Abstract
Background—There is currently no effective treatment for recurrent hormone refractory carcinomas of the prostate gland. An understanding of the underlying mechanisms responsible for the progression of these lesions is likely to be important for the development of new therapeutic approaches. Recently, it has been suggested that the transition to a hormone independent state is accompanied by increased proliferation and bcl-2 gene expression, as well as by a decreased apoptotic state.
Aim—To investigate the possible role of Bcl-2 and other cell cycle regulating proteins in the development of prostatic tumours.
Methods—Immunohistochemistry was used to study the relation between the expression of Bcl-2 and the androgen receptor, as well as p21WAF1/CIP1 (p21), and cyclin D1 status, in a series of 89 prostate cancer samples taken before androgen withdrawal treatment.
Results—Androgen receptor negative tumours expressed significantly higher amounts of Bcl-2 than those prostate carcinomas with low/medium androgen receptor values. However, in tumours expressing the highest amounts of androgen receptor, Bcl-2 expression was also high. A significant positive relation between Bcl-2 and p21 expression, as well as an inverse relation between Bcl-2 and cyclin D1 expression, was noted. Androgen receptor positive samples also expressed significantly higher amounts of cyclin D1.
Conclusions—These results suggest that p21 and cyclin D1 expression in prostatic cancer might be modulated by Bcl-2 and by androgens and in turn this could be relevant to the progression of prostatic cancer.
Keywords: prostatic cancer, hormone independency, Bcl-2, androgen receptor, p21WAF1/CIP1, cyclin D1
Tumour progression is usually described as an imbalance between proliferation and programmed cell death. It can be caused by alterations in genes that are essential for cell growth, differentiation, and apoptosis. The assessment of these genetic abnormalities, coupled with the precise measurement of proliferative potential, can provide important information about prognosis and response to treatment.
The prostate gland is dependent on the presence of circulating androgens to maintain its normal structure and function. Likewise, 70–80% of prostatic neoplasms are androgen sensitive tumours that undergo regression after chemical or surgical androgen ablation.1 The cell clones that are selected during androgen withdrawal treatment give rise to androgen independent prostate carcinomas. Such recurrent tumours are clinically more aggressive and the prognosis after relapse is poor.2 To date, no effective treatment has been developed for these hormone refractory carcinomas.
Recently, it has been postulated that the transition to a hormone independent state is accompanied by increased proliferation and Bcl-2 expression, as well as by a decreased rate of apoptosis.3 Apoptosis is an active ATP dependent process that, in the prostate, can be mediated via an increase in the intracellular calcium concentration.4 The bcl-2 gene encodes a 26 kDa protein Bcl-2, which prevents apoptosis when overexpressed in prostate cells.5 In previous studies, Bcl-2 expression was found to be more frequent in higher grade tumours and to predict disease progression.6 Therefore, the involvement of Bcl-2 in the development of hormone refractory prostatic tumours seems probable.7 In addition, the cyclin dependent kinase inhibitor, p21WAF1/CIP1 (p21), and cyclin D1 might be actively involved in these processes. Therefore, the aim of this study was to investigate the expression of Bcl-2 in relation to these various cell cycle regulating proteins.
Materials and methods
PATIENTS AND SPECIMENS
The expression of Bcl-2, the androgen receptor, p21, and cyclin D1 was analysed by immunohistochemistry in 89 paraffin wax embedded prostatic cancer samples (graded between 3 and 9 according to the Gleason system) before androgen withdrawal treatment. The samples were obtained from the Institute of Pathology, Palacký University, Olomouc and from the department of histopathology, New Cross Hospital, Wolverhampton.
MEASUREMENT
The numbers of positive cells were evaluated semiquantitatively by two independent investigators and by computer image analysis (LUCIA M; Laboratory Imaging, Prague, Czech Republic) in identical areas of parallel sections, and converted to a 0–3 scale (0, no positive cells; 1, up to 33.3% positive cells; 2, between 33.3% and 66.6% positive cells; 3, between 66.6% and 100% positive cells). Any disagreement between evaluators was checked and measurements were repeated.
IMMUNOHISTOCHEMISTRY
Primary antibodies to the androgen receptor (clone F39.4.1; Biogenex, San Ramon, Californa, USA), Bcl-2 (clone 124; Dako, Copenhagen, Denmark), p21 (clone WA1; MMCI, Brno, Czech Republic), and cyclin D1 (clone CD2.1; MMCI) were used in standard immunohistochemical methods. A biotinylated secondary antibody and streptavidin/biotin complexed with horseradish peroxidase (HRP) (streptABComplex/HRP duet kit; Dako) were also used and HRP activity was developed by means of 3,3′-diaminobenzidine.
STATISTICS
Statistical analysis was performed using a χ2 test for independence and homogeneity of variance (department of biometry, Palacký University, Olomouc, Czech Republic).
Results
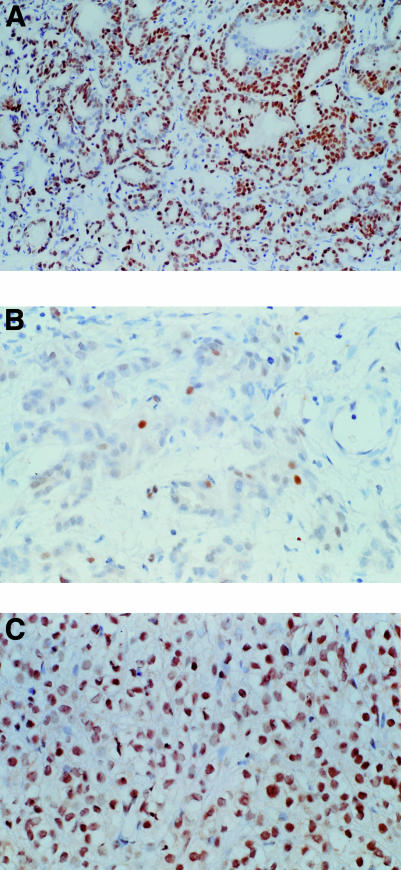
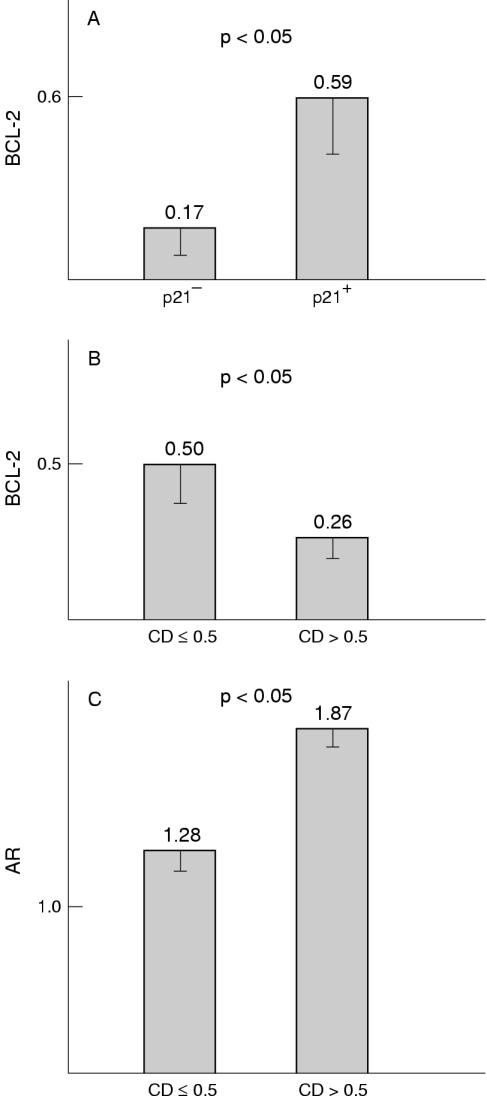
Figure 1A–C ▶ shows representative immunohistochemical results. Androgen receptor positive prostate cancers (more than 10% positive cells) occurred in both low (Gleason 3–5) as well as high (Gleason 8,9) grade carcinomas. In medium grade (Gleason 6,7) carcinomas expression of the androgen receptor was generally lower. These results were not significant because of the low number of high grade carcinomas. Analysis of the relation between Bcl-2 and androgen receptor expression revealed that Bcl-2 expression was significantly higher in androgen receptor negative prostatic cancers than in those samples with low/medium androgen receptor scores (score, 0.5–2.0; p < 0.05). However, in samples with the highest androgen receptor positivity, a slightly higher amount of Bcl-2 expression was noted, which was not significantly different from that found in androgen receptor negative samples. Bcl-2 expression was significantly higher in p21 positive tumours compared with those tumours in which p21 expression was absent (p < 0.05) (fig 2A ▶). There was also a significant inverse relation (p < 0.05) between expression of Bcl-2 and cyclin D1 (fig 2B ▶) and a significant positive relation (p < 0.05) between expression of the androgen receptor and cyclin D1 (fig 2C ▶). No other significant associations were seen, but a trend towards increasing p21 expression in cases with high concentrations of cyclin D1 was noted (data not shown).
Figure 1.
(A) Typical nuclear staining for the androgen receptor in prostatic cancer. (B) Nuclear staining for p21 in Bcl-2 positive prostatic cancer. (C) Intense nuclear staining for cyclin D1 in androgen receptor positive prostatic cancer.
Figure 2.
(A) Relation between Bcl-2 and p21 expression in prostatic cancer. (B) Relation between Bcl-2 and cyclin D1 (CD) expression in prostatic cancer. (C) Relation between androgen receptor (AR) and cyclin D1 expression in prostatic cancer.
Discussion
Recent reports suggest that combined endocrine treatment induces tumour regression by greatly reducing proliferation.8–10 However, other studies have also identified programmed cell death as the molecular mechanism underlying regression of androgen dependent human prostatic cancer after androgen ablation.8–14
Many recent papers have examined the role of p21 in the control of cell proliferation.15 This nuclear protein is an inhibitor of several cyclin dependent kinases and a component of the quaternary complex (including cyclin D1, specific cyclin dependent kinase, and proliferating cell nuclear antigen).16 The expression of p21 is variable among different human tissues,17 and has been implicated in the mechanisms of cell cycle arrest that allow DNA repair in response to wild-type, but not to mutant, p53.18–20 However, some recent findings demonstrate that p21 might also be induced in a p53 independent manner, for example by transforming growth factor β.15 p21 has also been postulated as an inducer of cyclin D1 in vitro and a significant association has been shown between p21 and cyclin D1 expression in breast cancer.21
Given the association between high concentrations of cyclin D1 and the increased proliferative activity found in some tumours, it was expected that overexpression of this protein would be associated with poor prognosis. Kallakury and co-workers22 analysed the expression of cyclin D1 in prostatic carcinoma and found that it was expressed in a minority of cases, where it was associated with a high Gleason grade. Detailed studies, however, did not show any significant association with recurrence or overall survival.23 On the other hand, overexpression of cyclin D1 was found more commonly in oestrogen receptor positive breast cancer, suggesting that cyclin D1 expression could be considered a marker of good prognosis.21
In untreated prostatic carcinoma, we found that there is a direct relation between androgen receptor and cyclin D1 expression, which is in accordance with the findings in breast cancer. This could be explained by steroid upregulation of cyclin D1. We have also found an association between increasing androgen receptor score and lower concentrations of the antiapoptotic protein Bcl-2. This supports the concept that Bcl-2 expression is developing in hormone independent prostate cancer. The finding of higher Bcl-2 concentrations in a few samples with the highest androgen receptor scores is in agreement with the theory that high grade prostatic cancers often synthesise high concentrations of mutant androgen receptor.24,25
One of the most interesting results was that increasing Bcl-2 expression was correlated with increasing concentrations of p21 but decreasing concentrations of cyclin D1. We suggest that this could be the consequence of a specific regulatory pathway in which Bcl-2, probably in accordance with androgens, upregulates the expression of p21, whereas the expression of cyclin D1 is suppressed. However, the relation between hormone responsiveness and expression of these various regulatory genes in prostate cancer remains unclear, although Chen et al have shown that p21 expression as well as expression of another cyclin dependent kinase inhibitor, p27, can be regulated, either directly or indirectly, by androgens.26
In conclusion, our results show that the expression of p21 and cyclin D1 in prostatic cancer might be modulated by Bcl-2 and androgens and that it may well be related to the progression of prostate cancer.
Acknowledgments
The work was supported in part by grants MSMT J14/98 151100001, IGA MZ CR 4020–3, and 4783−3.
References
- 1.Deneshgari F, Crawford ED. Endocrine therapy of advanced carcinoma of the prostate. Cancer 1993;71:1089–97. [DOI] [PubMed] [Google Scholar]
- 2.Stearns ME, McGarvey T. Prostate cancer: therapeutic, diagnostic, and basic studies. Lab Invest 1992;67:540–52. [PubMed] [Google Scholar]
- 3.Dorkin TJ, Neal DE. Basic science aspects of prostate cancer. Semin Cancer Biol 1997;8:21–7. [DOI] [PubMed] [Google Scholar]
- 4.Kyprianou N, English HF, Isaacs JT. Activation of Ca2+/Mg2+ dependent endonucleases as an early event in castration-induced prostatic cell death. Prostate 1989;13:103–18. [DOI] [PubMed] [Google Scholar]
- 5.Hochenberry DM, Nunez G, Milliman C. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 1990;348:334–6. [DOI] [PubMed] [Google Scholar]
- 6.Bubendorf L, Sauter G, Moch H, et al. Prognostic significance of Bcl-2 in clinically localized prostate cancer. Am J Pathol 1996;148:1557–65. [PMC free article] [PubMed] [Google Scholar]
- 7.Raffo AJ, Perlman H, Chen MW, et al. Overexpression of bcl-2 protects prostate cancer cells from apoptosis in vitro and confers resistance to androgen depletion in vivo. Cancer Res 1995;55:4438–45. [PubMed] [Google Scholar]
- 8.Magi-Galluzzi C, Montironi R, Giannulis G, et al. Prostatic invasive adenocarcinoma: effect of combination endocrine therapy (LHRH agonist and flutamide) on the expression and location of proliferating cell nuclear antigen (PCNA). Pathol Res Pract 1993;189:1154–60. [DOI] [PubMed] [Google Scholar]
- 9.Montironi R, Magi-Galluzzi C, Diamanti L, et al. Prostatic intra-epithelial neoplasia: expression and location of proliferating cell nuclear antigen (PCNA) in epithelial, endothelial and stromal nuclei. Virchows Arch 1993;422:185–92. [DOI] [PubMed] [Google Scholar]
- 10.Montironi R, Magi-Galluzzi C, Fabris G. Apoptotic bodies in prostatic intraepithelial neoplasia and prostatic adenocarcinoma following total androgen ablation. Pathol Res Pract 1995;191:873–80. [DOI] [PubMed] [Google Scholar]
- 11.Armas OA, Melamed A, Aprikian A, et al. Effect of preoperative androgen deprivation therapy in prostatic carcinoma. Lab Invest 1993;68:55A. [Google Scholar]
- 12.Colombel M, Symmans F, Gil S, et al. Detection of the apoptosis-suppressing oncoprotein bcl-2 in hormone-refractory human prostate cancers. Am J Pathol 1993;143:390–400. [PMC free article] [PubMed] [Google Scholar]
- 13.Kyprianou N, Bains AK, Jacobs SC. Induction of apoptosis in androgen-independent human prostate cancer cells undergoing thymineless death. Prostate 1994;25:66–75. [DOI] [PubMed] [Google Scholar]
- 14.Montironi R, Magi-Galluzzi C, Muzzonigro G, et al. Effects of combination endocrine treatment on normal prostate, prostatic intraepithelial neoplasia, and prostatic adenocarcinoma. J Clin Pathol 1994;47:906–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox LS. Multiple pathways control cell growth and transformation: overlapping and independent activities of p53 and p21Cip1/WAF1/Sdi1. J Pathol 1997;183:134–40. [DOI] [PubMed] [Google Scholar]
- 16.Xiong Y, Zhang H, Beach D. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell 1992;71:505–14. [DOI] [PubMed] [Google Scholar]
- 17.Fredersdorf S, Milne AW, Hall PA, et al. Characterization of a panel of novel anti-p21Waf1/Cip1 monoclonal antibodies and immunochemical analysis of p21Waf1/Cip1 expression in normal human tissues. Am J Pathol 1996;148:825–35. [PMC free article] [PubMed] [Google Scholar]
- 18.Li R, Waga S, Hannon GJ, et al. Differential effects by the p21 CDK inhibitor on PCNA-dependent DNA replication and repair. Nature 1994;371:534–7. [DOI] [PubMed] [Google Scholar]
- 19.Waga S, Hannon GJ, Beach D, et al. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature 1994;369:574–8. [DOI] [PubMed] [Google Scholar]
- 20.El Deiry WS, Tokino T, Velculeccu VE, et al. WAF1, a potential mediator of p53 tumor suppression. Cell 1993;75:817–25. [DOI] [PubMed] [Google Scholar]
- 21.Rey MJ, Fernandez PL, Jares P, et al. p21WAF1/Cip1 is associated with cyclin D1CCND1 expression and tubular differentiation but is independent of p53 overexpression in human breast carcinoma. J Pathol 1998;184:265–71. [DOI] [PubMed] [Google Scholar]
- 22.Kallakury BVS, Sheehan CE, Ambros RA, et al. The prognostic significance of p34cdc2 and cyclin D1 protein expression in prostate adenocarcinoma. Cancer 1997;80:753–63. [DOI] [PubMed] [Google Scholar]
- 23.Barnes DM. Cyclin D1 in mammary carcinoma. J Pathol 1997;181:267–69. [DOI] [PubMed] [Google Scholar]
- 24.Chodak GW, Kranc DM, Puy LA, et al. Nuclear localization of androgen receptor in heterogeneous samples of normal, hyperplastic and neoplastic human prostate. J Urol 1992;147:798–803. [DOI] [PubMed] [Google Scholar]
- 25.Habib FK, Odoma S, Busuttil A, et al. Androgen receptors in cancer of the prostate. Correlation with the stage and grade of the tumor. Cancer Res 1986;47:2351–6. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Robles AI, Martinez LA, et al. Expression of G1 cyclins, cyclin-dependent kinases, and cyclin-dependent kinase inhibitors in androgen-induced prostate proliferation in castrated rats. Cell Growth Differ 1996;7:1571–8. [PubMed] [Google Scholar]