Abstract
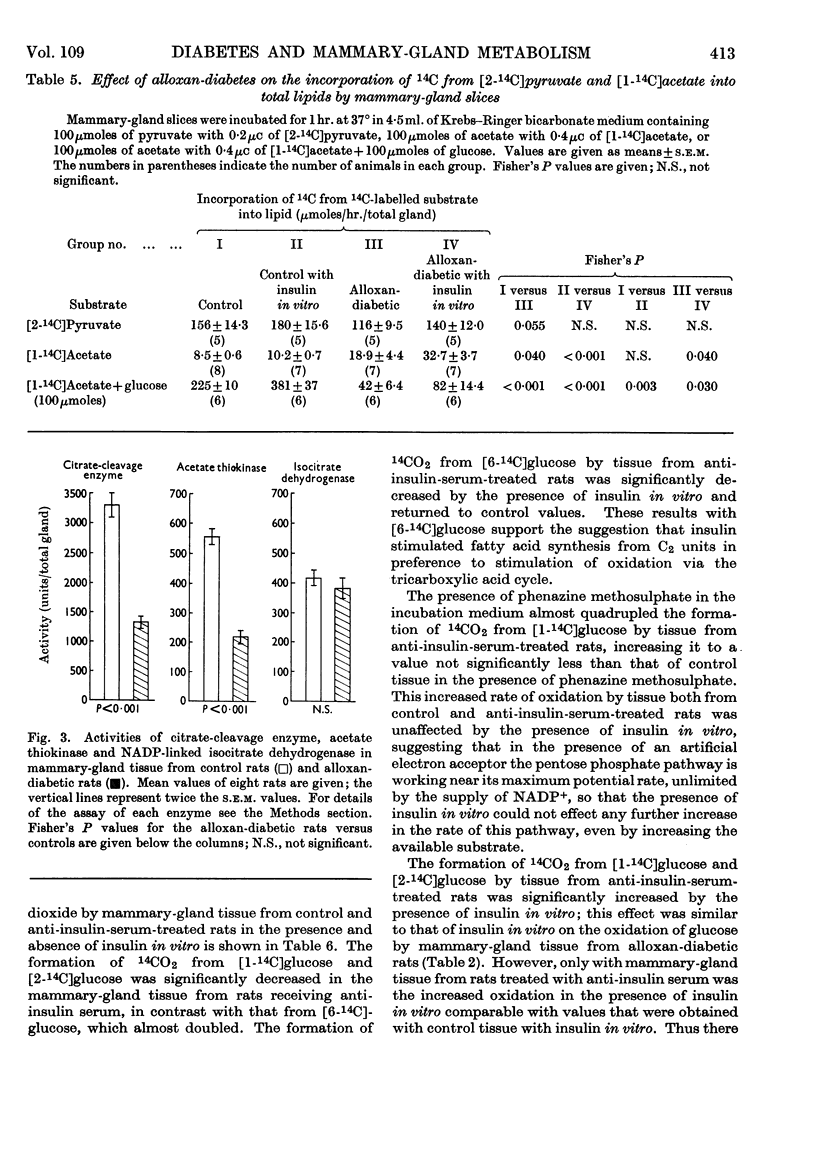
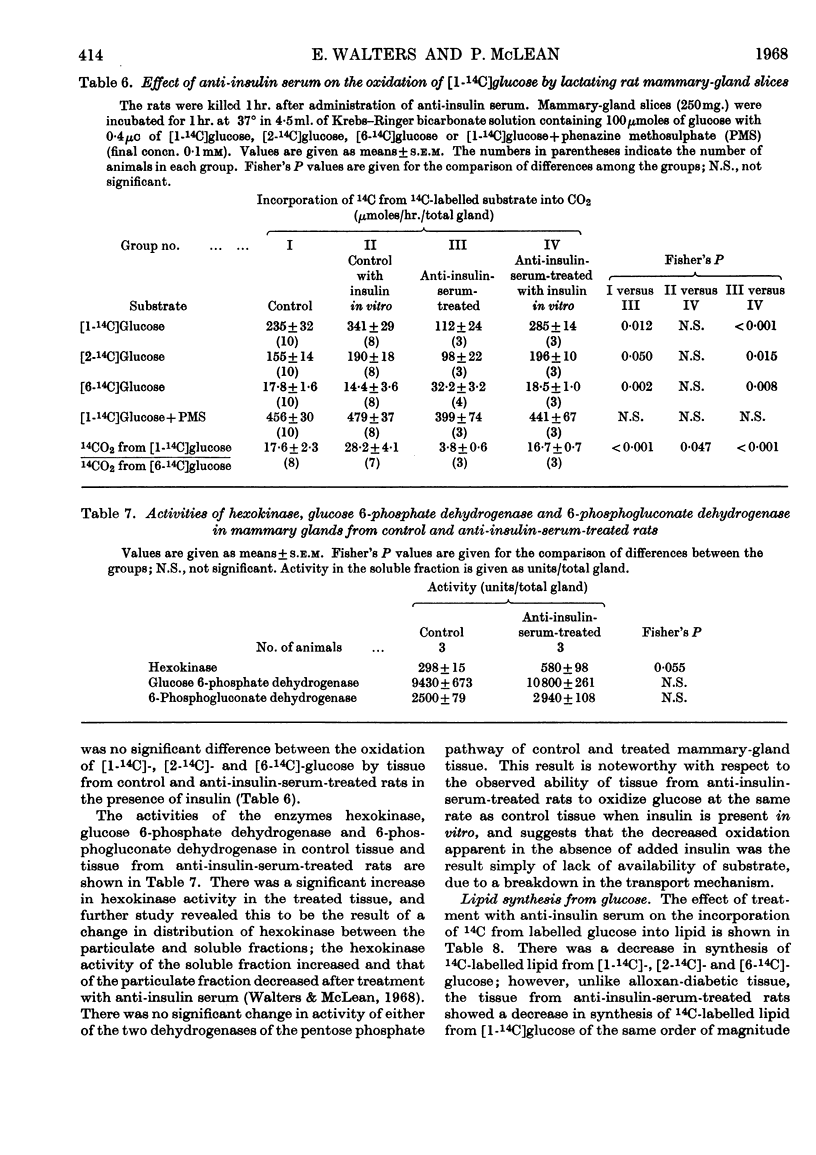
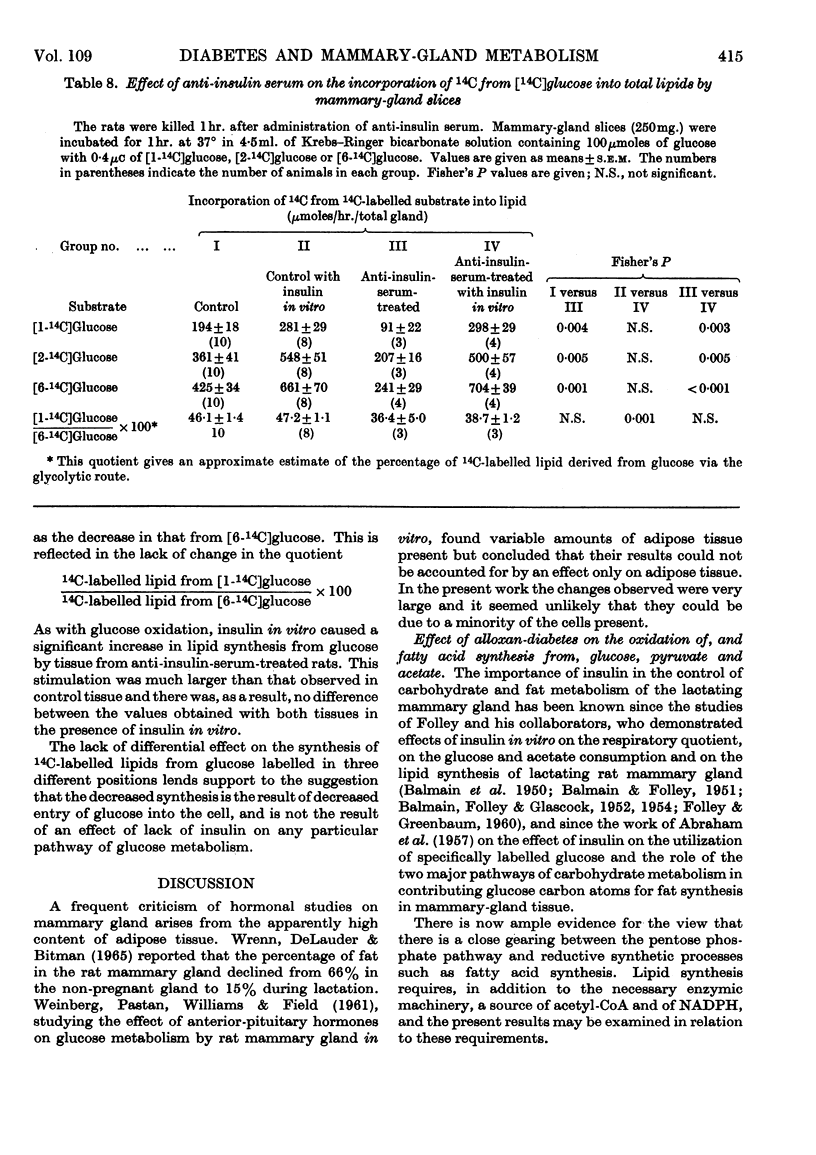
1. The overall metabolic changes in lactating mammary gland in alloxan-diabetic and anti-insulin-serum-treated rats were assessed by measurement of the incorporation of 14C from specifically labelled glucose, pyruvate and acetate into carbon dioxide and lipid, together with measurements of enzymes concerned with the pentose phosphate pathway and with citrate metabolism. 2. Alloxan-diabetes depressed the rate of formation of 14CO2 from [1-14C]glucose and [2-14C]glucose to approx. 10% of the control rate; this was partially reversed by addition of insulin in vitro. The quotient Oxidation of [1-14C]glucose/Oxidation of [6-14C]glucose fell from a value of 17·6 in the control group to 3·9 in the diabetic group and was restored to 14·3 in the presence of insulin in vitro. In keeping with these results it was shown that glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase activities were significantly decreased in alloxan-diabetic rats. 3. Alloxan-diabetes depressed the decarboxylation and the oxidation of labelled pyruvate, but not the oxidation of labelled acetate. 4. The synthesis of lipid from specifically labelled glucose was greatly decreased, that from [2-14C]pyruvate was almost unchanged and that from [1-14C]acetate alone was increased in alloxandiabetic rats. However, the stimulation of lipid synthesis from acetate by glucose was small in the alloxan-diabetic rats compared with the controls. Insulin in vitro partially reversed all these effects. Both citrate-cleavage enzyme and acetate thiokinase activities were decreased in alloxan-diabetic rats. 5. Treatment of rats with anti-insulin serum depressed the formation of 14CO2 from [1-14C]glucose and [2-14C]glucose, but increased that from [6-14C]glucose. This was completely restored by the presence of insulin in vitro. The quotient Oxidation of [1-14C]glucose/Oxidation of [6-14C]glucose fell from a value of 17·6 in the control group to 3·8 in the anti-insulin-serum-treated group. There were no changes in the activity of glucose 6-phosphate dehydrogenase or 6-phosphogluconate dehydrogenase, but the hexokinase distribution changed and the content of the soluble fraction increased significantly. 6. The synthesis of lipid from specifically labelled glucose was depressed in anti-insulin-serum-treated rats; this effect was completely reversed by addition of insulin in vitro to the tissue slices.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAHAM S., CADY P., CHAIKOFF I. L. Effect of insulin in vitro in pathways of glucose utilization, other than Embden-Meyerhof, in rat mammary gland. J Biol Chem. 1957 Feb;224(2):955–962. [PubMed] [Google Scholar]
- ABRAHAM S., MATTHES K. J., CHAIKOFF I. L. Role of TPNH in fatty acid synthesis from acetate by normal and diabetic rat-liver homogenate fractions. Biochim Biophys Acta. 1959 Dec;36:556–558. doi: 10.1016/0006-3002(59)90207-0. [DOI] [PubMed] [Google Scholar]
- BALMAIN J. H., FOLLEY S. J. Further observations on the in vitro stimulation by insulin of fat synthesis by lactating mammary gland slices. Biochem J. 1951 Oct;49(5):663–671. [PMC free article] [PubMed] [Google Scholar]
- BALMAIN J. H., FOLLEY S. J., GLASCOCK R. F. Effects of insulin and of glycerol in vitro on the incorporation of [carboxy-14C] acetate into the fatty acids of lactating mammary gland slices with special reference to species differences. Biochem J. 1952 Oct;52(2):301–306. doi: 10.1042/bj0520301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALMAIN J. H., FOLLEY S. J., GLASCOCK R. F. Relative utilization of glucose and acetate carbon for lipogenesis by mammary gland slices, studies with tritium, 13C and 14C. Biochem J. 1954 Feb;56(2):234–239. [PMC free article] [PubMed] [Google Scholar]
- BALMAIN J. H., FRENCH T. H., FOLLEY S. J. Stimulation by insulin of in vitro fat synthesis by lactating mammary gland slices. Nature. 1950 May 20;165(4203):807–808. doi: 10.1038/165807a0. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloff-Chain A., Catanzaro R., Chain E. B. Influence of anti-insulin serum on glucose metabolism. I. In isolated adipose tissue. Diabetes. 1967 Jul;16(7):472–474. doi: 10.2337/diab.16.7.472. [DOI] [PubMed] [Google Scholar]
- CROFFORD O. B., RENOLD A. E. GLUCOSE UPTAKE BY INCUBATED RAT EPIDIDYMAL ADIPOSE TISSUE. CHARACTERISTICS OF THE GLUCOSE TRANSPORT SYSTEM AND ACTION OF INSULIN. J Biol Chem. 1965 Aug;240:3237–3244. [PubMed] [Google Scholar]
- CROFFORD O. B., RENOLD A. E. GLUCOSE UPTAKE BY INCUBATED RAT EPIDIDYMAL ADIPOSE TISSUE. RATE-LIMITING STEPS AND SITE OF INSULIN ACTION. J Biol Chem. 1965 Jan;240:14–21. [PubMed] [Google Scholar]
- FOLLEY S. J., GREENBAUM A. L. Insulin and metabolism of fatty acids. Br Med Bull. 1960 Sep;16:228–232. doi: 10.1093/oxfordjournals.bmb.a069840. [DOI] [PubMed] [Google Scholar]
- GLOCK G. E., MCLEAN P. A preliminary investigation of the hormonal control of the hexose monophosphate oxidative pathway. Biochem J. 1955 Nov;61(3):390–397. doi: 10.1042/bj0610390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOCK G. E., McLEAN P. Further studies on the properties and assay of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase of rat liver. Biochem J. 1953 Oct;55(3):400–408. doi: 10.1042/bj0550400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howanitz P. J., Levy H. R. Acetyl-CoA carboxylase and citrate cleavage enzyme in the rat mammary gland. Biochim Biophys Acta. 1965 Oct 4;106(2):430–433. doi: 10.1016/0005-2760(65)90056-1. [DOI] [PubMed] [Google Scholar]
- KORNACKER M. S., LOWENSTEIN J. M. CITRATE AND THE CONVERSION OF CARBOHYDRATE INTO FAT. THE ACTIVITIES OF CITRATE-CLEAVAGE ENZYME AND ACETATE THIOKINASE IN LIVERS OF STARVED AND RE-FED RATS. Biochem J. 1965 Jan;94:209–215. doi: 10.1042/bj0940209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADSEN J., ABRAHAM S., CHAIKOFF I. L. THE CONVERSION OF GLUTAMATE CARBON TO FATTY ACID CARBON VIA CITRATE. I. THE INFLUENCE OF GLUCOSE IN LACTATING RAT MAMMARY GLAND SLICES. J Biol Chem. 1964 May;239:1305–1309. [PubMed] [Google Scholar]
- Mansford K. R. Influence of anti-insulin serum on glucose metabolism. II. In the perfused heart. Diabetes. 1967 Jul;16(7):475–477. doi: 10.2337/diab.16.7.475. [DOI] [PubMed] [Google Scholar]
- McLean P., Brown J. Activities of some enzymes concerned with citrate and glucose metabolism in transplanted rat hepatomas. Biochem J. 1966 Mar;98(3):874–882. doi: 10.1042/bj0980874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti R. L., Abraham S. Effects of insulin on glucose metabolism by explants of mouse mammary gland maintained in organ culture. Biochim Biophys Acta. 1966 Aug 24;124(2):280–288. doi: 10.1016/0304-4165(66)90191-7. [DOI] [PubMed] [Google Scholar]
- RIVERA E. M., BERN H. A. Influence of insulin on maintenance and secretory stimulation of mouse mammary tissues by hormones in organ-culture. Endocrinology. 1961 Aug;69:340–353. doi: 10.1210/endo-69-2-340. [DOI] [PubMed] [Google Scholar]
- SHARMA C., MANJESHWAR R., WEINHOUSE S. EFFECTS OF DIET AND INSULIN ON GLUCOSE-ADENOSINE TRIPHOSPHATE PHOSPHOTRANSFERASES OF RAT LIVER. J Biol Chem. 1963 Dec;238:3840–3845. [PubMed] [Google Scholar]
- Srere P. A., Foster D. W. On the proposed relation of citrate enzymes to fatty acid synthesis and ketosis in starvation. Biochem Biophys Res Commun. 1967 Mar 9;26(5):556–561. doi: 10.1016/0006-291x(67)90101-5. [DOI] [PubMed] [Google Scholar]
- VAGELOS P. R. LIPID METABOLISM. Annu Rev Biochem. 1964;33:139–172. doi: 10.1146/annurev.bi.33.070164.001035. [DOI] [PubMed] [Google Scholar]
- WEINBERG A. N., PASTAN I., WILLIAMS H. E., FIELD J. B. Effects of anterior pituitary hormones on glucose metabolism by rat mammary gland in vitro. J Biol Chem. 1961 Apr;236:1002–1005. [PubMed] [Google Scholar]
- Walters E., McLean P. Effect of thyroidectomy on pathways of glucose metabolism in lactating rat mammary gland. Biochem J. 1967 Nov;105(2):615–623. doi: 10.1042/bj1050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrenn T. R., DeLauder W. R., Bitman J. Rat mammary gland composition during pregnancy and lactation. J Dairy Sci. 1965 Nov;48(11):1517–1521. doi: 10.3168/jds.S0022-0302(65)88509-5. [DOI] [PubMed] [Google Scholar]


