA and increase in cis-isomers in Figure 4(B), concurrent with isomers occurring in the internal standard chromatogram. Samples with significant isomerization of the internal standard (>10–15%) should be discarded. Results of previous work have concluded that the biological matrix can cause ∼7% isomerization of atRA into cis-isomers [41]: cis-isomers exceeding this proportion should be endogenous, provided that the internal standard shows no isomerization.
Figure 1. Structures of atRA and the internal standard all-trans-4,4-dimethyl-RA.
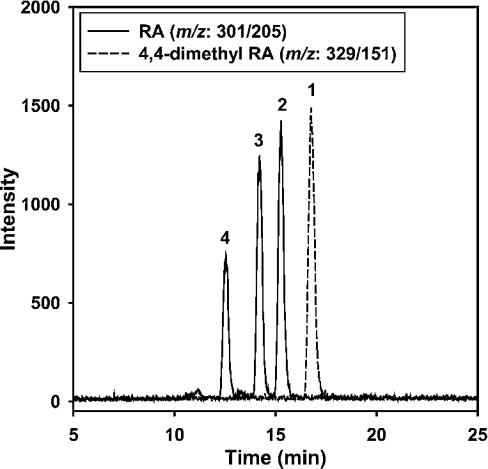
Figure 2. SRM chromatogram of RA standard solutions.
The solid line corresponds to m/z Q1:301.1/Q3:205.0. The broken line corresponds to m/z Q1:329.4/Q3:151.3. Peak identities are as follows: (1) 4,4-dimethyl-RA, (2) atRA, (3) 9-cis-RA and (4) 13-cis-RA. LC conditions are given in the Experimental section.
Figure 3. Similar isomerization rates of atRA (A and B) and 4,4-dimethyl-RA (C and D).
(A) and (C) show standard solutions (∼50 nM) exhibiting mild isomerization after 10 min of exposure to standard fluorescent room lights. (B) and (D) show standard solutions with severe isomerization after 30 min exposure to sunlight. Note the similar rate of production of isomers from the all-trans forms for both retinoids (tR≈16.2 min for atRA; tR≈19.8 for 4,4-dimethyl-RA) to cis-isomers (tR=10–16 min for RA and 12–18 min for 4,4-dimethyl-RA).
Figure 4. Application of internal standard with mouse kidney.
(A) Chromatogram for atRA with correct sample handling. Inset, 4,4-dimethyl-RA (internal standard) shows only all-trans form. (B) Chromatogram for atRA illustrating handling-induced isomerization by acid contamination. Inset, cis-isomers (arrows) of 4,4-dimethyl-RA. Note the decrease in atRA and increase in cis-isomers concurrent with cis-isomers in the internal standard. Peak identities: (1) 4,4-dimethyl-RA, (2) atRA, (3) 9-cis-RA and (4) 13-cis-RA. Peaks 5 and 6 probably indicate 9,13-di-cis-RA and 11-cis-RA respectively.
Percentage recovery
Recovery reflects extraction efficiency and handling losses. Percentage recovery was determined from the amount of 4,4-dimethyl-RA measured compared with the amount added. The average recovery (±S.E.M.) for all tissues was 80±2% (n=52). For serum, recovery was quantitative (101±2%; n=10). In tissue samples containing high levels of lipids, additional signals were observed in the 4,4-dimethyl-RA (m/z=329.4/151.3 transition at ∼tR=10 min, where tR is retention time); however, they did not interfere with quantification of 4,4-dimethyl-RA or its isomers. In samples containing fewer lipids, e.g. serum or cultured cell extracts, this additional signal was insignificant or non-existent.
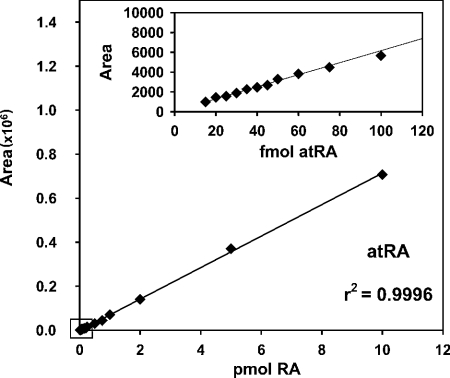
Linear range
Calibration curves for atRA (Figure 5), 9-cis-RA and 13-cis-RA established a linear working range of 20 fmol (0.3 ng/ml, 6 pg on column) to 10 pmol (150 ng/ml, 3 ng on column). Similar data were generated for 13-cis-RA and 9-cis-RA (results not shown). Note that, even at the very low end of the linear working range, the best-fit line coincided with the data. Linearity of each curve, as expressed by the correlation coefficient, r2, was >0.999 for atRA, 9-cis-RA and 13-cis-RA.
Figure 5. Calibration curve for atRA.
The linear working range is 20 fmol to 10 pmol. The inset shows the agreement of data points to the best-fit line at <100 fmol. Similar curves were generated for 9-cis-RA and 13-cis-RA.
Limits of detection and quantification
The lower LOD (limit of detection), defined by a signal/noise ratio of 3:1, was 10 fmol (0.15 ng/ml, 3 pg on column) (Figure 6). The lower LOQ (limit of quantification), defined by a signal/noise ratio of 10:1, was 20 fmol (0.3 ng/ml, 6 pg on column). The amount of sample that can be applied without contaminating the instrument constrains the upper limit of this assay.
Figure 6. Representative data showing the lower LOQ (20 fmol) and lower LOD (10 fmol) for atRA.
Precision and accuracy
Precision, measured by the instrumental CV (coefficient of variance), ranged from 0.5 to 2.9%. The instrumental CV was obtained by repeat measurements of standard samples on the same day. Accuracy, the agreement between the applied and measured amounts, was >97% above 100 fmol and >90% at lower values.
Reproducibility
Reproducibility of the assay was evaluated in terms of the intra-assay CV (same-day) and inter-assay CV (different day). Intra-assay and inter-assay variations were obtained using mouse liver and mouse kidney samples. For intra-assay variation, multiple aliquots of mouse liver from several mice were extracted and analysed. The intra-assay CV was 5.4±0.4% (n=9 mice, two analyses each mouse). For inter-assay variation, samples of minced tissues (liver or kidney) were frozen immediately, stored at −80 °C for 2 days, and homogenized on the day of analysis. The inter-assay CV was 8.9±1% (n=13 samples). Homogenized liver and kidney also were analysed for inter-assay variation. Homogenized samples frozen immediately and stored at −80 °C for 1 day gave comparable results (within 10%) with those of freshly analysed samples; however, homogenized samples stored for 1 month gave values that were ∼50% less than for samples analysed immediately. Therefore it should be possible to store tissue homogenates overnight in the freezer, but long-term storage of homogenized samples should be avoided, even at −80 °C.
Sample size
The ability to analyse very limited samples derives from sensitivity. We analysed routinely ∼10–20 mg wet weight of tissue samples, ∼10-fold less than has been reported for most RA assays. The ability to analyse small tissue samples allowed direct quantification of RA in specific brain loci, and represents one improvement offered by this assay.
RA in wild-type and CRBP-null mice
We quantified RA in mouse tissues and serum to demonstrate the utility of this assay (Table 1). atRA concentrations in the four tissues examined were close, ranging from 7 to 10 pmol/g of tissue (∼7–10 nM), whereas serum atRA was lower at ∼2 pmol/ml. Tissue 13-cis-RA concentrations ranged from 3 to 4 pmol/g of tissue; serum was ∼1 pmol/ml. CRBP-null mice do not exhibit abnormalities characteristic of vitamin A deficiency, implying that steady-state RA levels were not changed sufficiently to cause gross symptoms, but RA levels have not been reported [33]. We therefore quantified RA in CRBP-null mice. Liver atRA values were decreased in CRBP-null mice by ∼30% (P<0.05), but other tissues of CRBP-null animals had normal atRA compared with age-matched wild-type animals. The reduction in CRBP-null mice liver atRA, while apparently insufficient to permit vitamin-A-deficiency symptoms, suggests a function of CRBP in liver RA homoeostasis. Lack of change in extrahepatic tissues may reflect continuing and perhaps accelerated influx of retinol and/or RA at the expense of liver, and may represent one mechanism of accelerated liver retinyl ester loss in CRBP-null mice.
Table 1. RA concentrations in mouse tissues.
Quantification was performed with 2–4-month-old male mice fed with a stock diet. RA values in CRBP-null mice were statistically equivalent to those in wild-type, except for liver. atRA values for liver had P<0.05 between wild-type and null mice, and 13-cis-RA values had P<0.085 between wild-type and CRBP-null. Values are the means±S.E.M. (the number of mice assayed is given in parentheses) expressed in pmol/g of tissue.
| Wild-type C57BL/6 | CRBP-null | |||
|---|---|---|---|---|
| Sample | atRA | 13-cis-RA | atRA | 13-cis-RA |
| Liver | 9.6±1.0 (12) | 3.6±0.6 (12) | 7.0±0.6 (11) | 2.2±0.2 (9) |
| Kidney | 7.0±1.3 (12) | 3.1±0.4 (12) | 8.2±1.5 (9) | 4.5±1.3 (11) |
| Testis | 9.3±1.4 (8) | 2.9±0.9 (7) | 11.3±0.8 (11) | 2.0±0.2 (9) |
| Brain | 7.7±0.9 (8) | 4.2±1.3 (7) | 7.7±1.3 (11) | 3.9±1.1 (9) |
| Serum | 1.9±0.4 (6) | 1.2±0.3 (3) | 2.3±0.2 (6) | 1.1±0.2 (3) |
RA in specific brain loci
No direct rigorous analytical quantification of RA has been made in brain loci. To address this, we quantified RA in the hippocampus, cortex, striatum and cerebellum of 12-month-old female mice (Table 2). Mouse striatum had triple the amount of atRA of whole brain and almost double the atRA amount of hippocampus; cortex had the lowest concentration. The amount of 13-cis-RA relative to atRA in the cortex and the hippocampus was higher (70 and 93%) than in other brain loci, whole brain and other tissues.
Table 2. RA concentrations in whole brain and brain loci.
Quantification was carried out with brains from 12-month-old female C57BL/6 mice fed with a stock diet. Values are the means±S.E.M. (the number of mice assayed is given in parentheses) expressed in pmol/g of tissue.
| Sample | atRA | 13-cis-RA |
|---|---|---|
| Brain (whole) | 5.4±0.4 (4)* | 1.5±0.4 (5) |
| Cerebellum | 10.7±0.3 (4)* | 3.0±0.8 (4) |
| Cortex | 2.6±0.4 (8)* | 1.8±0.3 (8) |
| Hippocampus | 8.4±1.2 (8) | 7.8±0.8 (8) |
| Striatum | 15.3±4.7 (5) | 8.0±2.2 (5) |
* Statistically different from each other (P<0.001).
Comparison with other validated assays
Our results for normal mouse serum compare well with results of other validated assays [19,41,42]. Results of assays based on HPLC with UV detection have reported values in mouse serum of 1.08±0.33 ng/g (n=2, ∼3.6 pmol/ml) for atRA and 0.32±0.14 ng/g (n=2, ∼1.1 pmol/ml) for 13-cis-RA in adult NMRI female mice [41] or 0.42 ng/ml (n=6, ∼1.4 pmol/ml) with a relative S.D. of 16.7% for atRA and <0.3 ng/ml (n=6, <1.0 pmol/ml) for 13-cis-RA in an unknown age/strain of mouse [42]. We previously used GC (gas chromatography)/MS to quantify total RA in human serum, and obtained values ranging from 8.6 to 22 pmol/ml. Human serum RA levels are typically higher than those of mouse [41]. Because the GC/MS values reflect total RA, other isomers besides atRA and 13-cis-RA (e.g. 9,13-di-cis-RA) were probably measured, as pointed out in the original publication [19].
We observed no signal for 9-cis-RA above ∼0.2 pmol/g of tissue. Quantification methods for isomers other than atRA, 13-cis-RA and 9-cis-RA are currently being developed.
Summary
Instrumental methods for measuring RA have included HPLC with UV detection, HPLC with electrochemical detection, GC/MS and LC/MS. HPLC with UV detection has the benefit of ease and economy, but has a lower LOQ of ∼1 pmol and does not provide mass identification [38,43]. Even recent advances in column technology and column switching capabilities have lowered detection limits only ∼10-fold [41,42]. HPLC with electrochemical detection has sensitivity in the femtomolar range, but lacks the definite mass identification of analytes of MS, is subject to interference from other analytes and has solvent/electrode/flow dependent sensitivity [44–47]. GC/MS affords sensitivity, with a lower LOD of <250 fmol, but GC/MS requires derivatization [19,48]. LC/MS-based assays offer mass identification of analytes, but do not have the potential sensitivity or the enhanced specificity of SRM. Existing LC/MS assays have various limitations, including use of single-quadrupole systems, which have less sensitivity than systems with multiple MS events, no incorporation of an internal standard or incorporation of a less similar compound (to RA) as an internal standard, or focus only on compound identification/qualitative information [22,34,35,49]. Non-instrumental methods, such as in vitro detection systems based on reporter gene expression, have not been developed into analytically rigorous assays, are not specific for all-trans-RA (e.g. 3,4-didehydro-RA, 9-cis-RA, 4-oxo-RA, 4-hydroxy-RA and 4-hydroxy-retinol all produce signals), are not quantitative, and can give both false-positive and false-negative results [28,29,50]. Additionally, because reporter detection systems reflect RAR activation, they cannot evaluate retinoid presence in real time, and may reflect receptor sensitization, not just retinoid binding.
Triple-quadrupole LC/MS/MS should enhance current methods of RA quantification by offering sensitivity, specificity, no requirement for derivatization and definitive mass identification. The LC/MS/MS assay reported in the present paper takes advantage of these characteristics to provide direct analysis of low amounts of RA with small sample aliquots (∼10–20 mg of tissue), minimum work-up and definitive mass identification. Direct measurements of endogenous RA should have a substantial impact on investigating the relationships between RA and memory formation, cognitive dysfunction, aging and various neurological diseases, such as Alzheimer's disease, Parkinson's disease, Huntington's disease, schizophrenia and depression.
Acknowledgments
This work was supported by NIH (National Institutes of Health) grants DK36870 and AG13566 (J. L. N.) and NIH NRSA (National Research Service Award)-Kirschstein Postdoctoral Training Grant DK66924 (M. A. K.). We are grateful to Lu Chen for guidance with the brain dissections. We are grateful to Pierre Chambon and Norbert Ghyselinck for the gift of CRBP-null mice.
References
- 1.Wolf G. Multiple functions of vitamin A. Physiol. Rev. 1984;64:873–937. doi: 10.1152/physrev.1984.64.3.873. [DOI] [PubMed] [Google Scholar]
- 2.Saari J. C. Biochemistry of visual pigment regeneration: the Friedenwald lecture. Invest. Ophthalmol. Vis. Sci. 2000;41:337–348. [PubMed] [Google Scholar]
- 3.Stephensen C. B. Vitamin A, infection, and immune function. Annu. Rev. Nutr. 2001;21:167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- 4.Sun S. Y., Lotan R. Retinoids and their receptors in cancer development and chemoprevention. Crit. Rev. Oncol. Hematol. 2002;41:41–55. doi: 10.1016/s1040-8428(01)00144-5. [DOI] [PubMed] [Google Scholar]
- 5.Napoli J. L. Vitamin A: retinoids. In: Lennarz W. J., Lane M. D., editors. Encyclopedia of Biological Chemistry, vol. 4. Elsevier: St. Louis; 2004. pp. 354–359. [Google Scholar]
- 6.Mey J., McCaffery P. Retinoic acid signaling in the nervous system of adult vertebrates. Neuroscientist. 2004;10:409–421. doi: 10.1177/1073858404263520. [DOI] [PubMed] [Google Scholar]
- 7.Chiang M. Y., Misner D., Kempermann G., Schikorski T., Giguere V., Sucov H. M., Gage F. H., Stevens C. F., Evans R. M. An essential role for retinoid receptors RAR-β and RXR-γ in long-term potentiation and depression. Neuron. 1998;21:1353–1361. doi: 10.1016/s0896-6273(00)80654-6. [DOI] [PubMed] [Google Scholar]
- 8.Etchamendy N., Enderlin V., Marighetto A., Vouimba R.-M., Pallet V., Jaffard R., Higueret P. Alleviation of selective age-related relational memory deficit in mice by pharmacologically induced normalization of brain retinoid signaling. J. Neurosci. 2001;21:6423–6429. doi: 10.1523/JNEUROSCI.21-16-06423.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Misner D. L., Jacobs S., Shimizu Y., de Urquiza A. M., Solomin L., Perlmann T., De Luca L. M., Stevens C. F., Evans R. M. Vitamin A deprivation results in reversible loss of hippocampal long-term synaptic plasticity. Proc. Natl. Acad. Sci. U.S.A. 2001;98:11714–11719. doi: 10.1073/pnas.191369798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etchamendy N., Enderlin V., Marighetto A., Pallet V., Higueret P., Jaffard R. Vitamin A deficiency and relational memory deficit in adult mice: relationships with changes in brain retinoid signaling. Behav. Brain Res. 2003;145:37–49. doi: 10.1016/s0166-4328(03)00099-8. [DOI] [PubMed] [Google Scholar]
- 11.Denisenko-Nehrbass N. L., Jarvis E., Scharff C., Nottebohm F., Mello C. V. Site-specific retinoic acid production in the brain of adult songbirds. Neuron. 2000;27:359–370. doi: 10.1016/s0896-6273(00)00043-x. [DOI] [PubMed] [Google Scholar]
- 12.Zelyaznik N., Schrage K., McCaffery P., Mey J. Activation of retinoic acid signaling after sciatic nerve injury: up-regulation of cellular retinoid binding proteins. Eur. J. Neurosci. 2003;18:1033–1040. doi: 10.1046/j.1460-9568.2003.02834.x. [DOI] [PubMed] [Google Scholar]
- 13.Krezel W., Ghyselinck N., Samad T. A., Dupe V., Kastner P., Borrelli E., Chambon P. Impaired locomotion and dopamine signaling in retinoid receptor mutant mice. Science. 1998;279:863–867. doi: 10.1126/science.279.5352.863. [DOI] [PubMed] [Google Scholar]
- 14.Goodman A. B., Pardee A. B. Evidence for defective retinoid transport and function in late onset Alzheimer's disease. Proc. Natl. Acad. Sci. U.S.A. 2003;100:2901–2905. doi: 10.1073/pnas.0437937100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corcoran J. P. T., So P. L., Maden M. Disruption of retinoid signaling pathway causes a deposition of amyloid β in the adult rat brain. Eur. J. Neurosci. 2004;20:896–902. doi: 10.1111/j.1460-9568.2004.03563.x. [DOI] [PubMed] [Google Scholar]
- 16.McCaffery P. J., Adams J., Maden M., Rosa-Molinar E. Too much of a good thing: retinoic acid as an endogenous regulator of neural differentiation and exogenous teratogen. Eur. J. Neurosci. 2003;18:457–472. doi: 10.1046/j.1460-9568.2003.02765.x. [DOI] [PubMed] [Google Scholar]
- 17.Sucov H. M., Evans R. M. Retinoic acid and retinoic acid receptors in development. Mol. Neurobiol. 1995;10:169–184. doi: 10.1007/BF02740674. [DOI] [PubMed] [Google Scholar]
- 18.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 2001;10:940–954. [PubMed] [Google Scholar]
- 19.Napoli J. L., Pramanik B. C., Williams J. B., Dawson M. I., Hobbs P. D. Quantification of retinoic acid by gas–liquid chromatography–mass spectrometry: total versus all-trans retinoic acid in human plasma. J. Lipid Res. 1985;26:387–392. [PubMed] [Google Scholar]
- 20.Tang G. W., Russell R. M. 13-cis-retinoic acid is an endogenous compound in human serum. J. Lipid Res. 1990;31:175–182. [PubMed] [Google Scholar]
- 21.Barua B. A., Olson J. A. Retinoyl β-glucuronide: an endogenous compound of human blood. Am. J. Clin. Nutr. 1986;43:481–485. doi: 10.1093/ajcn/43.4.481. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt C. K., Volland J., Hamscher G., Nau H. Characterization of a new endogenous vitamin A metabolite. Biochim. Biophys. Acta. 2002;1583:237–251. doi: 10.1016/s1388-1981(02)00212-3. [DOI] [PubMed] [Google Scholar]
- 23.Ruberte E., Friederich V., Chambon P., Moriss-Kay G. Retinoic acid receptors and cellular retinoid binding proteins: their differential pattern of transcription during early morphogenesis in mouse embryos. Development. 1991;111:45–60. doi: 10.1242/dev.111.1.45. [DOI] [PubMed] [Google Scholar]
- 24.Yamagata T., Momoi M. Y., Yanagisawa M., Kumagai H., Yamakado M., Momoi T. Changes of the expression and distribution of retinoic acid receptors during neurogenesis in mouse embryos. Dev. Brain Res. 1994;77:163–176. doi: 10.1016/0165-3806(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 25.Krezel W., Kastner P., Chambon P. Differential expression of retinoid receptors in the adult mouse central nervous system. Neuroscience. 1999;89:1291–1300. doi: 10.1016/s0306-4522(98)00342-x. [DOI] [PubMed] [Google Scholar]
- 26.Giguere V. Retinoic acid receptors and cellular retinoid binding proteins: complex interplay in retinoid signaling. Endocr. Rev. 1994;15:61–79. doi: 10.1210/edrv-15-1-61. [DOI] [PubMed] [Google Scholar]
- 27.Maden M. Role and distribution of retinoic acid during CNS development. Int. Rev. Cytol. 2001;209:1–77. doi: 10.1016/s0074-7696(01)09010-6. [DOI] [PubMed] [Google Scholar]
- 28.Luo T., Wagner E., Grün F., Dräger U. C. Retinoic acid signaling in the brain marks the formation of optic projections, maturation of the dorsal telencephalon, and function of limbic sites. J. Comp. Neurol. 2004;470:297–316. doi: 10.1002/cne.20013. [DOI] [PubMed] [Google Scholar]
- 29.Wagner M. A. Use of reporter cells to study endogenous retinoid sources in embryonic tissues. Methods Enzymol. 1997;282:98–107. doi: 10.1016/s0076-6879(97)82099-x. [DOI] [PubMed] [Google Scholar]
- 30.Zetterstrom R. H., Lindqvist E., Mata de Urquiza A., Tomac A., Eriksson U., Perlmann T., Olson L. Role of retinoids in the CNS: differential expression of retinoid binding proteins and receptors and evidence for presence of retinoic acid. Eur. J. Neurosci. 1999;11:407–416. doi: 10.1046/j.1460-9568.1999.00444.x. [DOI] [PubMed] [Google Scholar]
- 31.Dawson M. I., Hobbs P. D., Chan R. L., Chao W. R. Retinoic acid analogues with ring modifications: synthesis and pharmacological activity. J. Med. Chem. 1981;24:1214–1223. doi: 10.1021/jm00142a018. [DOI] [PubMed] [Google Scholar]
- 32.Barua A. B., Furr H. C. Properties of retinoids. Mol. Biotechnol. 1998;10:167–182. doi: 10.1007/BF02760863. [DOI] [PubMed] [Google Scholar]
- 33.Ghyselinck N. B., Bavik C., Sapin V., Mark M., Bonnier D., Hindelang C., Dierich A., Nilsson C. B., Hakansson H., Sauvant P., et al. Cellular retinol-binding protein I is essential for vitamin A homeostasis. EMBO J. 1999;18:4903–4914. doi: 10.1093/emboj/18.18.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCaffery P., Evans J., Koul O., Volpert A., Reid K., Ullman M. D. Retinoid quantification by HPLC/MSn. J. Lipid Res. 2002;43:1143–1149. doi: 10.1194/jlr.d200012-jlr200. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y., Chang W. Y., Prins G. S., van Breeman R. B. Simultaneous determination of all-trans, 9-cis, 13-cis retinoic acid and retinol in rat prostate using liquid chromatography–mass spectrometry. J. Mass Spectrom. 2001;36:882–888. doi: 10.1002/jms.189. [DOI] [PubMed] [Google Scholar]
- 36.van Breeman R. B., Nikolic D., Xu X., Xiong Y., van Lieshout M., West C. E., Schilling A. B. Development of a method for quantification of retinol and retinyl palmitate in human serum using high-performance liquid chromatography–atmospheric pressure chemical ionization–mass spectrometry. J. Chromatogr. A. 1998;794:245–251. doi: 10.1016/s0021-9673(97)01138-2. [DOI] [PubMed] [Google Scholar]
- 37.De Hoffmann E., Charette J., Stroobant V. Paris: John Wiley & Sons; 1996. Mass Spectrometry: Principles and Applications. [Google Scholar]
- 38.Napoli J. L. Quantification of physiological levels of retinoic acid. Method Enzymol. 1986;123:112–124. doi: 10.1016/s0076-6879(86)23015-3. [DOI] [PubMed] [Google Scholar]
- 39.Barua A. B., Furr H. C. Properties of retinoids: structure, handling, and preparation. Methods Mol. Biol. 1998;89:3–28. doi: 10.1385/0-89603-438-0:3. [DOI] [PubMed] [Google Scholar]
- 40.Napoli J. L., Horst R. L. Quantitative analyses of naturally occurring retinoids. Methods Mol. Biol. 1998;89:29–40. doi: 10.1385/0-89603-438-0:29. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt C. K., Brouwer A., Nau H. Chromatographic analysis of endogenous retinoids in tissues and serum. Anal. Biochem. 2003;315:36–48. doi: 10.1016/s0003-2697(02)00662-0. [DOI] [PubMed] [Google Scholar]
- 42.Wyss R., Bucheli F. Determination of endogenous levels of 13-cis-retinoic acid (isotretinoin), all-trans-retinoic acid (tretinoin), and their 4-oxo metabolites in human and animal plasma by high-performance liquid chromatography with automated column switching and ultraviolet detection. J. Chromatogr. B Biomed. Sci. Appl. 1997;700:31–47. doi: 10.1016/s0378-4347(97)00303-4. [DOI] [PubMed] [Google Scholar]
- 43.Gundersen T. E., Blomhoff R. Qualitative and quantitative liquid chromatographic determination of natural retinoids in biological samples. J. Chromatogr. A. 2001;935:13–43. doi: 10.1016/s0021-9673(01)01043-3. [DOI] [PubMed] [Google Scholar]
- 44.Hagen J. J., Washco K. A., Monning C. A. Determination of retinoids by reversed-phase capillary liquid chromatography with amperometric electrochemical detection. J. Chromatogr. B Biomed. Sci. Appl. 1996;677:225–231. doi: 10.1016/0378-4347(95)00465-3. [DOI] [PubMed] [Google Scholar]
- 45.Sakhi A. K., Gundersen T. E., Ulven S. M., Blomhoff R., Laundanes E. Quantitative determination of endogenous retinoids in mouse embryos by high-performance liquid chromatography with online solid-phase extraction, column switching, and electrochemical detection. J. Chromatogr. A. 1998;828:451–460. doi: 10.1016/s0021-9673(98)00676-1. [DOI] [PubMed] [Google Scholar]
- 46.Ulven S. M., Gundersen T. E., Weedon M. S., Landaas V. O., Sakhi A. K., Fromm S. H., Geronimo B. A., Moskaug J. O., Blomhoff R. Identification of endogenous retinoids, enzymes, binding proteins, and receptors during early post-implantation development in the mouse: important role of retinal dehydrogenase type 2 in synthesis of all-trans-retinoic acid. Dev. Biol. 2000;220:379–391. doi: 10.1006/dbio.2000.9634. [DOI] [PubMed] [Google Scholar]
- 47.Toth K., Stulik K., Kutner W., Feher Z., Linder E. Electrochemical detection in liquid flow techniques: characterization and classification. Pure Appl. Chem. 2004;76:1119–1138. [Google Scholar]
- 48.Napoli J. L. Quantification and characteristics of retinoid synthesis from retinol and β-carotene in tissue fractions and established cell lines. Methods Enzymol. 1990;189:470–482. doi: 10.1016/0076-6879(90)89324-b. [DOI] [PubMed] [Google Scholar]
- 49.Chithalen J. V., Luu L., Petkovich M., Jones G. HPLC–MS/MS analysis of products generated from all-trans-retinoic acid using recombinant human CYP26A. J. Lipid Res. 2002;43:1133–1142. doi: 10.1194/jlr.m100343-jlr200. [DOI] [PubMed] [Google Scholar]
- 50.Wagner M., Han B., Jessell T. M. Regional differences in retinoid release from embryonic neural tissue detected by an in vitro reporter assay. Development. 1992;116:55–66. doi: 10.1242/dev.116.1.55. [DOI] [PubMed] [Google Scholar]