Abstract
Lactate dehydrogenase (LDHA) activation induces tumorigenesis by activating tumor proliferation, growth, invasion, and metastasis. Whether LDHA mediates tumor metabolism that upon diffuse large B-cell lymphoma (DLBCL) occur remains unknown. Here, we investigated how LDHA adopt tumor metabolism after activation to regulate DLBCL-inducible. We investigated LDHA is highly expressed in peripheral blood mononuclear cells (PBMCs) of DLBCL patients. Knockdown of LDHA results in an increase in the apoptosis of cells, suppression of cell growth and migration in OCI-Ly1 and OCI-Ly10 cells. We show that LDHA gains a canonical enzyme activity to produce lactate and triggers NAD + in DLBCL cells. Furthermore, p-STAT5 was identified as a downstream target of LDHA, and the p-STAT5 protein level was significantly reduced related to decreased LDHA protein expression. Collectively, our findings identify the oncogenic role of LDHA in DLBCL and suggest that LDHA can be considered as a pivotal prognostic biomarker and a potential therapeutic target.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00277-024-06083-2.
Keywords: LDHA, Diffuse large B-cell lymphoma, Tumor metabolism, Cell proliferation, Signaling pathway
Introduction
Metabolic reprogramming represents well established cancer hallmarks [1]. Tumor metabolism as introduced by Otto Warburg in the early 20th century promotes survival, proliferation, and therapeutic resistance [2]. It has been well-established that cancer cells are under constant oxidative stress, as reflected by elevated basal level of reactive oxygen species (ROS), due to increased metabolism driven by aberrant cell growth [3].
Oxidative stress serves an important role in carcinogenesis [4–8]. The associations between oxidative stress and hematological malignancies are rarely reported [9]. DLBCL is the most common subtype of non-Hodgkin lymphoma (NHL) accounting for 30–35% of all nodal lymphomas [10]. The role of oxidative stress as a prognostic factor for DLBCL has been studied in recent years [11–13].
In tumor anaerobic glycolysis, LDHA preferentially catalyzes pyruvate to lactate and which is undisputed known as a vital checkpoint [14]. LDHA is elevated in many types of cancer and is reported to be associated with tumor proliferation, growth, invasion, and metastasis [15, 16]. Inhibition of LDHA can limit the energy supply in cancer cells, thereby reducing metastasis and invasion of cancer cells [17]. Many studies have suggested that LDHA is a prognostic factor and a potential target for treatment in DLBCL [18–22]. Metabolism-related gene LDHA was highly expressed in malignant B cells in DLBCL tissue which was clustered using scRNA-seq [23]. Some studies have demonstrated that LDHA gene was upregulated in glycolysis/gluconeogenesis signaling pathway [22]. However, LDHA-related tumor metabolism reprogramming in DLBCL remains unclear.
Previous studies have demonstrated that LDHA amplify ROS in cancer cells in response to oxidative stimuli, which indicated that LDHA knockout significantly decreased the oxidative stress in tumors [24]. But how LDHA physiological relevance with ROS in DLBCL remains unknown. In our research, we present a molecular mechanism of cell metabolism that explains how LDHA promotes DLBCL development through cytoplasm localization of LDHA and the product of canonical enzyme activity of lactate.
Materials and methods
Gene expression and bioinformatics analysis
This study utilized data downloaded from the TCGA database (https://portal.gdc.cancer.gov). RNA-seq data from the TCGA-Diffuse large B-cell lymphoma (DLBC) project were submitted to the STAR workflow and extracted in TPM format to obtain gene expression and clinical data. Data processing and visualization were conducted using R software (version 4.2.1), where clinically irrelevant duplicate data were removed. Appropriate statistical methods were selected based on the characteristics of the data format (R package “stats” and R package “car”) for statistical analysis, and R package “ggplot2” was used for data visualization. This study adhered to the Helsinki Declaration (2013 revised version) and complied with the publication guidelines issued by TCGA. The authors did not conduct any research involving humans or animals.
Clinical samples
This study was approved by the biomedical ethical committee of Shandong Provincial Hospital affiliated to Shandong First Medical University (Jinan, China). Peripheral blood samples were obtained from DLBCL patients and healthy control donors from 2018 to 2022 in Shandong Provincial Hospital.
Isolation of human peripheral blood mononuclear cells
Place fresh heparinized blood into 15 ml conical centrifuge tubes. Slowly layer the Ficoll solution (Solarbio, P8900) underneath the blood/PBS mixture by placing the tip of the pipet containing the Ficoll at the bottom of the sample tube. Use 3 ml Ficoll per 10 ml blood/PBS mixture. Centrifuge 30 min with no brake. Transfer the mononuclear cell layer to another centrifuge tube. Wash cells by adding excess HBSS and centrifuging 10 min.
Cell culture
OCI-Ly1 cells and OCI-Ly10 cells were in RPMI-1640 enriched with 10% fetal bovine serum. The medium contained 1% penicillin/streptomycin mixture. All cells were incubated in a humidified atmosphere of 5% CO2 at 37 °C. Authentication testing of OCI-Ly1 and OCI-Ly10 cell lines have been performed by Shanghai Biowing Applied Biotechnology Co., Ltd via STR profiling. STR profiles match the standards recommended for OCI-Ly1 and OCI-Ly10 cell lines authentication.
Reagents
Propidium iodide (PI), APC BrdU Flow Kit and PE Annexin V Apoptosis Detection Kit were purchased from BD Biosciences. Hydrogen peroxide solution (H2O2) and N-acetyl-L-cysteine (NAC) were purchased from Sigma Aldrich. FX11 was purchased from MedChem Express. Anti-phospho-STAT5, total STAT5, GAPDH, tubulin and LDHA were purchased from Cell Signaling Technology. Lactate Assay Kit was purchased from Sigma Aldrich. NAD+/NADH Assay Kit (WST-8) and H2O2 Assay Kit were purchased from Beyotime.
Cell transfection
The green fluorescent protein (GFP)-puro-tagged lentivirus vectors encoding LDHA shRNA or Con shRNA were purchased from Vigene Biosciences (Jinan, China). Stable cell lines expressing Con shRNA and LDHA shRNA were generated by transfection of Con shRNA or LDHA shRNA lentivirus vectors into OCI-Ly1 and OCI-Ly10 cells according to the manufacturers’ instruction, and screened for expression with 2 µg/mL puromycin (Solarbio, P8230). Infection efficiencies of the transfected cells were validated using western blot assays.
Western blot analysis
Protein extracts from cells were prepared using cell lysis buffer. Total protein was subjected to SDS-PAGE and transferred to PVDF membrane (Millipore). Antibodies against STAT5, p-STAT5, LDHA, Tubulin or GAPDH were incubated overnight at 4 °C with primary antibody and visualized using electro-chemi-luminescence kit (Santa Cruz, sc-2048) by darkroom exposure and film development technology or by chemiluminescence signal detected with an Amersham Imager 600 imaging system (General Electric, USA).
Protein crosslinking assay
Cells treated with or without H2O2 for 6 h and cells were lysed by lysis buffer. The supernatants of cell lysates were added with 0.025 wt% glutaraldehyde for 30 min at 37 °C. The reactions were terminated by a final concentration of 50 mM Glycine. Samples were then separated and analyzed by western blotting with LDHA antibody.
ROS measurement
ROS was measured using reactive oxygen species detection kit (Beyotime, S0033). H2DCFDA was hydrolyzed to DCFH in the cells. Next, DCFH was oxidized to DCF with high fluorescence intensity by an oxidizing agent in the cells. The cells were incubated with H2DCFDA for 30 min and washed by 1640 medium for three times. At last, the solution was detected with BD FACS Calibur and analyzed with FCS express software.
Lactate content measurement
Intracellular lactate contents of cells and serum lactate contents were determined by using Lactate Assay Kit (MAK064, Sigma-Aldrich). Con shRNA and LDHA shRNA of OCI-Ly1 and OCI-Ly10 cells were cultured in RPMI-1640 supplemented with 10% FBS and treated with H2O2 (50 µM) for 6 h. The cell pellet and serum were collected for lactate content measurement according to the manufacturer’s instructions.
NAD+/NADH determination
Cell pellets were washed with phosphate buffered saline (PBS) and re-suspended with precooled extraction buffer. The lysate was centrifuged at 12,000 g for 10 min at 4 °C, and supernatant was measured using an NAD+/NADH Assay Kit with WST-8 (Beyotime, S0175).
Cell proliferation assay
Cell proliferation assay was conducted using the Cell Counting Kit-8 (CCK-8) assay kit (0.05 mg/ml, Promega). DLBCL cells were seeded at a density of 5000 cells/well in 96-well plates for 24 h and subsequently incubated with CCK-8 for 4 h at 37 °C. The optical density was detected at 450 nm by using SpectraMax® iD3 multi-mode microplate readers.
Cell migration assay
Cell migration assay was performed using transwell chambers in 24-well plates (Corning, #3422). In brief, OCI-Ly1 or OCI-Ly10 cells were plated in the upper chamber in serum-free 1640 medium at 1 × 105 cells per well. The bottom chamber contained a 10% FBS 1640 medium. Cells were allowed to migrate for 24 h in a humidified chamber at 37 °C with 5% CO2. After incubation, cells that have migrated are located in the lower layer. The total migrated cell count was determined using a cell counter.
Cell apoptosis assay
OCI-Ly1 or OCI-Ly10 cells were cultured in 6-well plates to 70–80% confluence. The cells were treated with H2O2 (50 µM) for 6 h. Then, the cells were collected by trypsinization and washed with ice-cold PBS. The 7-AAD/Annexin V-PE assay or PI/Annexin V-FITC assay were performed using a PharmingenTM Annexin V Apoptosis Detection Kit (BD Biosciences, #559763 or #556547) according to the manufacturer’s instructions. Briefly, the harvested cells were incubated in 1× binding buffer containing 5 µL Annexin V and 5 µL 7-AAD or PI for 30 min at room temperature in the dark. The cell apoptosis was measured and analyzed by BD FACS Calibur or Beckman Coulter.
BrdU incorporation
BrdU incorporation was performed using a BD Pharmingen™ APC BrdU Flow Kit (BD Biosciences, #552598) according to the manufacturer’s instructions. For BrdU labeling experiment, BrdU (final 20 μm) was added to the medium for 2 h before cells were collected and cells were harvested. Measurements of the immunofluorescent cells were performed with BD FACS Calibur and analyzed with FCS express software.
Statistical analysis
The statistical significance of differences between various groups in the experiment was calculated with the two-tailed paired/unpaired t-test, and error bars represent standard deviation (SD). Statistical analyses were performed using GraphPad Prism 5. Correlation between LDHA expression and clinical features of patients with DLBCL was calculated with chi-square test using SPSS 27.0. Bioinformatics analysis was performed using R software (R version 4.2.1) and calculated using Wilcoxon rank sum test. P values below 0.05 were considered statistically significant. * p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001. Data are shown as mean ± SEM.
Results
Increased expression of LDHA in patients with DLBCL
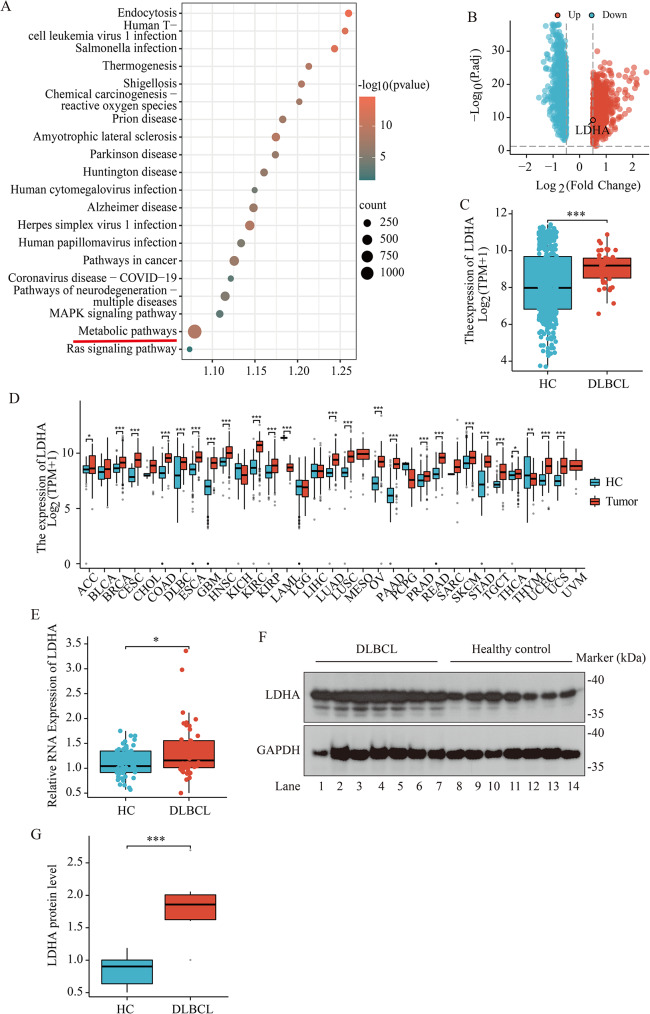
In order to investigate the regulatory role of tumor metabolism in DLBCL, we plan to screen universally effective tumor metabolic regulators from the RNA-seq results of 48 DLBCL patients in TCGA (portal.gdc.cancer.gov/). The KEGG enrichment analysis of RNA-seq results in TCGA suggest that the metabolic pathway is the most genes enrichment pathway (Fig. 1A). We found that the tumor metabolism-related gene LDHA was included in the most genes enrichment metabolic pathway (Table S1).
Fig. 1.
LDHA levels are elevated in peripheral blood mononuclear cells of DLBCL patients. (A) KEGG pathways were shown by bubble graphs. KEGG analyses of genes from RNA-Seq results of 48 DLBCL patients in TCGA (portal.gdc.cancer.gov/). Genes obtained from RNA-Seq were enriched through DAVID database (https://david.ncifcrf.gov/). We sorted the top 20 pathways in an enrichment descending order and obtained the KEGG pathway, which were filtered based on their p < 0.05. The metabolic pathway is highlighted with a red line. (B) DEGs in the GSE83632 dataset were visualized by the volcano map (http://bioinformatics.com.cn/). The high expression of LDHA in DLBCL was examined in the GSE83632 dataset (the location of the LDHA was circled in the figure). |log fold change (FC)| > 0.5 and adjusted P-value < 0.05. (C) Difference in LDHA expression between DLBCL patients (DLBCL) and healthy control individuals (HC). Data are presented as means ± SEM. ** p < 0.01 (Wilcoxon rank sum test). (D) Differential expression of LDHA in various cancers between patients with tumor (Tumor) and healthy control individuals (HC). Data are presented as means ± SEM. * p < 0.05, ** p < 0.01 (Wilcoxon rank sum test). (E) Quantitative RT-PCR analysis of LDHA in PBMCs between DLBCL patients (DLBCL, n = 46) and the healthy control individuals (HC, n = 54). Data were normalized to Gapdh mRNA levels. Data are presented as means ± SEM. * p < 0.05 (Wilcoxon rank sum test). (F, G) Western blotting indicated enhanced LDHA protein expression in PBMCs of DLBCL patients, n = 7 for DLBCL patients (DLBCL) and n = 7 for healthy control individuals (HC) for two independent experiments. Data are presented as means ± SD. *** p < 0.001 (Student’s t-Tests, Unpaired test, Two-tailed)
To verify the validity of LDHA in DLBCL, we selected independent samples from two separate studies to verify the differences in LDHA gene expression. The public database from GSE83632 included 76 DLBCL patients and 87 healthy donors, and the statistical results show that |log fold change (FC)| > 0.5 and adjusted P-value < 0.05. 748 up-regulated and 721 down-regulated differentially expressed genes (DEGs) were identified in GSE83632 dataset in DLBCL samples relative to healthy controls (Fig. 1B). We found LDHA was shown in up-regulated DEGs of the volcano plots (Fig. 1B). KEGG enrichment analysis of these LDHA related DEGs in GSE83632 dataset revealed that the most genes enrichment pathway of DEGs was metabolic pathway (Fig. S1a, Table S2).
Another public dataset from GSE12453 included 42 lymphocytic and histiocytic (L&H) lymphoma B-cell originate cases and 25 healthy donors and other malignant B-cell originate cases, and the statistical results show that |log fold change (FC)| > 0.5 and adjusted P-value < 0.05. 1731 up-regulated and 1365 down-regulated differentially expressed genes (DEGs) were identified in GSE12453 dataset in lymphoma B cells relative to normal naïve B-cells and other malignant B cells (Fig. S1b). We found LDHA was shown in up-regulated DEGs of the volcano plots (Fig. S1b). KEGG enrichment analysis of these LDHA related DEGs in GSE12453 dataset revealed that the most genes enrichment pathway of DEGs was also the metabolic pathway (Fig. S1c) (Table S3). Therefore, the LDHA gene is reliable as a tumor metabolic biomarker for DLBCL.
To confirm an association between LDHA expression and DLBCL, we collected gene expression data for 47 DLBCL patients from the TGCA database and 444 healthy donors from the GTEx database. The results indicated that the expression of the LDHA gene in patients with DLBCL was significantly higher compared with that in healthy donors (Fig. 1C). LDHA expression in 33 common tumors was analyzed and found to be significantly overexpressed in 22 tumor types, including DLBCL (Fig. 1D). In order to verify the functional role of LDHA in DLBCL of our hospital, we next verified the expression of LDHA in DLBCL, finding higher levels of LDHA mRNA and protein in PBMCs of DLBCL patients than healthy donors (Fig. 1E, F and G).
Thus, the above large-scale data and clinical findings indicate that LDHA was highly expressed and might be an independent prognostic indicator for patients with DLBCL.
Analysis of the prognostic value of LDHA in DLBCL
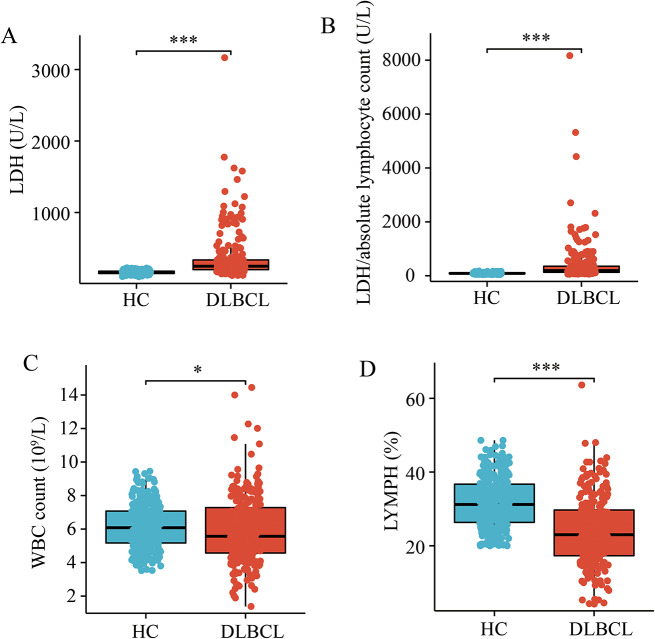
Recent studies have shown that in DLBCL, the higher serum level of lactate dehydrogenase (LDH) and a high LDH to ALC (absolute lymphocyte count) ratio were significantly correlated with the inferior survival outcomes [25, 26]. We retrospectively reviewed the medical records of 237 patients diagnosed as pathologically proven DLBCL between June 2018 and September 2022 and 320 people in the healthy control group.
There were 116 males and 121 females in DLBCL group. The average age was 56.51 ± 15.74 years old, ranging from 6 to 87. The healthy control group contains 136 males and 184 females, ageing from 18 to 82 years old. The average age was 44.37 ± 15.00 years old. This study was approved by the biomedical ethical committee of Shandong Provincial Hospital affiliated to Shandong First Medical University. We investigated comparatively the total serum LDH activity and LDH to ALC ratio in patients with DLBCL (n = 237) and healthy control (n = 320). Mean blood serum LDH in the diagnostic samples was 163.9 U/L among controls and 363.4 U/L among DLBCL patients (Fig. 2A). This difference was significant in LDH to ALC ratio. Mean blood serum LDH to ALC ratio was 89.57 U/L among controls and 409.9 U/L among DLBCL patients (Fig. 2B). To exclude the effect of cell number on serum LDH value, we counted white blood cells (WBC) and lymphocytes. WBC counts were lower in the DLBCL group than in healthy donors (Fig. 2C), with a more significant reduction in lymphocytes (Fig. 2D).
Fig. 2.
Increased serum LDH in DLBCL patients. (A, B) Serum LDH (a) and the ratio of serum LDH to absolute lymphocyte count (b) in DLBCL patients (DLBCL). Serum LDH levels in DLBCL group were higher than those in the healthy control group (HC). Data are presented as means ± SEM. *** p < 0.001 (Wilcoxon rank sum test). (C, D) WBC (c) and lymphocyte (%) (d) in peripheral blood of DLBCL group (DLBCL) and of the healthy control group (HC). Data are presented as means ± SEM. * p < 0.05, *** p < 0.001 (Wilcoxon rank sum test)
The clinical implications of LDHA upregulation in patients with DLBCL were further validated in our hospital. Increased expression of LDHA was associated with elevated serum LDH (Table 1), indicating a positive correlation between high LDHA expression and DLBCL disease progression.
Table 1.
Evaluation the differences between samples with high and low expression of LDHA, related to Fig. 1
| Clinical variables | No. of patients | LDHA expression | p value | |
|---|---|---|---|---|
| High | Low | |||
| Age (years) | ||||
| < 60 | 17 | 5 | 12 | 0.417 |
| ≥60 | 29 | 12 | 17 | |
| Gender | ||||
| Male | 27 | 12 | 15 | 0.2098 |
| Female | 19 | 5 | 14 | |
| Serum LDH | ||||
| Normal | 33 | 9 | 24 | 0.03016* |
| Elevated | 13 | 8 | 5 | |
| WBC | ||||
| Normal | 44 | 15 | 29 | 0.05894 |
| Elevated | 2 | 2 | 0 | |
| Lymphocytes | ||||
| Normal | 45 | 16 | 29 | 0.1867 |
| Elevated | 1 | 1 | 0 | |
| Lymphocytes % | ||||
| Normal | 44 | 15 | 29 | 0.05894 |
| Elevated | 2 | 2 | 0 | |
*P< 0.05
LDH: Lactate dehydrogenase; WBC: white blood cell
Increased lactate accumulation in DLBCL patients and LDHA knock down reduced glycolysis activation in DLBCL cells
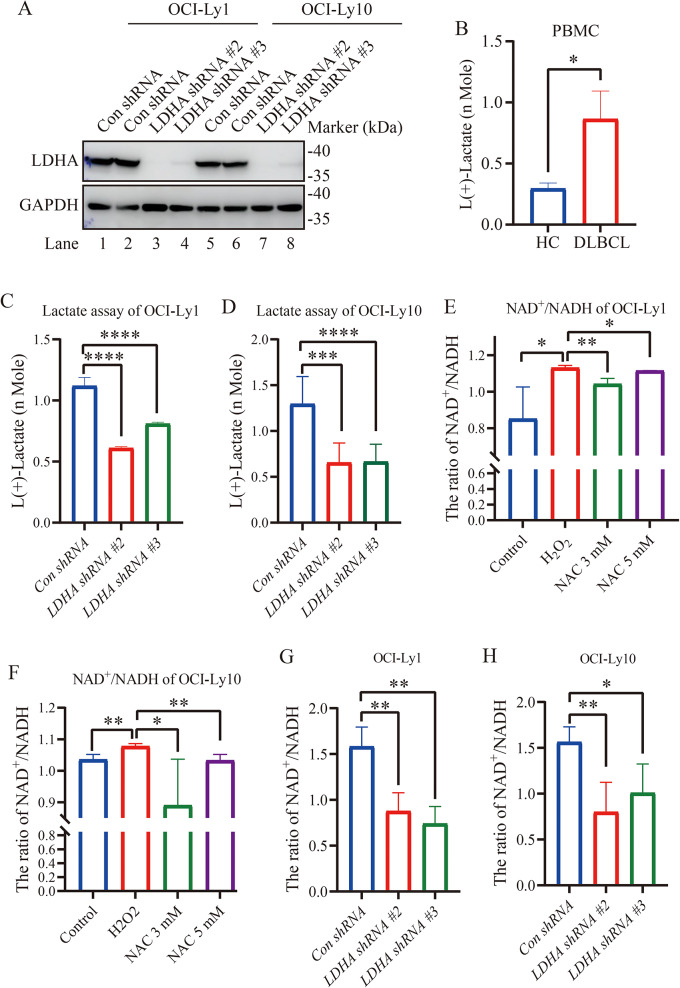
To explore the role of LDHA in DLBCL cells, stable knockdown of LDHA in OCI-Ly1/OCI-Ly10 cells were established. LDHA shRNA #2 and LDHA shRNA #3, two of the three lentivirus-mediated RNA interference vectors against LDHA, exhibited highest efficacy of LDHA knockdown (Fig. 3A).
Fig. 3.
LDHA knockdown suppresses lactate and NAD+/NADH production. (A) Immunoblotting of LDHA in OCI-Ly1 and OCI-Ly10 cells stably transfected with lentivirus-scramble shRNA (Con shRNA) or LDHA shRNA. LDHA expression was reduced in LDHA knockdown OCI-Ly1/OCI-Ly10 cells. (B) Enhanced lactate concentration in PBMCs of DLBCL patients. Lactate concentration in each well with equal number of cells was measured (n = 7 for DLBCL patients (DLBCL) and n = 6 for the healthy control group (HC)). Data are presented as means ± SD. * p < 0.05 (Student’s t-Tests, Unpaired test, Two-tailed). (C, D) Decreased lactate concentration in LDHA shRNA cells compared with Con shRNA in OCI-Ly1/OCI-Ly10 cells. Lactate concentration in each well with an equal number of cells was measured (n = 8 for each group). Data are presented as means ± SD. *** p < 0.001, **** p < 0.0001 (Student’s t-Tests, Unpaired test, Two-tailed). (E, F) The NAD+/NADH ratio was measured in OCI-Ly1/OCI-Ly10 cells treated with H2O2 or H2O2 + NAC. NAD + and NADH levels were measured to calculate the NAD+/NADH ratio in biological replicates of OCI-Ly1/OCI-Ly10 cells (n = 4 for each group). Data are presented as means ± SD. * p < 0.05, ** p < 0.01 (Student’s t-Tests, Unpaired test, Two-tailed). (G, H) The NAD+/NADH ratio was measured in Con shRNA and LDHA shRNA cells treated with H2O2. NAD + and NADH levels were measured to calculate the NAD+/NADH ratio in biological replicates of the LDHA knockdown stable cell line (n = 4 for each group). Data are presented as means ± SD. * p < 0.05, ** p < 0.01 (Student’s t-Tests, Unpaired test, Two-tailed)
LDHA plays an indispensable role in cancer development and possesses a well-defined canonical enzyme activity, catalyzing the conversion between pyruvate and lactate [15]. Lactate is produced from pyruvate by LDHA, which is frequently overexpressed in tumor cells and is important for cell growth. We quantified the direct substrate of LDHA, including lactate. We investigated comparatively lactate in patients with DLBCL and healthy control. As expected, lactate, the product of LDHA activity, accumulated by ~ 1.6-fold in PBMCs of DLBCL patients (Fig. 3B). We measured lactic acid production and the cytosolic NAD+/NADH redox state in the Con shRNA and LDHA shRNA OCI-Ly1/OCI-Ly10 cells. In comparison with the Con shRNA cells, LDHA knockdown significantly reduced levels of lactate production in OCI-Ly1/OCI-Ly10 cells (Fig. 3C and D). NAD+/NADH increased in OCI-Ly1/OCI-Ly10 cells upon H2O2 treatment and H2O2-induced NAD+/NADH was reversed by NAC supplement (Fig. 3E and F). LDHA silencing decreased the ratios of NAD+/NADH in OCI-Ly1/OCI-Ly10 cells (Fig. 3G and H). Collectively, these results indicate that LDHA knockdown reduced glycolysis in DLBCL.
Oxidative stress-induced LDHA expression in DLBCL cells
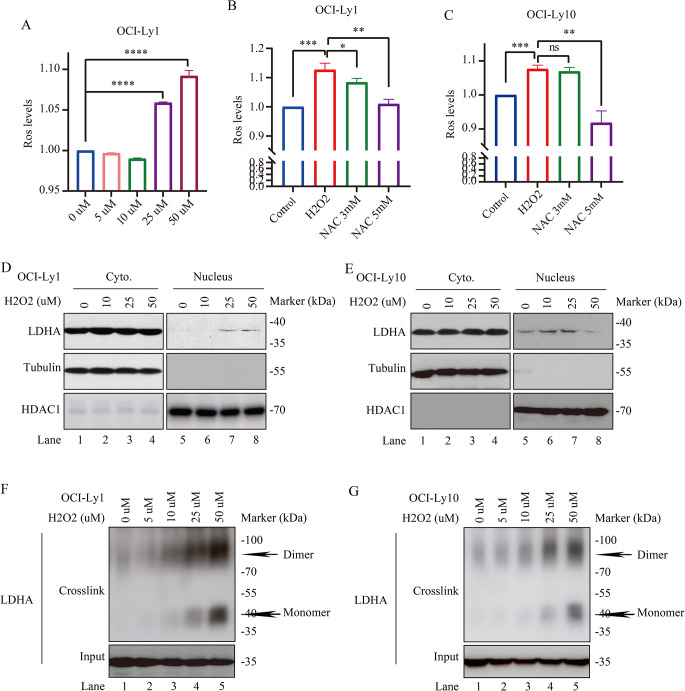
The excessive and inappropriate production of ROS can cause oxidative stress and is implicated in DLBCL. The present study wants to see LDHA response to H2O2 induced oxidative stress. The cellular ROS levels were measured upon H2O2 treatment in DLBCL cells under the same condition. To this end, we treated OCI-Ly1 cells with H2O2 and found that ROS production increased in a dose-dependent manner (Fig. 4A). Notably, supplement with a ROS scavenger NAC reduced ROS production in OCI-Ly1/OCI-Ly10 cells (Fig. 4B and C). These data indicated that ROS production is dependent on H2O2.
Fig. 4.
Increased endogenous LDHA protein expression in OCI-Ly1/OCI-Ly10 cells upon H2O2 treatment. (A, B, C) Ros production is profoundly increased in a H2O2 dose-dependent manner. Ros levels in OCI-Ly1 cells upon different doses of H2O2 treatment, as indicated (n = 4 for each group). Cellular ROS levels were measured in OCI-Ly1/OCI-Ly10 cells, with or without 3 mM and 6 mM NAC treatment for 6 h, followed by H2O2 treated for 6 h, then ROS production was measured (n = 3 for each group). Data are presented as means ± SD. ** p < 0.01, *** p < 0.001, **** p < 0.0001 (Student’s t-Tests, Unpaired test, Two-tailed). (D, E) H2O2 mediates LDHA cytoplasm and nuclear localization. OCI-Ly1/OCI-Ly10 cells were treated with or without H2O2 for 6 h as indicated for cytoplasm and nuclear isolating, followed by blotting with LDHA, Tubulin, and HDAC1. (F, G) OCI-Ly1/OCI-Ly10 cell extracts with or without H2O2 treatment were crosslinked with 0.025% glutaraldehyde and analyzed by western blotting using LDHA antibody. Dimeric and monomeric LDHA were indicated. The loading inputs for gel filtration are shown below
LDHA plays an indispensable role in cancer development and possesses a well-defined canonical enzyme activity, catalyzing the conversion between pyruvate and lactate [15]. It is well known that LDHA localizes at cytoplasm exerting its lactate-producing activity and LDHA localizes at nuclear exerting its noncanonical enzyme activities [27, 28]. To investigate the potential role of LDHA in oxidative stress-induced DLBCL, we treated OCI-Ly1/OCI-Ly10 cells with H2O2 and found that LDHA was mainly localized in the cytoplasm of OCI-Ly1/OCI-Ly10 cells, and only very few amount of LDHA was expressed in the nucleus upon H2O2 treatment (Fig. 4D, E).
As the oligomerization state of metabolic enzymes closely links to their catalytic activity [29–31], we measured monomer and dimer fractions of LDHA upon H2O2 treatment. Protein crosslinking assay and gel filtration were performed. LDHA monomer and dimer were dramatically increased by H2O2 treatment (Fig. 4F and G). Taken together, these results demonstrate that ROS-induced mainly cytoplasm location of LDHA, accompanied with its dimer and monomer fractions, confers LDHA with an enzyme activity.
LDHA promotes cell proliferation, migration and decreased apoptosis of OCI-Ly1/OCI-Ly10 cells in vitro
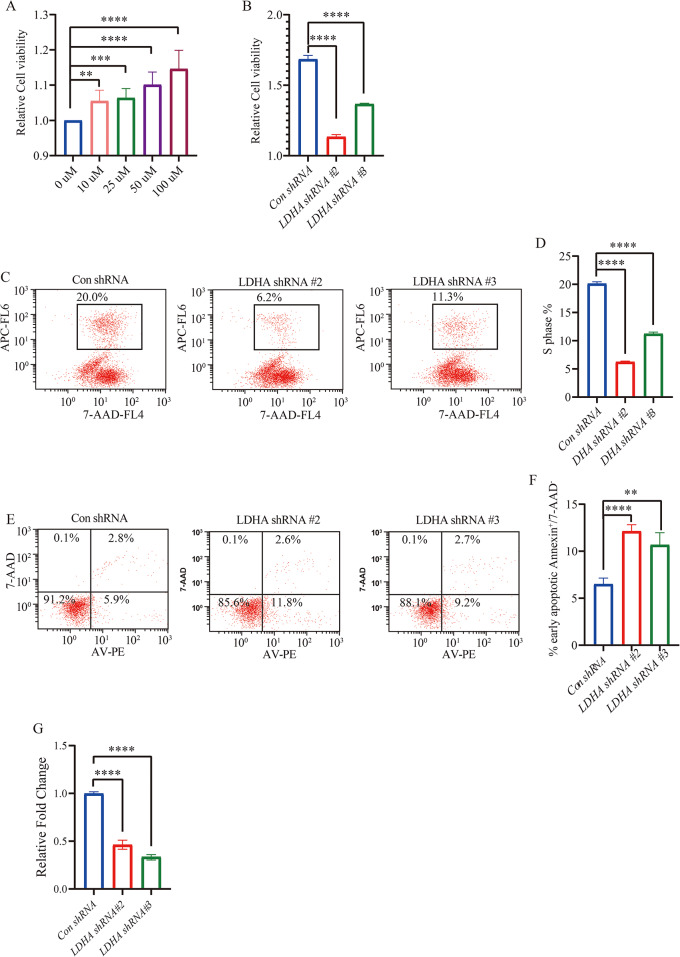
Cell proliferation of OCI-Ly1/OCI-Ly10 cells were evaluated by CCK-8 assay. Results showed that H2O2 promoted proliferation of OCI-Ly1/OCI-Ly10 cells (Fig. 5A, Fig. S2a) and knockdown of LDHA resulted in substantial growth suppression of the OCI-Ly1/OCI-Ly10 cells (Fig. 5B and Fig. S2b).
Fig. 5.
LDHA knockdown promoted OCI-Ly1 cells apoptosis and decreased proliferation and migration. (A) CCK8 assay of cellular proliferation upon a series H2O2 treatment by using Con shRNA and LDHA shRNA of OCI-Ly1 stable cell line upon H2O2 treatment (n = 6 for each group). Data are presented as means ± SD. ** p < 0.01, *** p < 0.001, **** p < 0.0001 (Student’s t-Tests, Unpaired test, Two-tailed). (B) CCK8 assay of cellular proliferation by using Con shRNA and LDHA shRNA of OCI-Ly1 stable cell line upon H2O2 treatment (n = 3 for each group). Data are presented as means ± SD. **** p < 0.0001 (Student’s t-Tests, Unpaired test, Two-tailed). (C, D) Con shRNA and LDHA shRNA of OCI-Ly1 cells were cultured in the presence of H2O2. The cells were labeled with BrdU for 2 h before collecting and stained with 7-AAD and anti-BrdU antibody, analyzed by flow cytometry. BrdU-positive cells are gated and their percentages are indicated (C) and quantificated (D) (n = 3 for each group). Data are presented as means ± SD. **** p < 0.0001 (Student’s t-Tests, Unpaired test, Two-tailed). (E, F) Annexin V/7-AAD staining for the determination of apoptosis of Con shRNA and LDHA shRNA OCI-Ly1 cells treated with H2O2 (n = 4 for each group). Data are presented as means ± SD. ** p < 0.01, **** p < 0.0001 (Student’s t-Tests, Unpaired test, Two-tailed). (G) Cellular migration in OCI-Ly1 cells with Con shRNA and LDHA shRNA treated with H2O2 was determined by transwell assay (n = 5 for each group). Data are presented as means ± SD. **** p < 0.0001 (Student’s t-Tests, Unpaired test, Two-tailed)
To examine the ability of LDHA to promote the proliferation of cells from a quiescent state, we measured BrdU incorporation in Con shRNA and LDHA shRNA groups. BrdU incorporation was significantly reduced in LDHA shRNA group compared with Con shRNA group under H2O2 treatment (Fig. 5C and D and S2c). We also investigated whether decreased LDHA could affect the apoptosis of DLBCL cells by flow cytometry. Significant increase was observed in cell apoptosis of LDHA shRNA cells compared with Con shRNA group (Fig. 5E and F and Fig. S2d). Enhanced migration is an important characteristic of tumor, we next examined the migration of DLBCL cells. The transwell assay revealed that cellular migration activity decreased in the LDHA shRNA group compared with the Con shRNA group upon H2O2 treatment (Fig. 5G and Fig. S2e).
These data suggested that LDHA mediated cell proliferation, migration, and apoptosis for DLBCL cells stimulated with H2O2 in vitro, which might impact the progression of DLBCL.
LDHA activates p-STAT5 expression in DLBCL patients and in OCI-Ly1/OCI-Ly10 cells
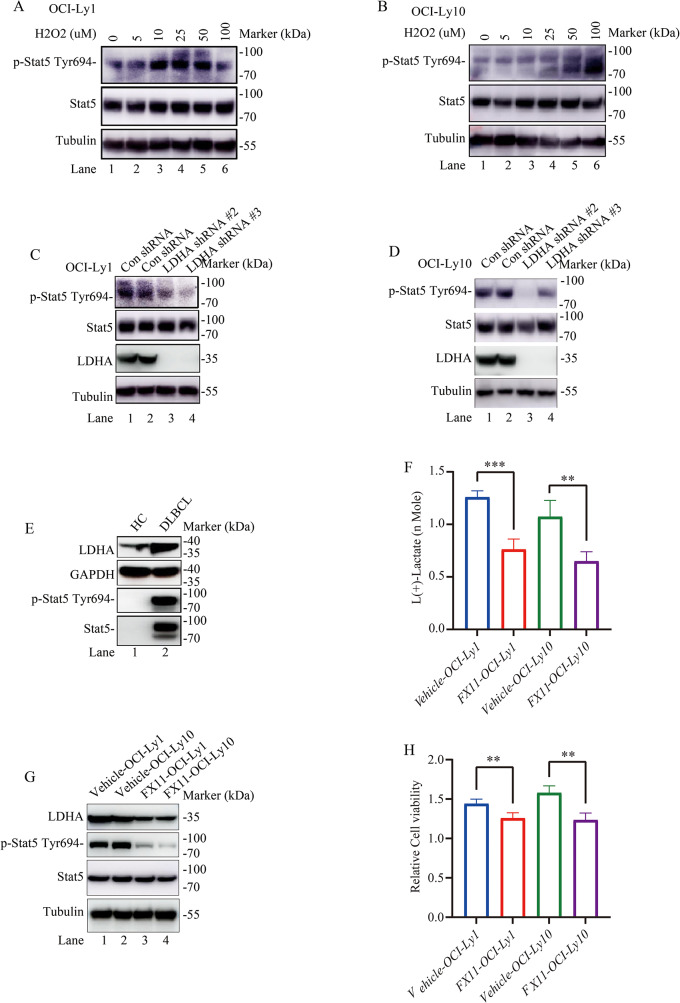
Gene set enrichment analysis of DLBCL related genes scored highly under JAK-STAT signaling pathway in the GSE12453 dataset in DLBCL samples relative to normal naïve B-cells (Fig. S1c). So metabolic pathway related enzyme LDHA is potentially involved in JAK-STAT signaling pathway in DLBCL (Fig. S1c). STAT has been proved to be associating with regulation of energy metabolism in the Warburg effect [32]. LDHA was one key enzyme responsible for the maintenance of tumor glycolysis in aerobic conditions which was up-regulated by STAT at both the gene and protein level [32]. STAT5 is one of seven members of the STAT family of proteins and has a key role in the generation of B-cell lymphoma [33, 34]. To further test whether H2O2 is essential for STAT5 activation, p-STAT5 protein expression was generated for western blotting analysis. LDHA was dramatically increased by H2O2 treatment (Fig. 6A and B). We performed western blotting to measure protein expression in LDHA shRNA group. p-STAT5 protein expression were down-regulated in LDHA shRNA group compared to Con shRNA group upon H2O2 treatment (Fig. 6C and D). Also, p-STAT5 and STAT5 protein expression were expressed higher in DLBCL patients than in healthy control group (Fig. 6E). Furthermore, the protein levels of p-STAT5 changed in response to LDHA, further validating p-STAT5 as one signaling pathway which LDHA regulated.
Fig. 6.
LDHA is important for STAT5 phosphorylation in OCI-Ly1/OCI-Ly10 cells. (A, B) H2O2 treatment increased endogenous p-STAT5 protein expression in OCI-Ly1/OCI-Ly10 cells. Cells were exposed to H2O2 (0, 5, 10, 25, 50, and 100 µM) for 8 h. Whole cell lysates were prepared and p-STAT5 and total STAT5 protein levels were analyzed by immunoblot. β-tubulin was used as a loading control (Replication once). (C, D) Protein levels of p-STAT5 and total STAT5 upon LDHA knockdown in OCI-Ly1/OCI-Ly10 cells treated with H2O2. We constructed LDHA knockdown stable cell line in OCI-Ly1/OCI-Ly10 cells. Cells were lysed and p-STAT5, total STAT5 and LDHA protein levels were analyzed by immunoblot and quantified upon 50 µM H2O2. β-tubulin was used as a loading control (Replication for two separated experiments). (E) Protein levels of p-STAT5, total STAT5 and LDHA in DLBCL patients and healthy control (HC). GAPDH was used as a loading control (Replication for four separated experiments). (F) Decreased lactate concentration in OCI-Ly1/OCI-Ly10 cells treated with 25 µM FX11 (n = 4 for each group). Data are presented as means ± SD. ** p < 0.01, *** p < 0.001 (Student’s t-Tests, Unpaired test, Two-tailed). (G) Protein levels of p-STAT5, total STAT5 and LDHA in OCI-Ly1/OCI-Ly10 cells treated with 25 µM FX11. Tubulin was used as a loading control (Replication once). (H) Decreased cell proliferation in OCI-Ly1/OCI-Ly10 cells treated with 25 µM FX11 (n = 4 for each group). Data are presented as means ± SD. ** p < 0.01 (Student’s t-Tests, Unpaired test, Two-tailed)
The conversion of pyruvate to lactate by LDHA is the central mechanism in our model by which NAD + is regenerated. Thus, we asked whether inhibiting LDHA activity could also reduce lactate. As such, we utilized the LDHA-specific chemical inhibitor FX11 in this study. In further support of our model LDHA knockdown suppresses lactate production, inhibiting LDHA with FX11 also reduced lactate production, p-STAT5 expression and OCI-Ly1/OCI-Ly10 cell proliferation (Fig. 6F, G and H). Cumulatively, these data support an in vitro model whereby perturbation of LDHA with LDHA inhibitor or LDHA shRNA disrupts cell balance in DLBCL cells.
Discussion
Metabolism-related gene LDHA was highly expressed in DLBCL tissue. Here, we have demonstrated an unknown mechanism by which LDHA promoted DLBCL. This paper shows that increased expression of LDHA is associated with DLBCL incidence. The data support the notion that LDHA promoted DLBCL by inducing metabolic programming and found that LDHA overexpression in DLBCL by increasing lactate levels. This is compatible with metabolic protumor functions of LDHA in DLBCL. Additional studies are necessary to validate these novel results.
LDHA preferentially catalyzing pyruvate to lactate in tumor anaerobic glycolysis [14]. Serum LDH is a key enzyme in glycolysis of DLBCL patients [35]. We have shown increased serum LDH levels in the large cohort of DLBCL patients. It indicated that glycolysis is the main way to increase energy supply in DLBCL patients. Similarly, there is an increased fraction of PBMCs expressing higher protein levels of LDHA and lactate in DLBCL patients compared to control group.
It is well known that LDHA localizes at cytoplasm exerting its lactate-producing activity [36]. Along this line, we found that subcellular detection of LDHA protein might provide additional prognostic information, as LDHA was mainly localized in cytoplasmic upon H2O2 treatment in OCI-Ly1/OCI-Ly10 cells. We verified if LDHA exerted the enzyme activity to catalyze pyruvate to lactate in DLBCL cells. Recent studies also discovered canonical enzyme activity of LDHA, catalyzing pyruvate to lactate with increased ratio of NAD+/NADH redox state upon H2O2 treatment. At first glance, the association of increased overall and cytoplasmic expression of LDHA with prognosis of DLBCL may be readily explained by the role of LDHA in the Warburg effect. Several studies have demonstrated that aberrant activation of LDHA has been found to be closely related to diverse cancers [37, 38]. LDHA is mainly located in the cytoplasm, acting as the glycolysis regulator through the pyruvate/lactate ratio, but it has also been reported inside mitochondria and is present in the nucleus [27, 39]. In our study, LDHA is mainly located in the cytoplasm. Whether LDHA located in the nucleus or mitochondria and its function in DLBCL cells will be studied in the future.
LDHA contains 332 amino acids, usually existing as a tetramer [36]. In this study, we investigated whether LDHA exists as a tetramer. Interestingly, H2O2 induced ROS accumulation promotes mainly cytoplasma localization of LDHA mono and dimer in OCI-Ly1/OCI-Ly10 cells of our research. Our results revealed an important role for LDHA-mediated oxidative stress in DLBCL. In our study, mono and dimer LDHA overexpressed exhibited oxidative stress by increasing ROS upon H2O2.
Lactate metabolism has been proven to be associated with cell proliferation and survival [36]. LDHA is found in cancer cells correlating with tumor proliferation and migration potential [37]. Depending on LDHA level, cells could exhibit the proliferation, apoptosis and migration. In our study, LDHA over expression promotes proliferation and migration and with reduced apoptosis. Our study indicated that targeting metabolic reprogramming through LDHA might be an effective strategy to induce DLBCL.
Previous study has demonstrated that LDHA was up-regulated by STAT [32]. As STAT5 is one of seven members of the STAT family of proteins and has a key role in the generation of B-cell lymphoma [33, 34]. Our results also showed that reduced expression of LDHA could downregulate oxidative stress mediator p-STAT5 expression. Thus, we suspected that LDHA-mediated oxidative stress promotes proliferation and migration and with reduced apoptosis might go through the p-STAT5 signalling pathway.
Currently, LDHA testing is not widely implemented in clinical practice, and the detection reagents have yet to be standardized. This study serves as a preliminary investigation into the metabolic role and implications of LDHA in the growth of DLBCL. However, the absence of subtype analysis represents a limitation of this research. Future studies intend to enroll a larger cohort of patients and perform subtype analyses in order to elucidate the factors contributing to the prognostic differences among DLBCL subtypes.
Another limitation of this study is that we primarily conducted our research at the in vitro cellular level. As our research progresses, we aim to investigate the regulatory mechanism of LDHA. We plan to conduct in vivo experiments in mice.
In conclusion, we demonstrate that the cytoplasmic cytoplasma localization of LDHA in DLBCL is sufficient to trigger antioxidant responses and activate STAT5 pathway, leading to cell survival and proliferation under oxidative stress. As such, blocking LDHA may offer more opportunities to DLBCL prevention and ROS-based cancer therapies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank YL. Hou for the flow cytometric analysis. We thank HY. Zhang for blood routine samples collection and transport.
Author contributions
Na Zhou wrote the main manuscript text and Jialin Zhang, Qifeng Lu and Wei Liu prepared figures. All authors reviewed the manuscript.
Funding
This work was supported by the natural science foundation of Shandong Province of China (Grant #ZR2023MH157) and the national natural science foundation of China (Grant #82300898).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This retrospective study was carried out using the case series of our hospital. The study was approved by the Ethics Committee of Shandong Provincial Hospital (NSFC: No. 2024 − 152) and was conducted in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was waived by our Institutional Review Board because of the retrospective nature of our study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jialin Zhang and Qifeng Lu contributed equally to this work.
References
- 1.Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674 [DOI] [PubMed] [Google Scholar]
- 2.Warburg O (1956) On the origin of cancer cells. Science 123(3191):309–314 [DOI] [PubMed] [Google Scholar]
- 3.Aboelella NS et al (2021) Oxidative stress in the Tumor Microenvironment and its relevance to Cancer Immunotherapy. Cancers (Basel), 13(5) [DOI] [PMC free article] [PubMed]
- 4.Tsukioka T et al (2012) Preoperative serum oxidative stress marker as a strong indicator of nodal involvement in clinical stage I lung adenocarcinoma. Int J Clin Oncol 17(3):250–255 [DOI] [PubMed] [Google Scholar]
- 5.Gencer M et al (2006) Association of serum reactive oxygen metabolite levels with different histopathological types of lung cancer. Respiration 73(4):520–524 [DOI] [PubMed] [Google Scholar]
- 6.Suzuki Y et al (2013) Hepatocellular carcinoma patients with increased oxidative stress levels are prone to recurrence after curative treatment: a prospective case series study using the d-ROM test. J Cancer Res Clin Oncol 139(5):845–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inokuma T et al (2009) Oxidative stress and tumor progression in colorectal cancer. Hepatogastroenterology 56(90):343–347 [PubMed] [Google Scholar]
- 8.Hou D et al (2018) Increased oxidative stress mediates the antitumor effect of PARP inhibition in ovarian cancer. Redox Biol 17:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nojima J et al (2011) Oxidation stress index’ as a possible clinical marker for the evaluation of non-hodgkin lymphoma. Br J Haematol 155(4):528–530 [DOI] [PubMed] [Google Scholar]
- 10.Zhang J et al (2015) Disruption of KMT2D perturbs germinal center B cell development and promotes lymphomagenesis. Nat Med 21(10):1190–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura H et al (2022) Clinical significance of oxidative stress for untreated patients with diffuse large B-cell lymphoma. Mol Clin Oncol 16(1):4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peroja P et al (2012) Oxidative stress and redox state-regulating enzymes have prognostic relevance in diffuse large B-cell lymphoma. Exp Hematol Oncol 1(1):2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mai Y et al (2016) An oxidative stress-based mechanism of doxorubicin cytotoxicity suggests new therapeutic strategies in ABC-DLBCL. Blood 128(24):2797–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji Y et al (2017) Corrigendum: adenylate kinase hCINAP determines self-renewal of colorectal cancer stem cells by facilitating LDHA phosphorylation. Nat Commun 8:16000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie H et al (2014) Targeting lactate dehydrogenase–a inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor-initiating cells. Cell Metab 19(5):795–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussien R, Brooks GA (2011) Mitochondrial and plasma membrane lactate transporter and lactate dehydrogenase isoform expression in breast cancer cell lines. Physiol Genomics 43(5):255–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koukourakis MI et al (2014) Lactate dehydrogenase 5 isoenzyme overexpression defines resistance of prostate cancer to radiotherapy. Br J Cancer 110(9):2217–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao S et al (2022) Fbw7 inhibits the progression of activated B-Cell like diffuse large B-Cell lymphoma by targeting the positive Feedback Loop of the LDHA/lactate/miR-223 Axis. Front Oncol 12:842356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhalla K et al (2018) Role of hypoxia in diffuse large B-cell lymphoma: metabolic repression and selective translation of HK2 facilitates development of DLBCL. Sci Rep 8(1):744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong Y et al (2022) KIF23 is a potential biomarker of diffuse large B cell lymphoma: analysis based on bioinformatics and immunohistochemistry. Med (Baltim) 101(24):e29312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J et al (2017) AKT Hyperactivation and the potential of AKT-Targeted therapy in diffuse large B-Cell lymphoma. Am J Pathol 187(8):1700–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun C et al (2019) Gene expression profiles analysis identifies a novel two-gene signature to predict overall survival in diffuse large B-cell lymphoma. Biosci Rep 39(1) [DOI] [PMC free article] [PubMed]
- 23.Zhao Y et al (2022) Single-cell RNA-Seq and bulk RNA-Seq reveal Intratumoral Heterogeneity and Tumor Microenvironment characteristics in diffuse large B-Cell lymphoma. Front Genet 13:881345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu H et al (2021) Lactate dehydrogenases amplify reactive oxygen species in cancer cells in response to oxidative stimuli. Signal Transduct Target Ther 6(1):242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keane C et al (2018) A high LDH to absolute lymphocyte count ratio in patients with DLBCL predicts for a poor intratumoral immune response and inferior survival. Oncotarget 9(34):23620–23627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JH et al (2014) The highest prognostic impact of LDH among International Prognostic Indices (IPIs): an explorative study of five IPI factors among patients with DLBCL in the era of Rituximab. Ann Hematol 93(10):1755–1764 [DOI] [PubMed] [Google Scholar]
- 27.Liu Y et al (2018) Nuclear lactate dehydrogenase A senses ROS to produce alpha-hydroxybutyrate for HPV-induced cervical tumor growth. Nat Commun 9(1):4429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Go S et al (2021) The extracellular lactate-to-pyruvate ratio modulates the sensitivity to oxidative stress-induced apoptosis via the cytosolic NADH/NAD(+) redox state. Apoptosis 26(1–2):38–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosh R et al (2014) Allosteric inhibition of the IRE1alpha RNase preserves cell viability and function during endoplasmic reticulum stress. Cell 158(3):534–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Um JW et al (2012) Alzheimer amyloid-beta oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nat Neurosci 15(9):1227–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao X et al (2012) Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol Cell 45(5):598–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pitroda SP et al (2009) STAT1-dependent expression of energy metabolic pathways links tumour growth and radioresistance to the Warburg effect. BMC Med 7:68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malin S, McManus S, Busslinger M (2010) STAT5 in B cell development and leukemia. Curr Opin Immunol 22(2):168–176 [DOI] [PubMed] [Google Scholar]
- 34.Blix ES et al (2012) Phospho-specific flow cytometry identifies aberrant signaling in indolent B-cell lymphoma. BMC Cancer 12:478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma SY et al (2021) A prognostic immune risk score for diffuse large B-cell lymphoma. Br J Haematol 194(1):111–119 [DOI] [PubMed] [Google Scholar]
- 36.Lin Y, Wang Y, Li PF (2022) Mutual regulation of lactate dehydrogenase and redox robustness. Front Physiol 13:1038421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fantin VR, St-Pierre J, Leder P (2006) Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 9(6):425–434 [DOI] [PubMed] [Google Scholar]
- 38.Le A et al (2010) Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A 107(5):2037–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brooks GA et al (1999) Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc Natl Acad Sci U S A 96(3):1129–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.