Abstract
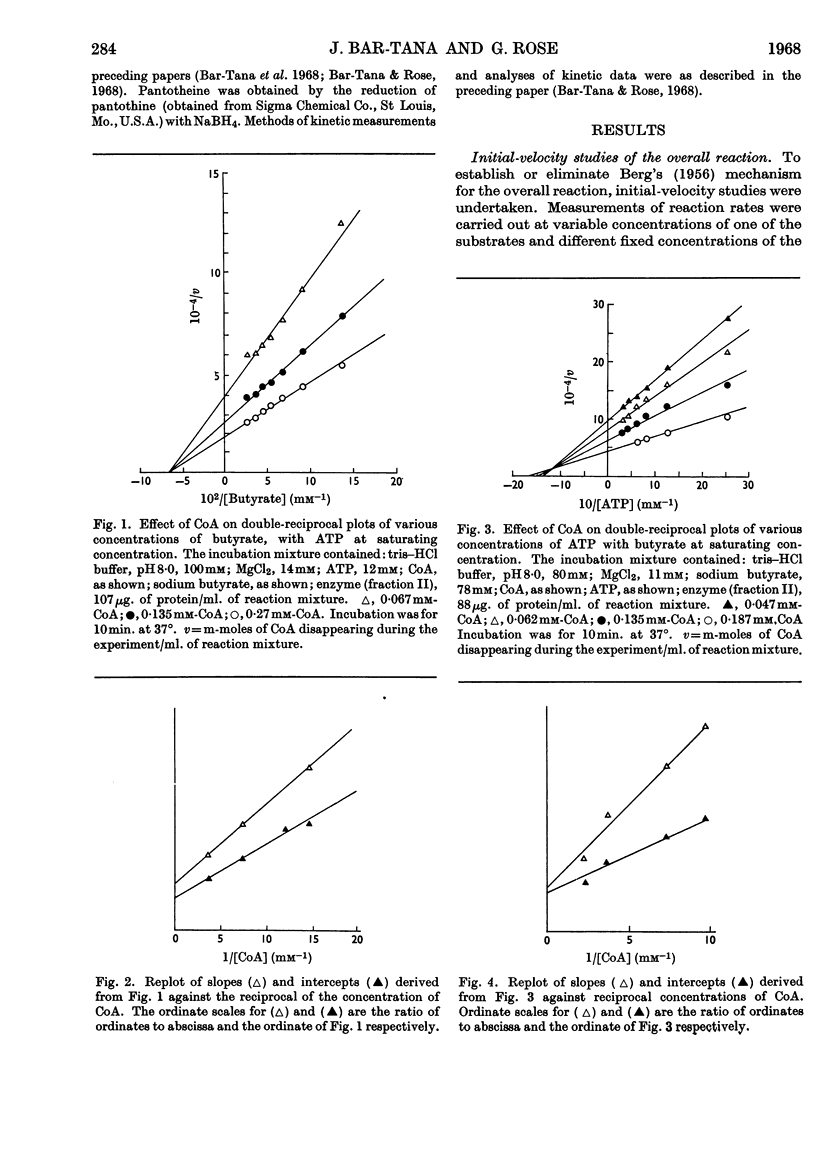
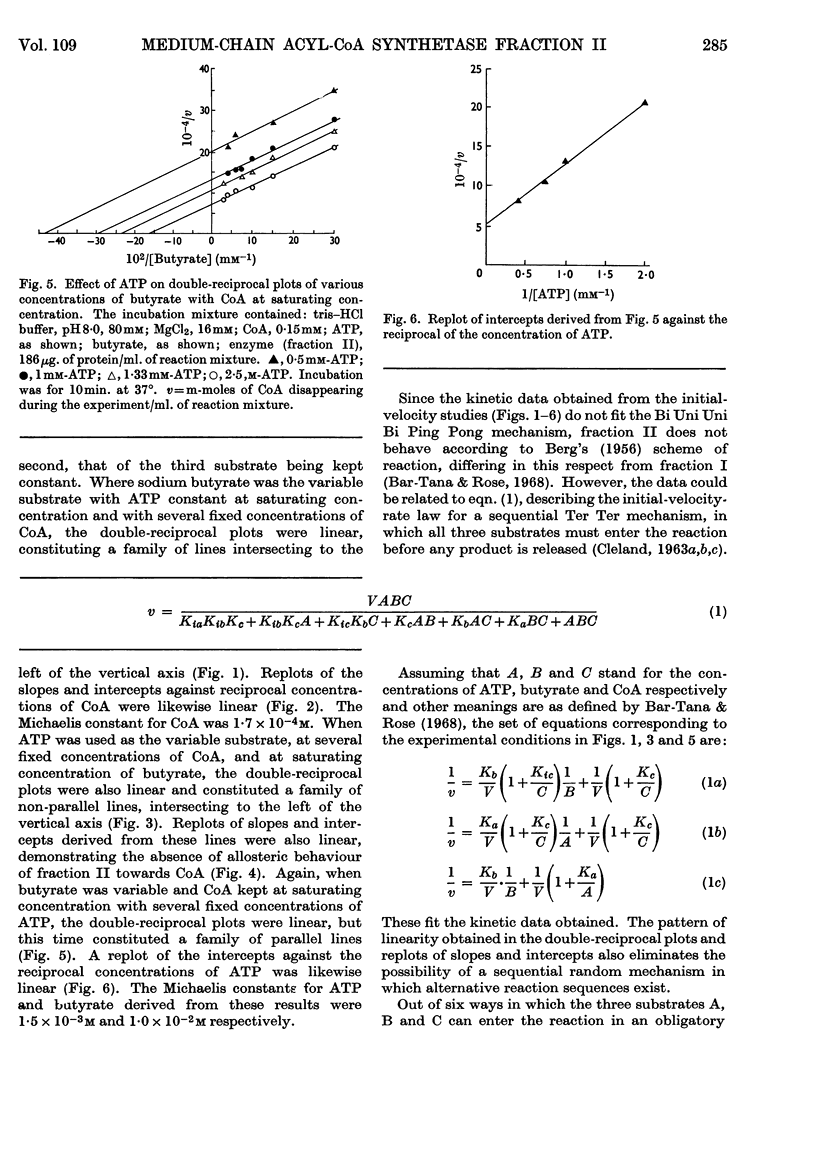
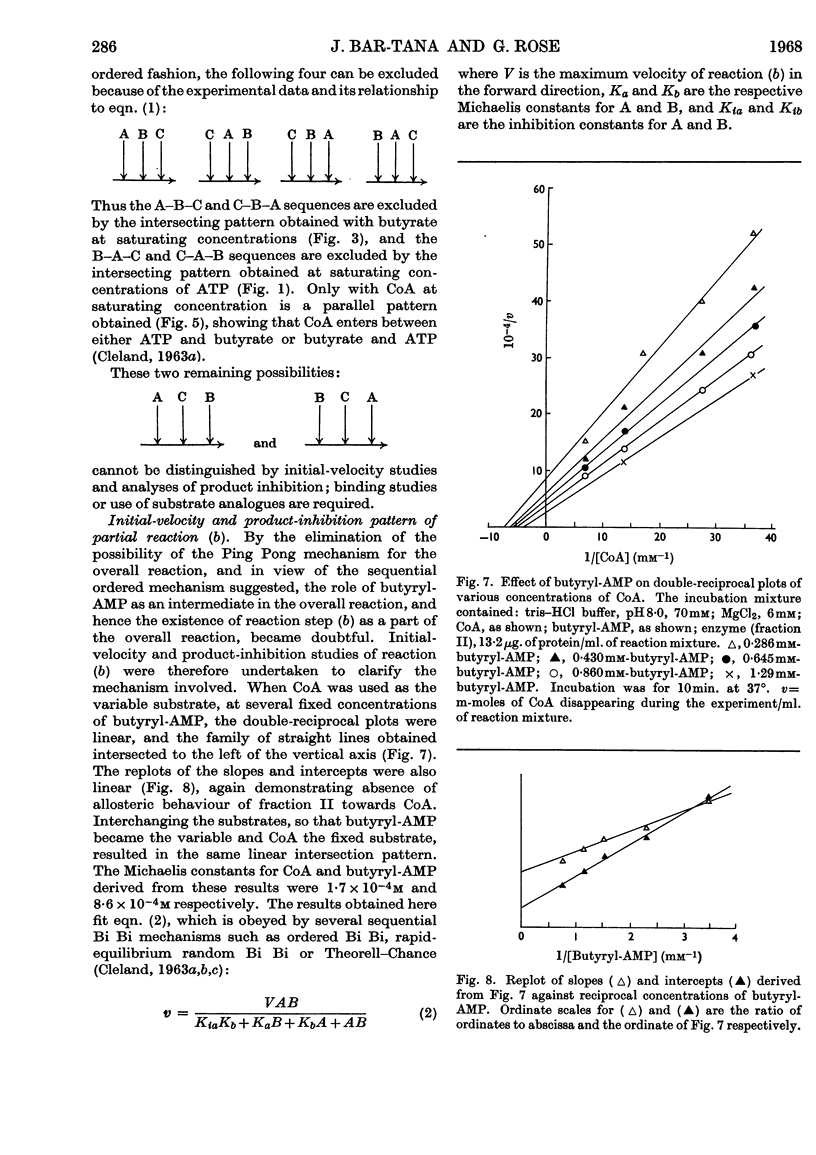
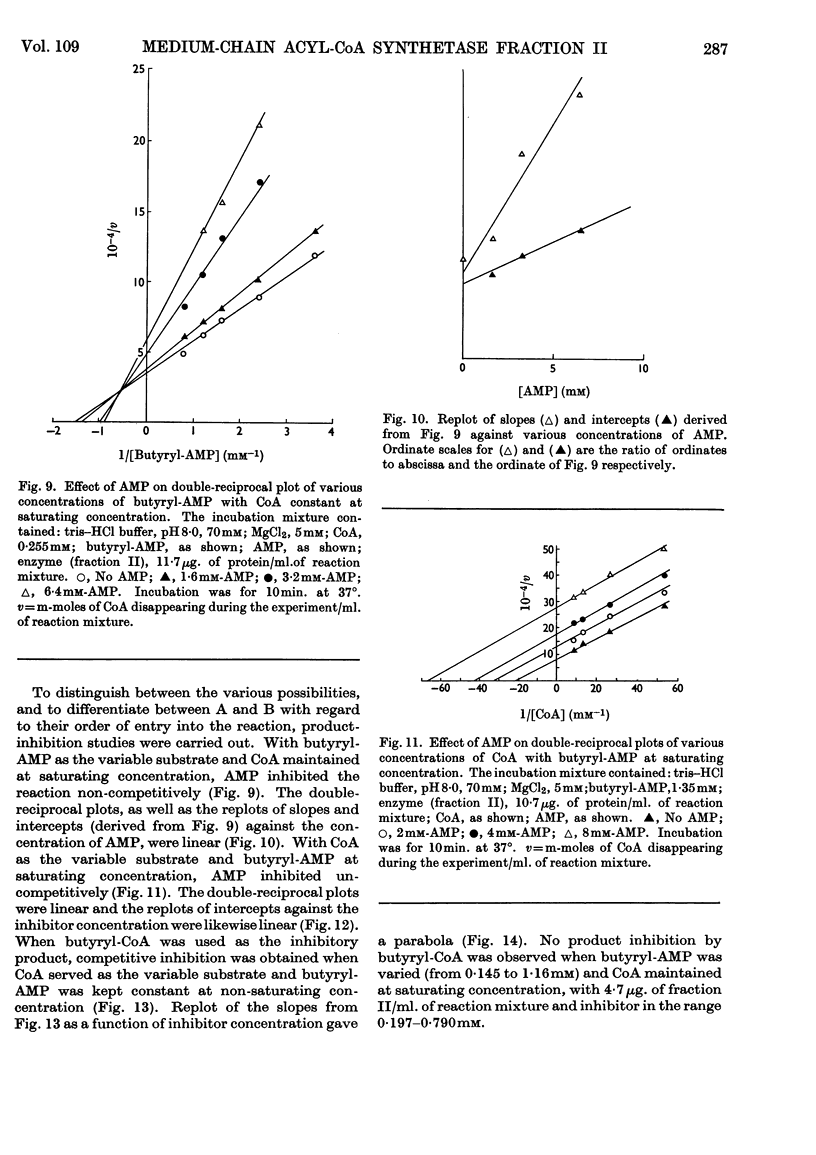
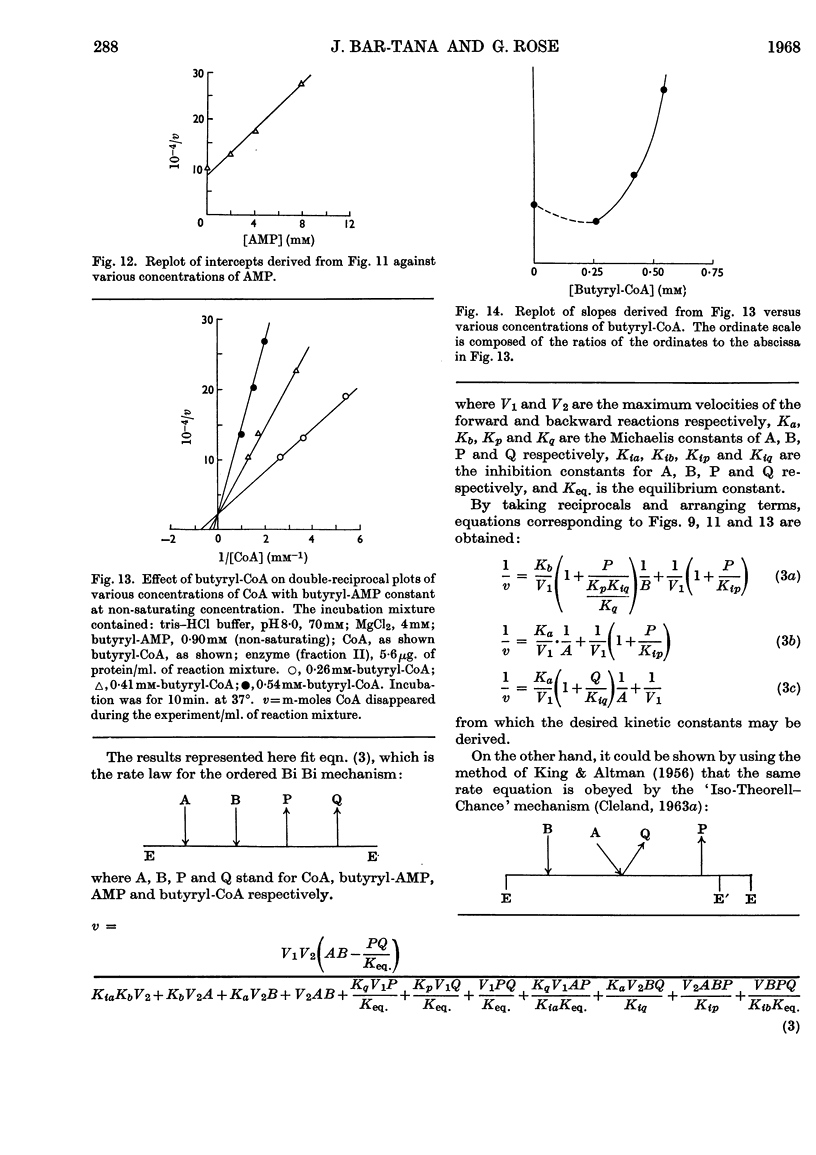
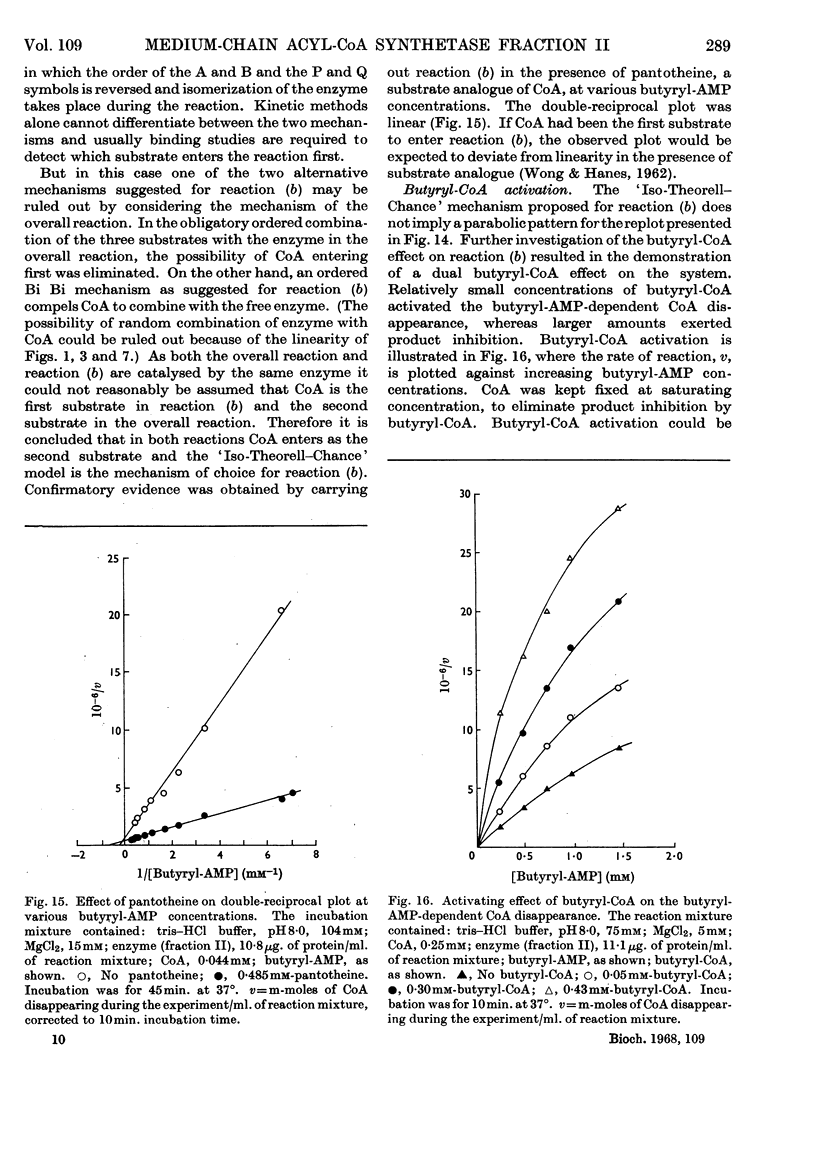
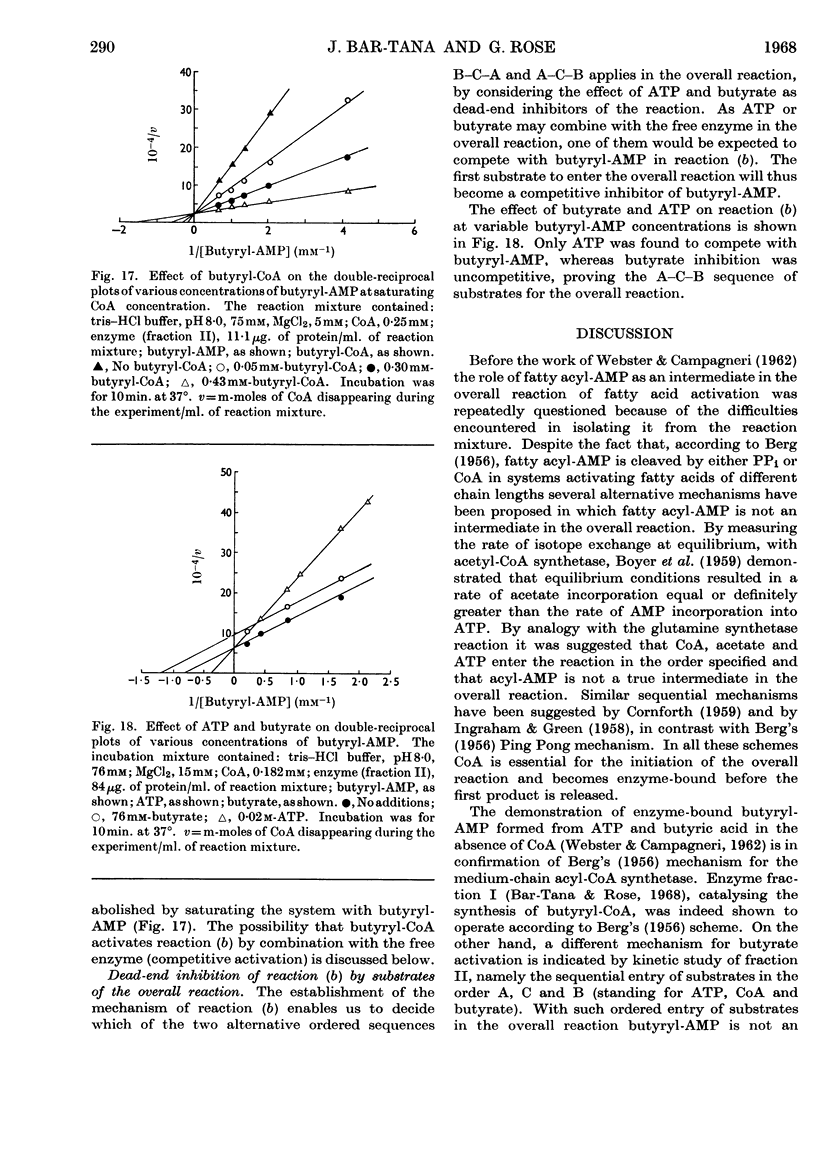
1. The mechanism of reaction of fatty acyl-CoA synthesis catalysed by fatty acyl-CoA synthetase from ox liver (fraction II; Bar-Tana, Rose & Shapiro, 1968) was investigated by a kinetic study of CoA disappearance dependent on butyrate plus ATP or butyryl-AMP (overall and partial reaction b respectively). 2. Contrary to findings with another enzyme (fraction I), a Bi Uni Uni Bi Ping Pong mechanism (Cleland, 1963a,b,c) corresponding to Berg's (1956) scheme of reaction was eliminated and an ordered Ter Ter mechanism with an A–C–B (standing for ATP, CoA and butyrate respectively) sequence of substrate entry for the overall reaction was established for fraction II. Partial reaction (b) was found to follow the `Iso-Theorell–Chance' mechanism. 3. Also, in contrast with results obtained with fraction I, no allosteric properties could be demonstrated with fraction II.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERG P. Acyl adenylates; an enzymatic mechanism of acetate activation. J Biol Chem. 1956 Oct;222(2):991–1013. [PubMed] [Google Scholar]
- BOYER P. D., MILLS R. C., FROMM H. J. Hypotheses for and some kinetic studies with glutamine synthetase and acetate thiokinase. Arch Biochem Biophys. 1959 Mar;81(1):249–263. doi: 10.1016/0003-9861(59)90194-8. [DOI] [PubMed] [Google Scholar]
- Bar-Tana J., Rose G., Shapiro B. Studies on medium-chain fatty acyl-coenzyme a synthetase. Purification and properties. Biochem J. 1968 Sep;109(2):269–274. doi: 10.1042/bj1090269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Tana J., Rose G. Studies on medium-chain fatty acyl-coenzyme a synthetase. Enzyme fraction I: mechanism of reaction and allosteric properties. Biochem J. 1968 Sep;109(2):275–282. doi: 10.1042/bj1090275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. III. Prediction of initial velocity and inhibition patterns by inspection. Biochim Biophys Acta. 1963 Feb 12;67:188–196. doi: 10.1016/0006-3002(63)91816-x. [DOI] [PubMed] [Google Scholar]
- INGRAHAM L. L., GREEN D. E. Role of magnesium in acetyl coenzyme A formation by acetothiokinase. Science. 1959 Apr 3;129(3353):895–896. doi: 10.1126/science.129.3353.896. [DOI] [PubMed] [Google Scholar]
- MAHLER H. R., WAKIL S. J., BOCK R. M. Studies on fatty acid oxidation. I. Enzymatic activation of fatty acids. J Biol Chem. 1953 Sep;204(1):453–468. [PubMed] [Google Scholar]
- WEBSTER L. T., Jr, CAMPAGNARI F. The biosynthesis of acetyl and butyryl adenylates. J Biol Chem. 1962 Apr;237:1050–1055. [PubMed] [Google Scholar]
- WONG J. T., HANES C. S. ISOTOPIC EXCHANGE AT EQUILIBRIUM AS A CRITERION OF ENZYMATIC MECHANISMS. Nature. 1964 Aug 1;203:492–494. doi: 10.1038/203492a0. [DOI] [PubMed] [Google Scholar]
- WONG J. T., HANES C. S. Kinetic formulations for enzymic reactions involving two substrates. Can J Biochem Physiol. 1962 Jun;40:763–804. [PubMed] [Google Scholar]


