Abstract
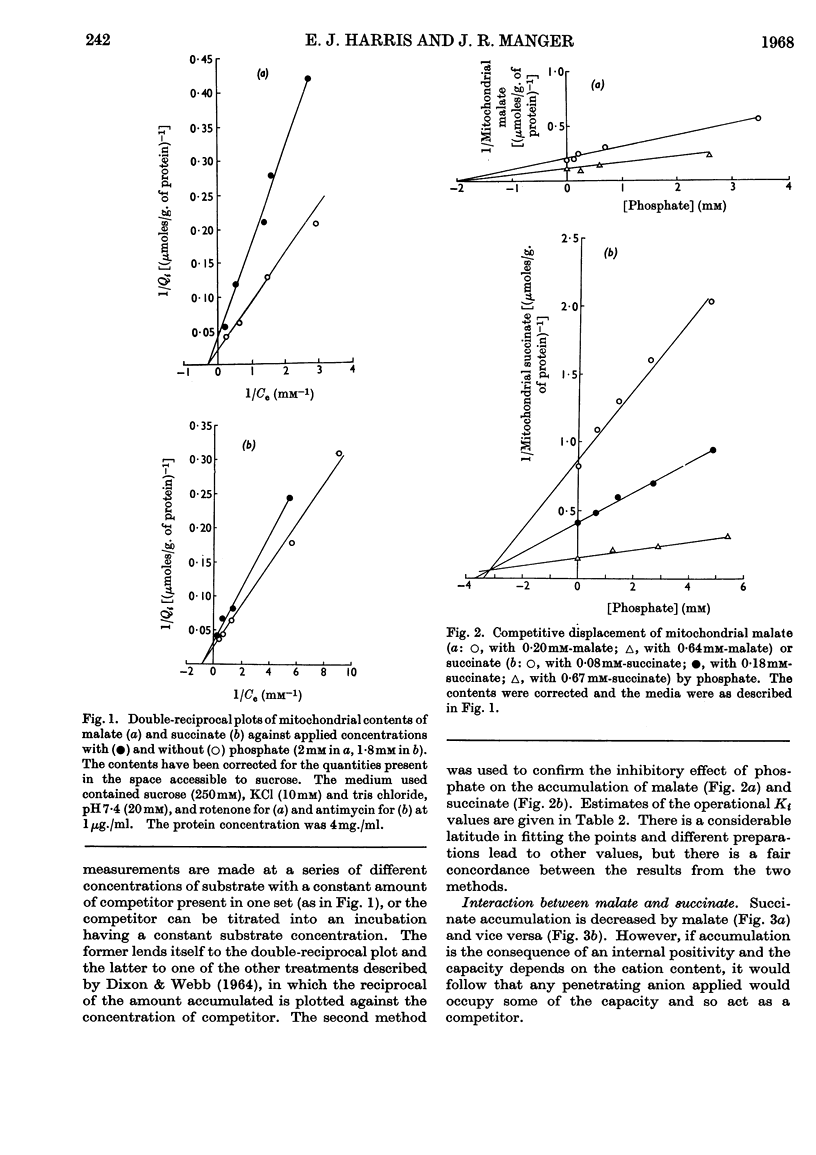
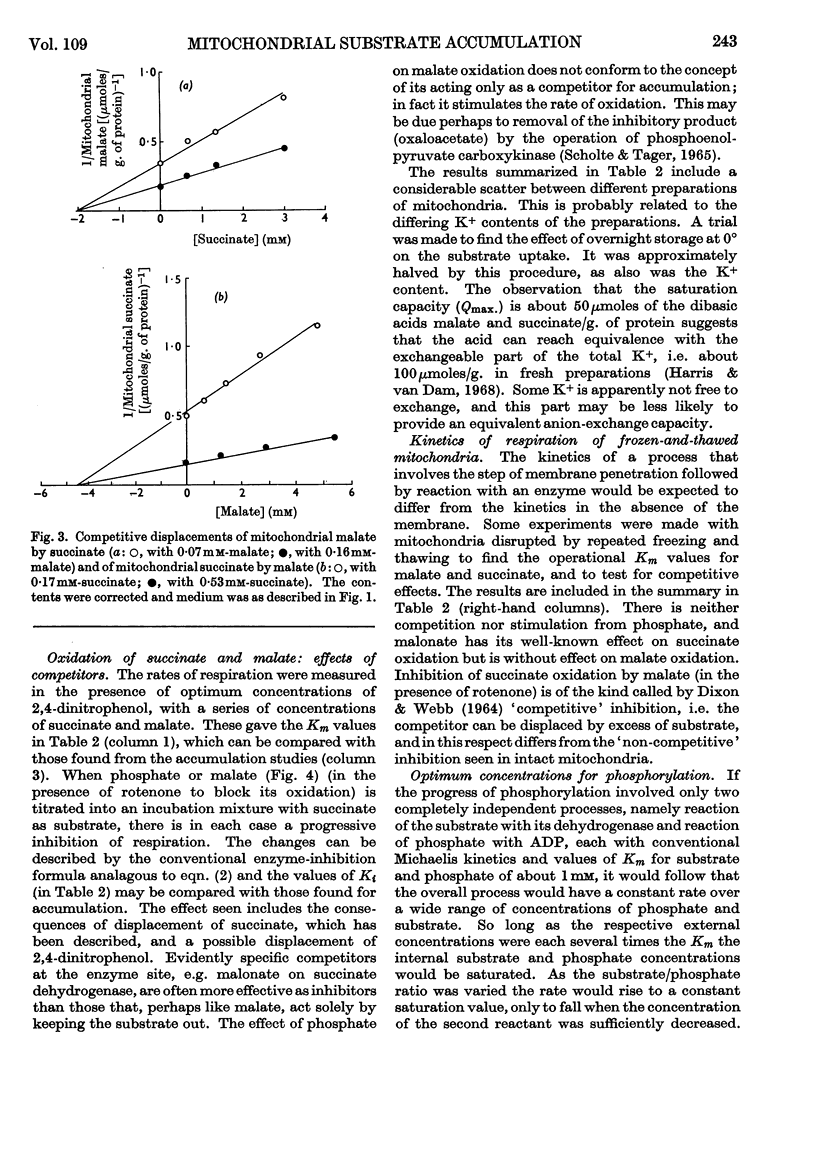
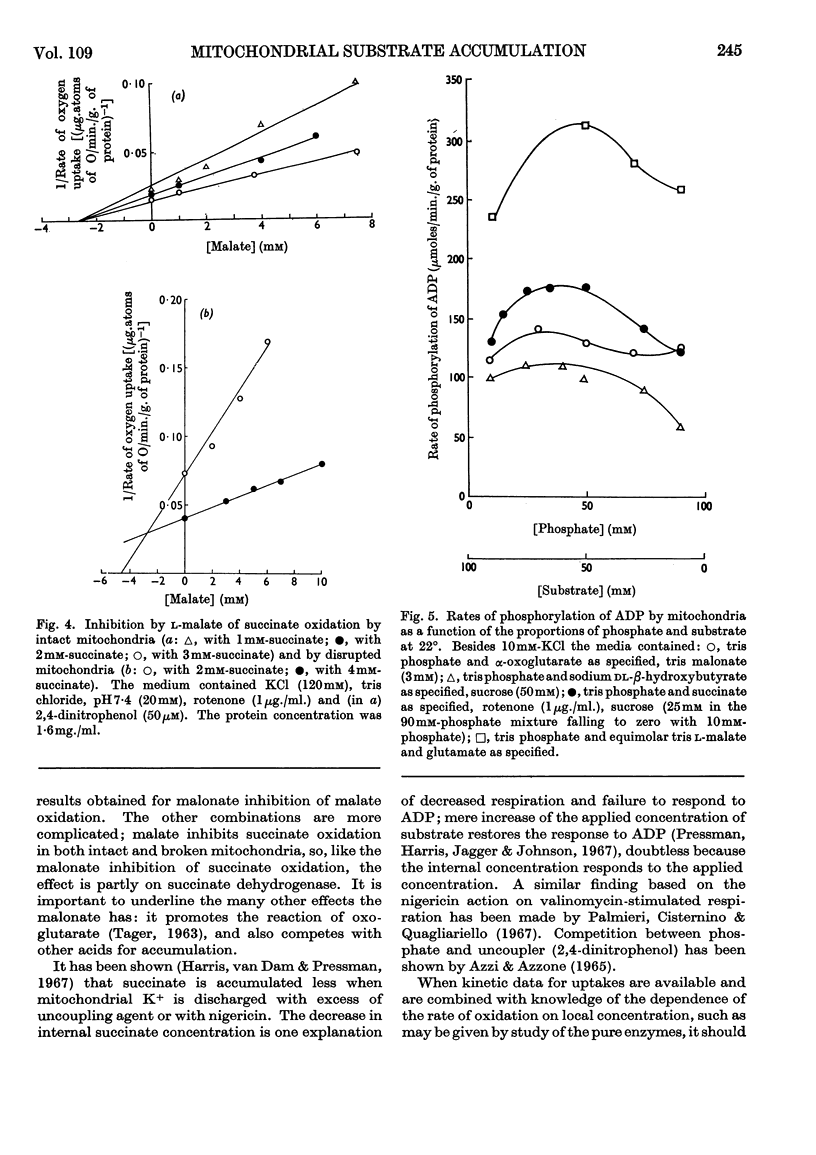
1. The concentration-dependence of the intramitochondrial accumulation of l-malate and succinate was measured and expressed in the form of adsorption isotherms. The accumulation, however, may arise because of an internal positive potential. 2. The competition for accumulation offered by some other anions, including phosphate, was measured and is expressed conventionally by additional terms in the adsorption equation. 3. The interactions between anions were also studied when one was acting as oxidized substrate. 4. In some examples there is a parallel between the effects of an added anion on both accumulation and oxidation; in other cases chemical participation of the added substance in metabolism is presumed to remove the correlation. 5. It is suggested that by combining kinetic data on penetration with stoicheiometric data on accumulation and specific reaction rates it may be possible to account for the rates of respiration obtained with intact mitochondria. 6. It is possible to show that there is a certain phosphate/substrate ratio for maximum phosphorylation rate with some substrates. This is to be expected when phosphate and substrate compete for accumulation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzi A., Azzone G. F. Swelling and shrinkage phenomena in liver mitochondria. I. Large amplitude swelling induced by inorganic phosphate and by ATP. Biochim Biophys Acta. 1965 Aug 24;105(2):253–264. doi: 10.1016/s0926-6593(65)80150-3. [DOI] [PubMed] [Google Scholar]
- Chappell J. B. The oxidation of citrate, isocitrate and cis-aconitate by isolated mitochondria. Biochem J. 1964 Feb;90(2):225–237. doi: 10.1042/bj0900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S. M., Williams G. R. The effect of malate and other dicarboxylic acids on mitochondrial isocitrate metabolism. J Biol Chem. 1966 Aug 25;241(16):3696–3700. [PubMed] [Google Scholar]
- GAMBLE J. L., Jr ACCUMULATION OF CITRATE AND MALATE BY MITOCHONDRIA. J Biol Chem. 1965 Jun;240:2668–2672. [PubMed] [Google Scholar]
- HAGIHARA B. Techniques for the application of polarography to mitochondrial respiration. Biochim Biophys Acta. 1961 Jan 1;46:134–142. doi: 10.1016/0006-3002(61)90656-4. [DOI] [PubMed] [Google Scholar]
- HASLAM R. J., KREBS H. A. Substrate competition in the respiration of animal tissues. The metabolic interactions of pyruvate and alpha-oxoglutarate in rat-liver homogenates. Biochem J. 1963 Mar;86:432–446. doi: 10.1042/bj0860432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. J. The dependence on dicarboxylic acids and energy of citrate accumulation in depleted rat liver mitochondria. Biochem J. 1968 Sep;109(2):247–251. doi: 10.1042/bj1090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. J., van Dam K. Changes of total water and sucrose space accompanying induced ion uptake or phosphate swelling of rat liver mitochondria. Biochem J. 1968 Feb;106(3):759–766. doi: 10.1042/bj1060759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. J., van Dam K., Pressman B. C. Dependence of uptake of succinate by mitochondria on energy and its relation to potassium retention. Nature. 1967 Mar 18;213(5081):1126–1127. doi: 10.1038/2131126a0. [DOI] [PubMed] [Google Scholar]
- Max S. R., Purvis J. L. Energy-linked incorporation of citrate into rat liver mitochondria. Biochem Biophys Res Commun. 1965 Dec 21;21(6):587–594. doi: 10.1016/0006-291x(65)90526-7. [DOI] [PubMed] [Google Scholar]
- Palmieri F., Cisternino M., Quagliariello E. Inhibition of uptake and oxidation of succinate in rat-liver mitochondria. Biochim Biophys Acta. 1967;143(3):625–627. doi: 10.1016/0005-2728(67)90068-0. [DOI] [PubMed] [Google Scholar]
- Pressman B. C., Harris E. J., Jagger W. S., Johnson J. H. Antibiotic-mediated transport of alkali ions across lipid barriers. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1949–1956. doi: 10.1073/pnas.58.5.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte H. R., Tager J. M. On the nature of the compartmentation of the substrate-linked phosphorylation in rat-liver mitochondria. Biochim Biophys Acta. 1965 Nov 22;110(2):252–258. doi: 10.1016/s0926-6593(65)80032-7. [DOI] [PubMed] [Google Scholar]
- TAGER J. M. Stimulation by malonate or isocitrate of the transfer of hydrogens from alpha-oxoglutarate to alpha-oxoglutarate plus ammonia in rat-liver mitochondria. Biochim Biophys Acta. 1963 Jun 11;73:341–343. doi: 10.1016/0006-3002(63)90322-6. [DOI] [PubMed] [Google Scholar]
- Tyler D. D., Gonze J., Estabrook R. W. Observations on the inhibitor sensitivity of the choline oxidation system. Arch Biochem Biophys. 1966 Aug;115(2):373–384. doi: 10.1016/0003-9861(66)90287-6. [DOI] [PubMed] [Google Scholar]
- WERKHEISER W. C., BARTLEY W. The study of steady-state concentrations of internal solutes of mitochondria by rapid centrifugal transfer to a fixation medium. Biochem J. 1957 May;66(1):79–91. doi: 10.1042/bj0660079. [DOI] [PMC free article] [PubMed] [Google Scholar]


