Abstract
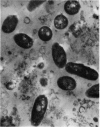
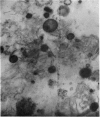
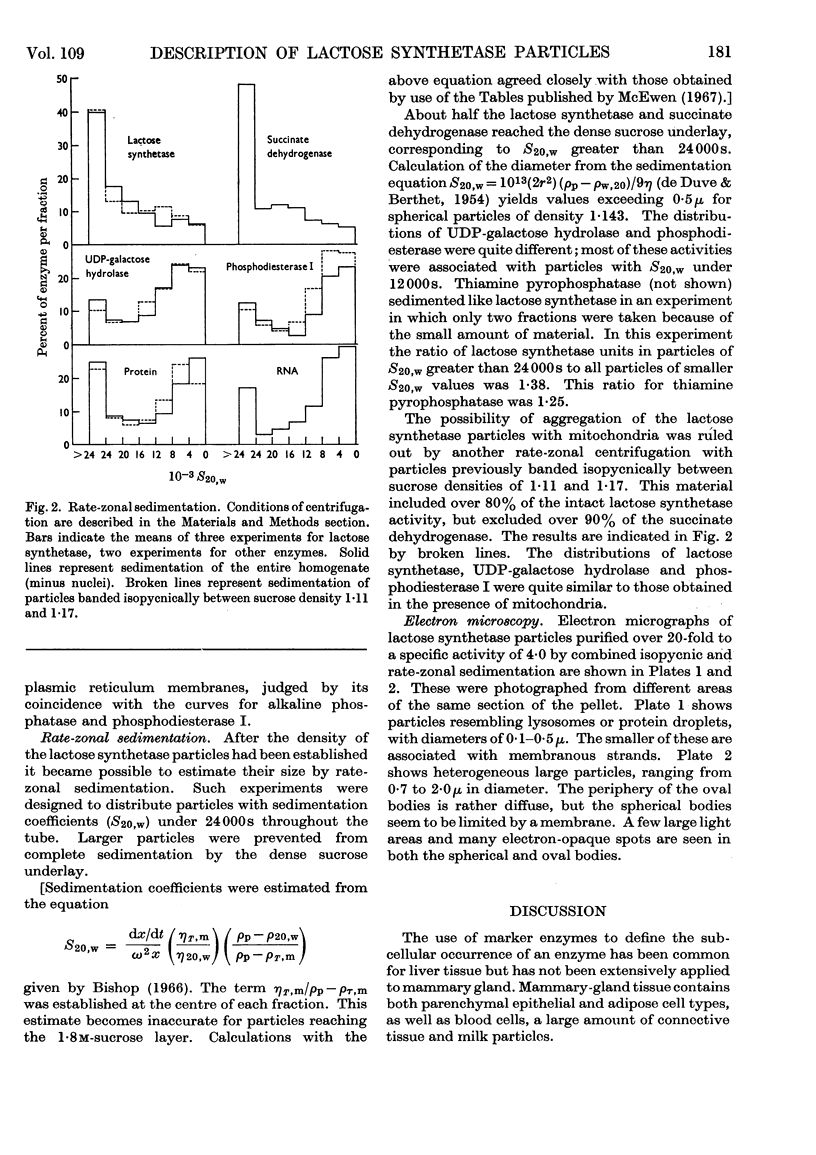
1. The conditions that promoted the solubilization of particulate lactose synthetase were effective for solubilizing the thiamine pyrophosphatase of the Golgi apparatus but differed from those effective for β-glucuronidase or acid phosphatase of lysosomes. 2. Lactose synthetase-containing particles did not bind Mg2+ or Cs+ ions, suggesting that they are not related to endoplasmic reticulum membranes. 3. Intact lactose synthetase and thiamine pyrophosphatase particles banded isopycnically at a density of 1·143 in a sucrose gradient. The dissociated `A' sub-unit of lactose synthetase, UDP-galactose hydrolase, p-nitrophenyl phosphate acid phosphatase, alkaline phosphatase and phosphodiesterase I were associated with particles of a broad density range from 1·12 to 1·20. Lysosomal enzymes β-glucuronidase, arylsulphatase and β-glycerophosphate acid phosphatase were associated with particles of density 1·20, 1·175 and 1·15 respectively. 4. Rate-zonal sedimentation studies indicated that lactose synthetase particles have S20,w values exceeding 24000s, corresponding to spherical particles of diameter exceeding 5·4×10−5cm. 5. Electron micrographs of lactose synthetase particles purified over 20-fold revealed small spherical bodies (0·1–0·5μ) resembling lysosomes, the smaller of which were attached to membranes, and larger heterogeneous spherical or oval bodies (0·7–1·8μ) resembling lipofuscin secretory granules. 6. The relationship between lactose synthetase particles and the Golgi origin of secretion granules is discussed.
Full text
PDF

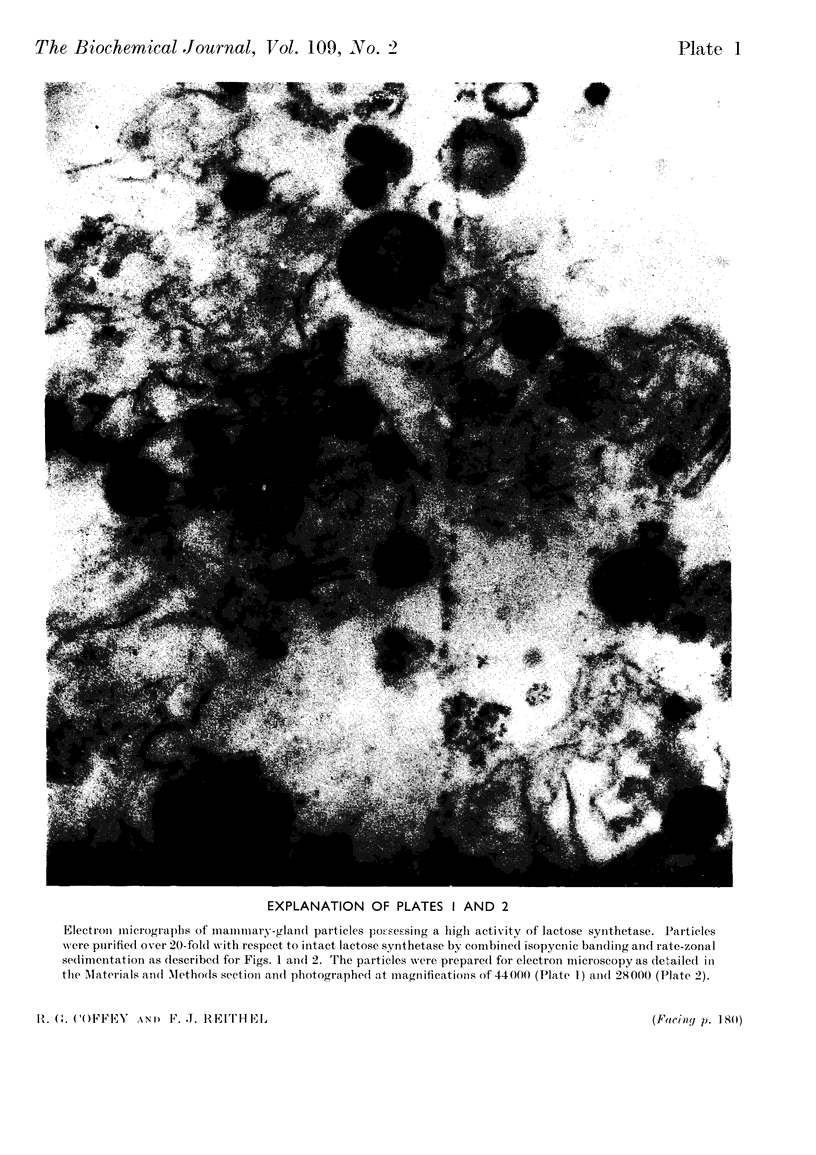



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- APPELMANS F., DE DUVE C. Tissue fractionation studies. 3. Further observations on the binding of acid phosphatase by rat-liver particles. Biochem J. 1955 Mar;59(3):426–433. doi: 10.1042/bj0590426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. M., Beard M. E. Alpha-hydroxy acid oxidase: localization in renal microbodies. Science. 1965 Sep 24;149(3691):1507–1509. doi: 10.1126/science.149.3691.1507. [DOI] [PubMed] [Google Scholar]
- Anderson N. G., Barringer H. P., Babelay E. F., Nunley C. E., Bartkus M. J., Fisher W. D., Rankin C. T., Jr The design and operation of the B-IV zonal centrifuge system. Natl Cancer Inst Monogr. 1966 Jun;21:137–164. [PubMed] [Google Scholar]
- Barber A. A., Rankin C. T., Jr, Anderson N. G. Lipid peroxidation in rat tissue particulates separated by zonal centrifugation. Natl Cancer Inst Monogr. 1966 Jun;21:333–344. [PubMed] [Google Scholar]
- Bishop B. S. Digital computation of sedimentation coefficients in zonal centrifuges. Natl Cancer Inst Monogr. 1966 Jun;21:175–188. [PubMed] [Google Scholar]
- Bowers W. E., Finkenstaedt J. T., de Duve C. Lysosomes in lymphoid tissue. I. The measurement of hydrolytic activities in whole homogenates. J Cell Biol. 1967 Feb;32(2):325–337. doi: 10.1083/jcb.32.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodbeck U., Ebner K. E. The subcellular distribution of the A and B proteins of lactose synthetase in bovine and rat mammary tissue. J Biol Chem. 1966 Dec 10;241(23):5526–5532. [PubMed] [Google Scholar]
- Coffey R. G., Reithel F. J. The lactose synthetase particles of lactating bovine mammary gland. Preparation of particles with intact lactose synthetase. Biochem J. 1968 Sep;109(2):169–176. doi: 10.1042/bj1090169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBAUM A. L., SLATER T. F., WANG D. Y. Lysosomal-like particles in the rat mammary gland. Nature. 1960 Oct 22;188:318–320. doi: 10.1038/188318a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McEwen C. R. Tables for estimating sedimentation through linear concentration gradients of sucrose solution. Anal Biochem. 1967 Jul;20(1):114–149. doi: 10.1016/0003-2697(67)90271-0. [DOI] [PubMed] [Google Scholar]
- ROY A. B. Comparative studies on the liver sulphatases. Biochem J. 1958 Mar;68(3):519–528. doi: 10.1042/bj0680519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEIDER W. C., KUFF E. L. On the isolation and some biochemical properties of the Golgi substance. Am J Anat. 1954 Mar;94(2):209–224. doi: 10.1002/aja.1000940203. [DOI] [PubMed] [Google Scholar]
- SELLINGER O. Z., BEAUFAY H., JACQUES P., DOYEN A., DE DUVE C. Tissue fractionation studies. 15. Intracellular distribution and properties of beta-N-acetylglucosaminidase and beta-galactosidase in rat liver. Biochem J. 1960 Mar;74:450–456. doi: 10.1042/bj0740450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S., Easter D. J., Dils R. Fatty acid biosynthesis. 3. Intracellular site of enzymes in lactating-rabbit mammary gland. Biochim Biophys Acta. 1966 Dec 7;125(3):445–455. [PubMed] [Google Scholar]
- WELLINGS S. R., DEOME K. B., PITELKA D. R. Electron microscopy of milk secretion in the mammary gland of the C3H/Crgl mouse. I. Cytomorphology of the prelactating and the lactating gland. J Natl Cancer Inst. 1960 Aug;25:393–421. [PubMed] [Google Scholar]
- el-Aaser A. A., Reid E., Klucis E., Alexander P., Lett J. T., Smith J. Resolution of the components in the microsomal fraction of liver in the B-IV zonal centrifuge. Natl Cancer Inst Monogr. 1966 Jun;21:323–332. [PubMed] [Google Scholar]