Abstract
Monoclonal antibodies are essential tools for many molecular immunology investigations. In particular, when used in combination with techniques such as epitope mapping and molecular modelling, monoclonal antibodies enable the antigenic profiling and visualisation of macromolecular surfaces. In addition, monoclonal antibodies have become key components in a vast array of clinical laboratory diagnostic tests. Their wide application in detecting and identifying serum analytes, cell markers, and pathogenic agents has largely arisen through the exquisite specificity of these unique reagents. Furthermore, the continuous culture of hybridoma cells that produce these antibodies offers the potential of an unlimited supply of reagent. In essence, when compared with the rather limited supply of polyclonal antibody reagents, the feature of a continuous supply enables the standardisation of both the reagent and the assay technique. Clearly, polyclonal and monoclonal antibodies have their advantages and disadvantages in terms of generation, cost, and overall applications. Ultimately, monoclonal antibodies are only produced when necessary because their production is time consuming and frustrating, although greatly rewarding (at least most of the time!). This is especially apparent when a monoclonal antibody can be applied successfully in a routine pathology laboratory or can aid in the clinical diagnosis and treatment of patients. In this article, the generation and application of monoclonal antibodies are demystified to enable greater understanding and hopefully formulate novel ideas for clinicians and scientists alike.
Keywords: monoclonal antibodies, hybridomas, “magic bullets”
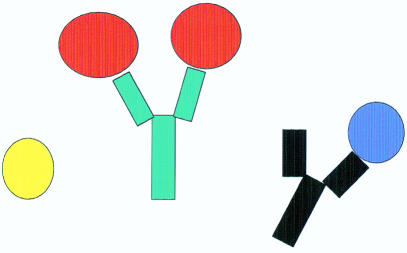
What are antibodies? For a lay person, the response might be that antibodies are special molecules in our blood and tissue fluids that help us fight infection. There are a variety of antibody molecules of different shapes and sizes, although the basic structure is essentially “Y” shaped, with the two tips designed to recognise and bind (fig 1 ▶) foreign agents (for example, bacteria), foreign substances, or harmful cells. The remainder of the molecule is associated with so called “effector functions”, which enable the antibody to interact with other immune cells, or serum proteins. In turn, these help do away with most unwanted company. Special molecules termed “monoclonal antibodies” can be obtained from cells grown in the laboratory, and it is these reagents that are useful in research and hospital laboratory diagnostic tests. This is because monoclonal antibodies are very specific for their intended targets. Of course, latterly, monoclonal antibodies have been termed “magic bullets” because they can be used as vehicles for delivering therapeutic agents to cancerous cells in the human body.
Figure 1.
Schematic representation of an antibody molecule highlighting the “Y” shaped structure.
Although simplistic, the preceding section encompasses a number of salient features appertaining to the structure, function, and applications of antibody molecules or immunoglobulins. Capitalising on this background, this article focuses on the theory and practical generation of murine monoclonal antibodies and their applications in the histopathological diagnosis and treatment of malignant disease.
Monoclonal antibodies
When a humoral immune response is provoked by an immunogen, such as tetanus toxoid, a plethora of antibodies are produced in an individual against different parts or regions of this foreign substance. These are termed antigenic determinants, or epitopes, which usually comprise six to eight amino acids. It should be appreciated that most antibodies recognise and interact with a three dimensional shape composed of “discontinuous” residues brought into juxtaposition by the folding of a molecule. Alternatively, antibodies can also recognise linear stretches of amino acids or “continuous” epitopes.1 Of course, an important concept to bear in mind is that each antibody molecule is specific for a single epitope, and that each antibody is the product of a single B cell clone. Thus, an antibody of unique specificity, derived from a single B cell clone, is termed a monoclonal antibody.
In our example cited above, tetanus toxoid would induce antibodies from numerous B cell clones; that is, this immunogen would produce a polyclonal antibody response. In contrast, the propagation of an isolated B cell clone would produce antibody of single specificity. However, a problem arises in that in tissue culture medium, B cells die within a few days of their isolation (for example, from a mouse spleen). Consequently, methods of conferring immortality on to B cells have been investigated. Indeed, immortality has been accomplished by means of viral transformation (for example, using Epstein-Barr virus) and/or fusion to cancerous cells to generate hybrids or “hybridomas”. In general, the former technique is used for the immortalisation of peripheral blood B cells (and production of human monoclonal antibodies), whereas myeloma cells have mainly been used in the production of murine monoclonal antibodies.2
Why monoclonal as opposed to polyclonal antibodies?
To their advantage, polyclonal antibodies detect a multiplicity of epitopes and therefore recognise antigen from different orientations: this may be important in certain assays where the detection of an analyte would be compromised by the use of a single epitope. In addition, polyclonal reagents are relatively simple and cheap to produce in the short term compared with monoclonal reagents. Furthermore, the use of larger animals (such as horses, goats, and rabbits) enables the recovery of a large volume (for example, 60 ml from a rabbit) of antibody rich serum. However, at some point a fresh batch will be sought as the original stock diminishes, which inevitability leads to the problem of batch to batch variation. This might include differences in antibody reactivity and titre, and thus polyclonal reagents in general suffer from a lack of reproducibility. In contrast, the continuous culture of B cell hybridomas offers a reproducible and potentially inexhaustible supply of antibody with exquisite specificity. Consequently, monoclonal antibodies enable the development of standardised and secure immunoassay systems.3 Overall, monoclonal antibodies serve as powerful tools for the investigation of macromolecules and cells,4 and have proved effective reagents in terms of specificity for clinical diagnostic tests. Latterly, of course, murine monoclonal reagents and their humanised counterparts have been used for clinical treatment with varying degrees of success.5,6
So how do you make a monoclonal antibody?
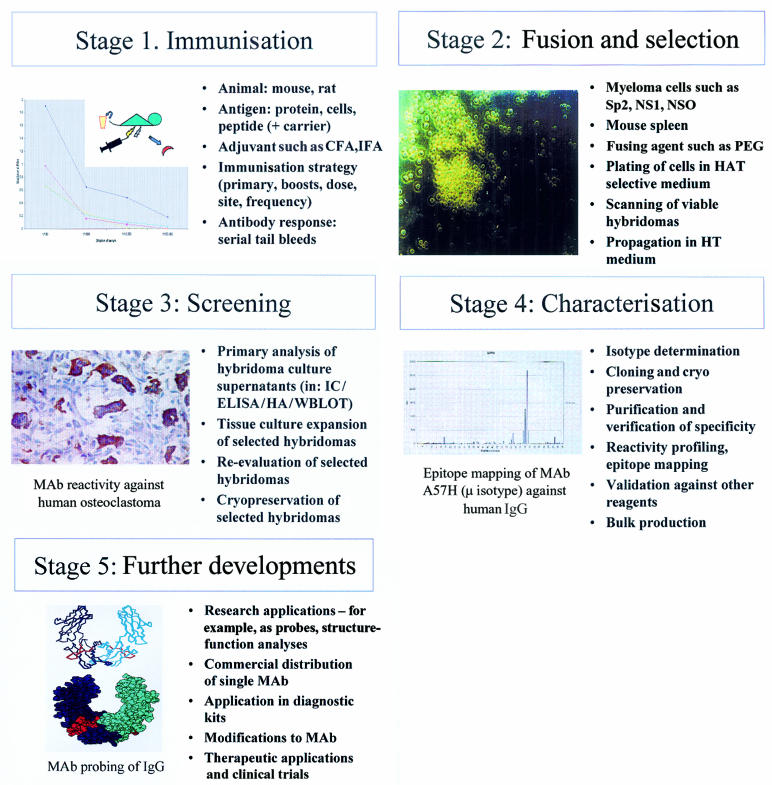
Let us start with a working definition: a monoclonal antibody is regarded as an antibody of single specificity, generated from the immortalisation of a plasma B cell in vitro. Although several recombinant approaches are possible,5,6 the process of demystifying monoclonal antibodies is best illustrated by the generation of murine monoclonal reagents. In essence, five main stages (fig 2 ▶) are highlighted: (1) immunisation, (2) fusion and selection, (3) screening, (4) characterisation, and (5) further developments.
Figure 2.
Five stages of generating a murine monoclonal antibody (MAb). (A) Immunisation, illustrating tail bleeds from mice immunised with Epstein-Barr virus (EBV) latent membrane protein 1 multiple antigenic synthetic peptide. (B) Fusion and selection, showing hybridoma PNG312G.4 (C) Screening, highlighting reactivity of MAb PNG211D against human osteoclastoma.8 (D) Characterisation, epitope mapping of MAb A57H against human IgG Fc. (E) Further developments, molecular modelling of monoclonal antibody A57H revealing an epitope (red) in the CH3 domain of IgG. CFA, complete Freund's adjuvant; ELISA, enzyme linked immunosorbent assay; HA, haemagglutination; HAT, hypoxanthine, aminopterin, and thymidine; HT, hypoxanthine and thymidine; IC, immunochemistry; IFA, incomplete Freund's adjuvant; PEG, polyethylene glycol; WBLOT, western blotting.
STAGE 1: IMMUNISATION
Substances that induce an immune response are usually foreign to the individual and are termed immunogens. In general, protein (50–100 μg), cells (1 × 107), multiple antigenic synthetic peptides, or a short peptide (6–18 amino acids) linked to a carrier protein (for example, keyhole limpet haemocyanin) can be used for the primary immunisation of Balb/c mice. More often than not, an immunogen will be delivered in conjunction with an adjuvant, which is regarded as a non-specific immune enhancer. Typical examples include Freund's complete/incomplete adjuvants and TiterMax™. Invariably, proteins are delivered subcutaneously whereas cells are given intraperitoneally. Regular boosting is needed to augment a polyclonal response, which can be monitored indirectly using tail bleeds. These offer sufficient serum to ascertain the antibody titre to a desired antigen usually in an assay system—for example, enzyme linked immunosorbent assay (ELISA)—that is ultimately required for the monoclonal reagent. The effect of boosting also encourages immunoglobulin class switching and the generation of higher affinity antibodies through somatic hypermutation. In general, IgG monoclonal antibodies are preferred because they are less prone to degradation, and may potentially be more useful as therapeutic reagents.6
Of course the end point, particularly for in vivo strategies, is to select an appropriate mouse (generally the best responder from tail bleeds) and remove (aseptically) antigenically responding B cells from its spleen (or lymph node) to obtain viable cells for hybridisation. It is noteworthy that although in vivo immunisation (including intrasplenic administration) is the favourite choice in many laboratories, there is also the opportunity for in vitro immunisation. In this case, cultured splenic cells are stimulated with only a minimal amount of antigen.
STAGE 2: FUSION AND SELECTION
The hybridisation process centres on the fusion of murine splenic B cells with histocompatible myeloma cells, such as Sp2/0. The latter (and various alternative myeloma cell lines, such as NS1, NSO, and X63Ag8) are preselected for a deficiency in the enzyme hypoxanthine guanine phosphoribosyltransferase (HGPRT)—for example, by culturing in medium containing 8-azaguanine. In essence, this enzyme is fundamental to the post-fusion hybridoma selection process. To understand this process it should be noted that cells possess two pathways of nucleotide biosynthesis: the de novo pathway and the salvage pathway, which uses HGPRT. Consequently, myeloma cells that are HGPRT negative are unable to use the salvage or “alternative” pathway for purine biosynthesis and are thus entirely reliant on the de novo pathway for survival. In the fusion process, splenic B cells are mixed with HGPRT negative myeloma cells and a fusing agent, such as polyethylene glycol. Hopefully, the mixing and centrifugation steps generate myeloma–splenic B cell hybridomas. Once these hybrid cells are formed and plated into tissue culture wells, the priority shifts towards removing unfused myeloma cells. This is necessary because the latter have the potential to outgrow other cells, particularly weakly established hybridomas. This situation is resolved by using a selective medium containing hypoxanthine, aminopterin, and thymidine, otherwise known as “HAT”. Of importance, is the fact that aminopterin blocks the de novo pathway—the only one available to HGPRT negative cells, and as a consequence all unfused myeloma cells will die. Of course, newly formed hybridomas survive this selection process because the salvage pathway enzyme is provided by its splenic B cell counterpart.
Unfortunately, some hybridomas are unstable and regress. Hence, meticulous attention should be given to the visual examination of hybridomas using an inverted microscope. A record of poorly growing, newly emerging, or established hybridomas provides credibility to immunoassay screening data. Once established, a given hybridoma colony will continually grow in culture medium (such as RPMI-1640 with antibiotics and fetal bovine serum) and produce antibody. Twenty to 30 days post-fusion, hybridomas can be propagated in “HT” medium (hypoxanthine and thymidine only) because aminopterin is no longer required.
STAGE 3: SCREENING
This stage focuses on identifying and selecting those hybridomas that produce antibody of appropriate specificity. The selection process must be ruthless otherwise numerous unwanted (at least to you!) hybridomas will compete for your time and incur unnecessary expense in terms of culture plates and medium. Invariably, a rapid “primary” screening system is used that tests the hybridoma culture supernatant for antibody reactivity and specificity. As an example, an Epstein-Barr viral associated protein or peptide can be coated on to plastic ELISA plates. After incubation of hybridoma culture supernatant, secondary enzyme labelled conjugate, and chromogenic substrate, a coloured product indicates a positive hybridoma. Alternatively, immunocytochemical screening might be more appropriate.
Ultimately, primary screening is necessary to “weed out” and eliminate non-specific hybridomas at the earliest opportunity. Obviously, it is important to screen supernatants with some degree of equity and, therefore, it might be wise to test hybridomas when at least three quarters confluent. Unfortunately, this approach means that screening becomes an almost daily task because not all hybridomas grow at similar rates. Of particular note, is the fact that slow growing (and often very stable) hybridomas can appear 25–30 days post-fusion, whereas most become established well before this time.
Hybridomas can initially be grown in multiwell plates and then, once selected, expanded to larger tissue culture flasks. This progression is necessary not only to maintain the well being of the hybridomas but also to provide sufficient cells for cryopreservation and supernatant for further investigations. As a rough guide, culture supernatant can yield anywhere between 1 and 60 μg/ml of monoclonal antibody: the latter being maintained at −20°C or lower until required. The numbers of hybridomas that can manageably be “taken through” in a given laboratory require continual validation. Furthermore, if a fusion has been particularly successful, some rationalisation of hybridomas will be needed; that is, selecting only those providing an intense immunocytochemical staining pattern. Of course, less favoured hybridomas can be cryopreserved and examined at a later date. What is important to bear in mind, is that the workload in generating hybridomas is generally exponential.
STAGE 4: CHARACTERISATION
Further analysis of a potential monoclonal antibody producing hybridoma in terms of reactivity, specificity, and crossreactivity can be achieved using culture supernatant or a purified immunoglobulin preparation. However, before any further work it is often necessary to re-clone hybridomas (for example, by limiting dilution) because an original colony might contain at least two populations of fused B cells. Unless resolved, a consequence of this situation could be ambiguous data resulting from antibodies of differing class, specificity, and affinity. For this reason, isotype determination serves not only to define the murine immunoglobulin class or subclass but also helps identify the presence of a single isotype—for example, IgG1 or a mixture, such as IgM and IgG2b. In addition, knowledge of a monoclonal antibody's isotype will help dictate the most appropriate column purification technique for a culture supernatant—for example, protein G for IgG1.
A crucial aspect of characterisation relates to monoclonal antibody profiling in different assay systems. This is especially pertinent for the antibody's potential as a diagnostic reagent because some monoclonal antibodies perform well in some systems but not others. This phenomenon, termed assay restriction,2,7 relates to how an antibody recognises its target epitope in the context of the assay system used. In this case, an important epitope could be masked, denatured, or rendered inaccessible by the immobilisation procedure adopted within a given technique. Characterisation also affords the opportunity to test against a wide panel of related antigens or tissue preparations, particularly if monoclonal antibodies are being targeted for histopathological purposes. Of course, these endeavours and the hand of serendipity might well lead to useful applications elsewhere, and thus help capitalise on the original investment of time, effort, and cost. Once certain of a hybridoma, bulk production of a monoclonal antibody can be achieved using surface expanded tissue culture flasks or hollow fibre systems, such as Technomouse.
It is noteworthy that although a hybridoma may be the fused product of a single B cell and produce a monoclonal antibody of exquisite specificity, this same antibody can in fact crossreact with other antigens or exhibit dual specificity.3 This corollary arises when an antibody combining site recognises more than one antigenic determinant, either because of some similarity in shape or chemical composition. Furthermore, the nuances of an assay system can also bias the exposure of a particular antigenic determinant or epitope. Consequently, stringent evaluation of a given monoclonal antibody and its target epitope is necessary,8 which may therefore include epitope mapping.1,9 This particular technique allows precise determination of key amino acid residues that are important for antibody recognition and binding. Further characterisation might also include affinity measurements of antigen–monoclonal antibody interactions using surface plasmon resonance (for example, BIACore or IBIS).
STAGE 5: FURTHER DEVELOPMENTS
Once derived, monoclonal antibodies can serve as investigative research tools, or find applications in diagnostic assays or as therapeutic agents. In addition to potential collaborative opportunities, commercial exploitation of monoclonal antibodies might provide some revenue for future research projects. Furthermore, epitope mapping of monoclonal antibodies in conjunction with molecular modelling can enable the visualisation and localisation of key antigenic regions on a molecule. This information might help to elucidate structure–function relations of proteins, carbohydrates, and other molecules of clinical relevance.
Of course, an ultimate goal of monoclonal specialists is to widen the application of antibodies for the clinical treatment of patients. Certain murine monoclonal antibodies have proved effective (depending on subclass) but might ultimately induce human antimouse responses. This problem has been circumvented either by cleavage of the immunogenic Fc portion of the immunoglobulin molecule or by recombinant methodologies. These have largely focused on producing chimeric antibodies containing a murine antibody recognition unit and human Fc region, or using a human IgG molecule and inserting murine complementary determining residues to retain antibody specificity.10,11 Clearly, further advances for so called magic bullets either alone (and reliant on the effector characteristics of the immunoglobulin isotype) or armed with radionucleotides or toxins will undoubtedly obtain further prominence.12
Monoclonal antibodies in the histopathological diagnosis of cancer and as agents in the treatment of malignant disease
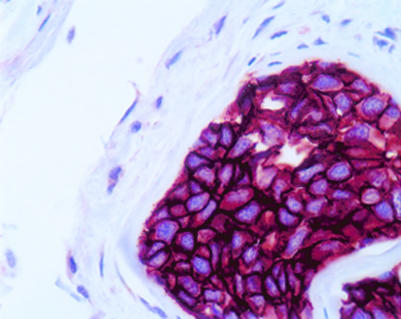
The application of monoclonal antibodies in diagnostic histopathology is particularly widespread, and these molecules can be used to classify tissues and tumours according to their expression of certain defined markers that reflect tissue or cellular genesis. For example, monoclonal antibodies against certain organ associated antigens, such as prostate specific antigen, placental alkaline phosphatase, human chorionic gonadotrophin, α fetoprotein and others, can assist the pathologist in establishing the nature of a primary tumour. In this regard, monoclonal antibodies have proved particularly useful in making the distinction between morphologically similar lesions, such as between mesothelioma and adenocarcinoma.13,14 This approach is also useful in the determination of the organ or tissue origin of undifferentiated metastases. The detection of occult metastases by immunocytological analysis of bone marrow and other tissue aspirates, as well as lymph nodes and other tissues, has also proved feasible with selected monoclonal antibodies (fig 3 ▶). Normal histopathological staining with haematoxylin and eosin is often not sensitive enough to detect small numbers of invasive or metastatic cells. When investigating the sentinel axillary lymph node for metastatic breast cancer—for example, the use of monoclonal antibodies to cytokeratin increases nodal positivity by up to 10%.15,16
Figure 3.
A monoclonal antibody directed against cytokeratin demonstrates micrometastases in smooth muscle of large bowel.
Certain markers detected by monoclonal antibodies can provide information on prognosis in patients with cancer. For example, the detection of the anti-apoptosis protein, BCL-2, is a poor prognostic indicator in a range of diverse tumour types, including ovarian and prostate cancer, and in both Hodgkin's and non-Hodgkin's lymphomas.17–20 Conversely, downregulation of the cyclin dependent kinase inhibitor, p27KIP1, has been shown to be associated with poor prognosis in breast, prostate, and colorectal cancer, and in non-Hodgkin's lymphomas.21–26 Monoclonal antibodies can also be useful in assessing the likely response to treatment in individual cases. For example, the routine immunohistochemical detection of oestrogen receptors has proved to be valuable in predicting those patients with breast cancer who will benefit from anti-oestrogen treatment.27,28
Monoclonal antibodies have enabled the identification of previously unknown cell molecules. Nowhere has this approach been more successful than in the recognition and distinction of the cell surface molecules of lymphocytes. Based on sets of monoclonal antibodies from different laboratories that appear to react with specific cell surface antigens, most surface markers of lymphocytes have been assigned a “cluster of differentiation” (CD) designation. Much of the data collection and the subsequent delineation of cell surface antigens to specific CD numbers has been carried out in a series of international workshops.29 Immunohistochemical studies have mapped the expression of various CD antigens to specific cells and tissues. This has led to their use as cell or tissue markers in the histopathological diagnosis of lymphomas. For example, markers of either B cell (such as CD20 or CD79) or T cell (such as CD3) differentiation may be used to make the distinction between B and T cell lymphomas or to distinguish undifferentiated lymphomas from other anaplastic tumours. Likewise, the cell surface receptor, CD30, is expressed by the malignant Hodgkin/Reed-Sternberg cells of Hodgkin's disease and also by Ki-anaplastic large cell lymphomas, and is useful in the discrimination of these disorders from other related neoplasms.30,31
Some CD antigens expressed on lymphoma cells have provided targets for immunotherapy approaches. Thus, rituximab (Rituxan™), a chimeric anti-CD20 monoclonal antibody containing human IgG1 and κ light chain constant regions with murine variable regions,32 has been used in the treatment of B cell lymphomas. In a phase II trial, rituximab treatment induced responses in 50% of patients with low grade B cell lymphomas and follicular lymphomas, with a median time to progression of 10.2 months.33 Side effects were associated with the first rituximab infusion and usually were mild to moderate. In a recently reported large phase II study of 166 patients with low grade or follicular lymphomas, an objective response was reported for 50% of patients, and side effects were identical to those described previously.34 Rituximab has also been shown to produce response rates of 37% and 33% in patients with high grade diffuse large B cell lymphoma and mantle cell lymphoma, respectively.35 The antilymphoma effects of rituximab are probably the result of complement and antibody dependent cell mediated cytoxicity,36 inhibition of cell proliferation, and induction of apoptosis.37 Lack of expression of CD20 on progenitor cells means that after rituximab treatment the normal B cell compartment can be replenished after the tumour has been partly or wholly ablated.
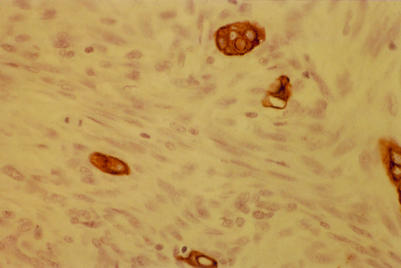
Similarly, identification of the overexpression of the oncogene, HER-2/neu (c-erbB-2), in breast cancer has led to the development of immunotherapy approaches for this disease based on the use of a monoclonal antibody. The HER-2/neu protein is a member of a family of closely related growth factor receptors that also includes the epidermal growth factor receptor (EGFR) or HER-1, HER-3, and HER-4.38 Overexpression of HER-2/neu in breast cancer has been demonstrated using a variety of approaches, including monoclonal antibody detection in both immunohistochemical (fig 4 ▶) and western blotting assays.39–42 In many of these studies, HER-2/neu amplification was associated with poor survival39–42 and poor response to treatment43–46; in particular, being a predictor of poor response to tamoxifen treatment.43–45 However, there have been inconsistencies in the results obtained using different methodologies, and some of the immunohistochemical assays in particular have occasionally produced conflicting data.46 It has been suggested that this might be the result of variations in specimen preparation procedures between and within laboratories.46
Figure 4.
The use of a monoclonal antibody in the immunocytochemical detection of high HER-2/neu expression in a metastatic deposit of breast cancer.
Despite these caveats, the preliminary results of clinical trials in humans using the anti-HER-2/neu monoclonal antibody, rhuMAB HER-2 (Herceptin™) have shown considerable promise. In a phase II study involving 45 women with HER-2/neu overexpressing metastatic breast cancer that was resistant to previous treatment, 11.6% of patients developed an objective response and 37% experienced stabilisation of their disease.47 In a recent phase III clinical trial of 469 patients with metastatic HER-2/neu overexpressing primary breast cancer, the use of Herceptin increased the time to disease progression and response rates when given in combination with either adriamycin-cytoxan or paclitaxel.48 Other approaches to anti-HER-2/neu treatment beyond the scope of this review include the use of antisense probes,49 and the induction of antitumour cytotoxic T cell responses by vaccination with peptides from HER-2/neu protein.50,51
The use of Rituxan and Herceptin are examples of the growing application of monoclonal antibodies in the clinical arena. The production and characterisation of antitumour human monoclonal antibodies or antibody fragments using phage display technology should enable the development of many more powerful agents with which to treat patients with cancer.
Conclusion
This article provides a number of salient points for the rationale in generating monoclonal antibodies and the steps necessary for their production. In addition, a number of applications have been cited for further reading. Using the mouse model and associated procedures of generating monoclonal antibodies certainly provides a means of demystifying monoclonal antibodies. However, it is noteworthy that other methodologies are available and that antibodies derived from recombinant phage antibody technology will become more prominent in the future. Furthermore, the process of generating hybridomas must always be considered a learning experience. Recently, multiple antigen peptides designed for an Epstein-Barr virus related amino acid sequence generated hybridomas that predominantly yielded monoclonal antibodies of the IgM isotype (PN Nelson et al, personal observation, 1999). In this case, the peptides were presumably unable to induce class switching of B cells. As a consequence, any future immunisation strategy will have to take this finding into consideration for the production of IgG antibodies. Finally, the task of generating hybridomas should not be undertaken lightly. A previous study3 highlighted the fact that only two in 576 hybridomas produced were potentially useful; that is, the return may be small but on occasions worthwhile—one of these antibodies, monoclonal antibody A57H, is commercially distributed as a pan-IgG reagent!
References
- 1.Nelson PN, Westwood OM, Jefferis R, et al. Characterisation of anti-IgG monoclonal antibody A57H by epitope mapping. Biochem Soc Trans 1997;25:373. [Google Scholar]
- 2.Nelson PN, Fletcher SM, De Lange GG, et al. Evaluation of monoclonal antibodies with putative specificity for human IgG allotypes. Vox Sang 1990;59:190–7. [DOI] [PubMed] [Google Scholar]
- 3.Nelson PN, Fletcher SM, MacDonald D, et al. Assay restriction profiles of three monoclonal antibodies recognizing the G3m(u) allotype: development of an allotype specific assay. J Immunol Methods 1991;138:57–64. [DOI] [PubMed] [Google Scholar]
- 4.Blottiere HM, Daculsi G, Anegon I, et al. Utilization of activated U937 monocytic cells as a model to evaluate biocompatibility and biodegradation of synthetic calcium phosphate. Biomaterials 1995;16:497–503. [DOI] [PubMed] [Google Scholar]
- 5.Hudson PJ. Recombinant antibody constructs in cancer therapy. Curr Opin Immunol 1999;11:548–57. [DOI] [PubMed] [Google Scholar]
- 6.Cragg MS, French RR, Glennie MJ. Signaling antibodies in cancer therapy. Curr Opin Immunol 1999;11:539–40. [DOI] [PubMed] [Google Scholar]
- 7.Jefferis R, Reimer CB, Skvaril F, et al. Evaluation of monoclonal antibodies having specificity for human IgG subclasses: results of an IUIS/WHO collaborative study. Immunol Lett 1985;10:223–8. [DOI] [PubMed] [Google Scholar]
- 8.Bull H, Choy M, Manyonda I, et al. Reactivity and assay restriction profiles of monoclonal and polyclonal antibodies to acid phosphatases: a preliminary study. Immunol Lett 2000 [In press.] [DOI] [PubMed]
- 9.Tzioufas AG, Yiannaki E, Sakarellos-Daitsiotis M, et al. Fine specificity of autoantibodies to La/SSB: epitope mapping and characterization. Clin Exp Immunol 1997;108:191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole MS, Stellrecht KE, Shi JD, et al. HuM291, a humanised anti-CD3 antibody, is immunosuppressive to T cells while exhibiting reduced mitogenicity in vitro. Transplantation 1999;68:563–71. [DOI] [PubMed] [Google Scholar]
- 11.Cole MS, Anasetti C, Tso JY. Human IgG2 variants of chimeric anti-CD3 are non-mitogenic to T cells. J Immunol 1997;159:3631–21. [PubMed] [Google Scholar]
- 12.Kreitman RJ. Immunotoxins in cancer therapy. Curr Opin Immunol 1999;11:570–8. [DOI] [PubMed] [Google Scholar]
- 13.Ordonez NG. The immunohistochemical diagnosis of epithelial mesothelioma. Hum Pathol 1999;30:313–23. [DOI] [PubMed] [Google Scholar]
- 14.Ordonez NG. Role of immunohistochemistry in differentiating epithelial mesothelioma from adenocarcinoma. Review and update. Am J Clin Pathol 1999;112:75–89. [DOI] [PubMed] [Google Scholar]
- 15.Pendas S, Dauway E, Cox CE, et al. Sentinel node biopsy and cytokeratin staining for the accurate staging of 478 breast cancer patients. Am Surg 1999;65:500–5. [PubMed] [Google Scholar]
- 16.Czerniecki BJ, Scheff AM, Callans LS, et al. Immunohistochemistry with pancytokeratins improves the sensitivity of sentinel lymph node biopsy in patients with breast carcinoma. Cancer 1999;85:1098–103. [PubMed] [Google Scholar]
- 17.Brink AA, Oudejans JJ, van den Brule AJ, et al. Low p53 and high bcl-2 expression in Reed-Sternberg cells predicts poor clinical outcome for Hodgkin's disease: involvement of apoptosis resistance? Mod Pathol 1998;11:376–83. [PubMed] [Google Scholar]
- 18.Gascoyne RD, Adomat SA, Krajewski S, et al. Prognostic significance of Bcl-2 protein expression and Bcl-2 gene rearrangement in diffuse aggressive non-Hodgkin's lymphoma. Blood 1997;90:244–51. [PubMed] [Google Scholar]
- 19.Henriksen R, Wilander E, Oberg K. Expression and prognostic significance of Bcl-2 in ovarian tumours. Br J Cancer 1995;72:1324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsushima H, Kitamura T, Goto T, et al. Combined analysis with Bcl-2 and p53 immunostaining predicts poorer prognosis in prostatic carcinoma. J Urol 1997;158:2278–83. [DOI] [PubMed] [Google Scholar]
- 21.Fredersdorf S, Burns J, Milne AM, et al. High level expression of p27(kip1) and cyclin D1 in some human breast cancer cells: inverse correlation between the expression of p27(kip1) and degree of malignancy in human breast and colorectal cancers. Proc Natl Acad Sci U S A 1997;94:6380–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porter PL, Malone KE, Heagerty PJ, et al. Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat Med 1997;3:222–5. [DOI] [PubMed] [Google Scholar]
- 23.Tan P, Cady B, Wanner M, et al. The cell cycle inhibitor p27 is an independent prognostic marker in small (T1a,b) invasive breast carcinomas. Cancer Res 1997;57:1259–63. [PubMed] [Google Scholar]
- 24.Thomas GV, Szigeti K, Murphy M, et al. Down-regulation of p27 is associated with development of colorectal adenocarcinoma metastases. Am J Pathol 1998;153:681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang RM, Naitoh J, Murphy M, et al. Low p27 expression predicts poor disease-free survival in patients with prostate cancer. J Urol 1998;159:941–5. [PubMed] [Google Scholar]
- 26.Erlanson M, Portin C, Linderholm B, et al. Expression of cyclin E and the cyclin-dependent kinase inhibitor p27 in malignant lymphomas—prognostic implications. Blood 1998;92:770–7. [PubMed] [Google Scholar]
- 27.Allred DC, Harvey JM, Berardo M, et al. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mol Pathol 1998;11:155–68. [PubMed] [Google Scholar]
- 28.Goussard J. Paraffin section immunocytochemistry and cytosol-based ligand-binding assays for ER and PR detection in breast cancer: the time has come for more objectivity. Cancer Lett 1998;132:61–6. [DOI] [PubMed] [Google Scholar]
- 29.Barclay AN, Brown MH, Law SKA, et al. The leukocyte antigen facts book, San Diego: Academic Press.
- 30.Stein H, Hummel M. Cellular origin and clonality of classic Hodgkin's lymphoma: immunophenotypic and molecular studies. Semin Hematol 1999;36:233–41. [PubMed] [Google Scholar]
- 31.Horie R, Watanabe T. CD30: expression and function in health and disease. Semin Immunol 1998;10:457–70. [DOI] [PubMed] [Google Scholar]
- 32.Maloney DG, Liles TM, Czerwinski DK, et al. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-c2b8) in patients with recurrent B-cell lymphoma. Blood 1994;84:2457–9. [PubMed] [Google Scholar]
- 33.Maloney D, Grillo-López A, White C, et al. IDEC-C2B8 (rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood 1997;90:2188–92. [PubMed] [Google Scholar]
- 34.McLaughlin P, Cabanillas F, Grillo-Lopez AJ, et al. IDEC-C2B8 anti-CD20 antibody: final report on a phase III pivotal trial in patients with relapsed low-grade or follicular lymphoma [abstract]. Blood 1996;88:90a. [Google Scholar]
- 35.Coiffier B, Haioun C, Ketterer N, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood 1998;92;1927–32. [PubMed]
- 36.Reff ME, Carner K, Chambers KS, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 1994;83:435–8. [PubMed] [Google Scholar]
- 37.Maloney DG, Smith B, Appelbaum FR. The anti-tumor effect of monoclonal anti-CD20 antibody therapy includes direct anti-proliferative activity and induction of apoptosis in CD20 positive non-Hodgkin's lymphoma cell lines [abstract]. Blood 1996;88:637a. [Google Scholar]
- 38.De Potter CR. The neu oncogene: more than just a prognostic indicator? Hum Pathol 1994;25:1264–8. [DOI] [PubMed] [Google Scholar]
- 39.Quenel N, Wafflart J, Bonichon F, et al. The prognostic value of c-erbB2 in primary breast carcinomas: a study on 942 cases. Breast Cancer Res Treat 1995;35:283–91. [DOI] [PubMed] [Google Scholar]
- 40.O'Malley FP, Saad Z, Kerkvliet N, et al. The predictive power of semiquantitative immunohistochemical assessment of p53 and c-erbB2 in lymph node negative breast cancer. Hum Pathol 1996;27:955–63. [DOI] [PubMed] [Google Scholar]
- 41.Borg A, Tandon AK, Sigurdsson H, et al. HER-2/neu amplification predicts poor survival in node-positive breast cancer. Cancer Res 1990;50:4332–7. [PubMed] [Google Scholar]
- 42.Molina R, Ciocca DR, Tandon AK, et al. Expression of HER-2/neu oncoprotein in breast cancer: a comparison of immunohistochemical and western blot techniques. Anticancer Res 1992;12:1965–91. [PubMed] [Google Scholar]
- 43.Wright C, Nicholson S, Angus B, et al. Relationship between c-erbB-2 protein product expression and response to endocrine therapy in advanced breast cancer. Br J Cancer 1992;65:118–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carlomagno C, Perrone F, Gallo C, et al. c-erb B2 overexpression decreases the benefit of adjuvant tamoxifen in early-stage breast cancer without axillary lymph node metastases. J Clin Oncol 1996;14:2702–8. [DOI] [PubMed] [Google Scholar]
- 45.Newby JC, Johnston SR, Smith IE, et al. Expression of epidermal growth factor receptor and c-erbB2 during the development of tamoxifen resistance in human breast cancer. Clin Cancer Res 1997;3:1643–51. [PubMed] [Google Scholar]
- 46.Ross JS, Fletcher JA. The HER-2/neu oncogene: prognostic factor, predictive factor and target for therapy. Semin Cancer Biol 1999;9:125–38. [DOI] [PubMed] [Google Scholar]
- 47.Baselga J, Tripathy D, Mendelsohn J, et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol 1996;14:737–44. [DOI] [PubMed] [Google Scholar]
- 48.Slamon D, Leyland-Jones B, Shak S. Addition of Herceptin™ (humanized anti-HER-2 antibody) to first line chemotherapy for HER2 overexpressing metastatic breast cancer markedly increases anticancer activity: a randomized multinational controlled phase III trial [abstract]. Proc ASCO 1998;17:98a. [Google Scholar]
- 49.Liu X, Pogo BG. Inhibition of erbB-2-positive breast cancer cell growth by erbB-2 antisense oligonucleotides. Antisense Nucleic Acid Drug Dev 1996;6:9–16. [DOI] [PubMed] [Google Scholar]
- 50.Brossart P, Stuhler G, Flad T, et al. Her-2/neu-derived peptides are tumor-associated antigens expressed by human renal cell and colon carcinoma lines and are recognized by in vitro induced specific cytotoxic T lymphocytes. Cancer Res 1998;58:732–6. [PubMed] [Google Scholar]
- 51.Disis ML, Grabstein KH, Cheaver MA. HER-2/neu peptide vaccines elicit T cell immunity to the HER-2/neu protein in patients with breast and ovarian cancer [abstract]. Proc ASCO 1998;17:97a. [Google Scholar]