Abstract
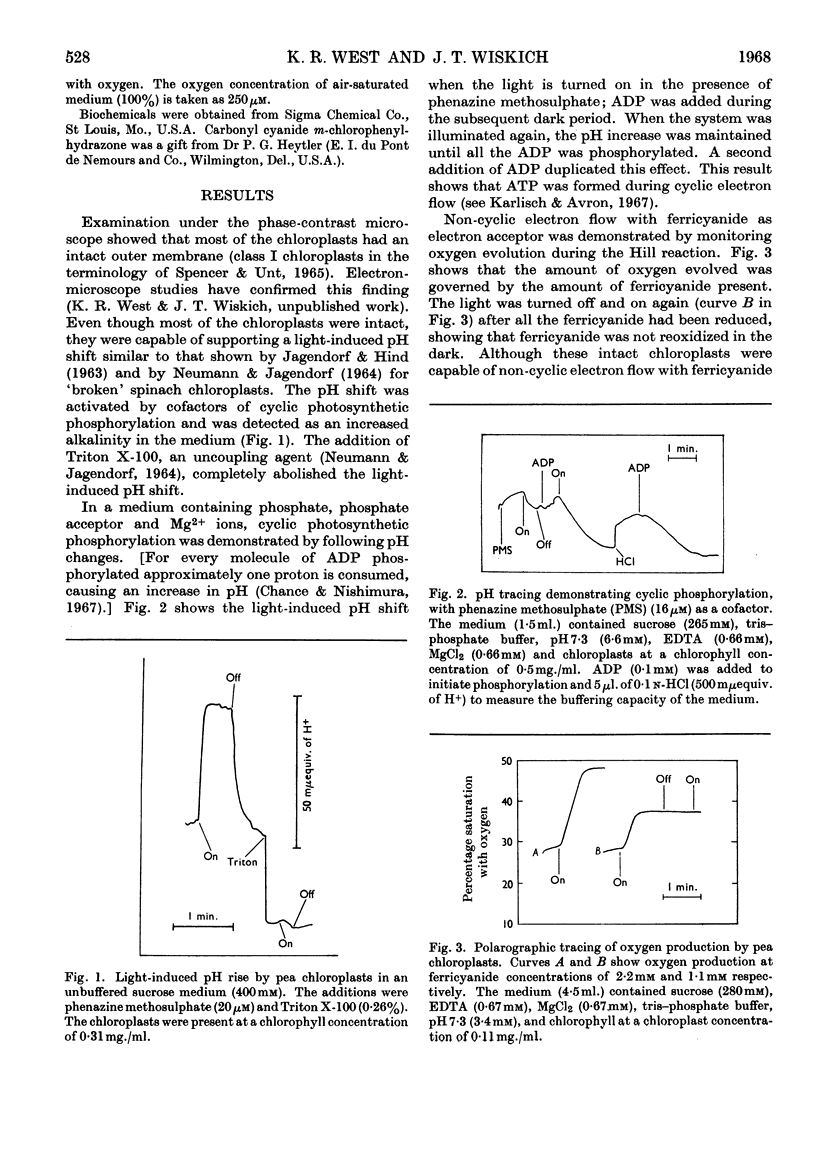
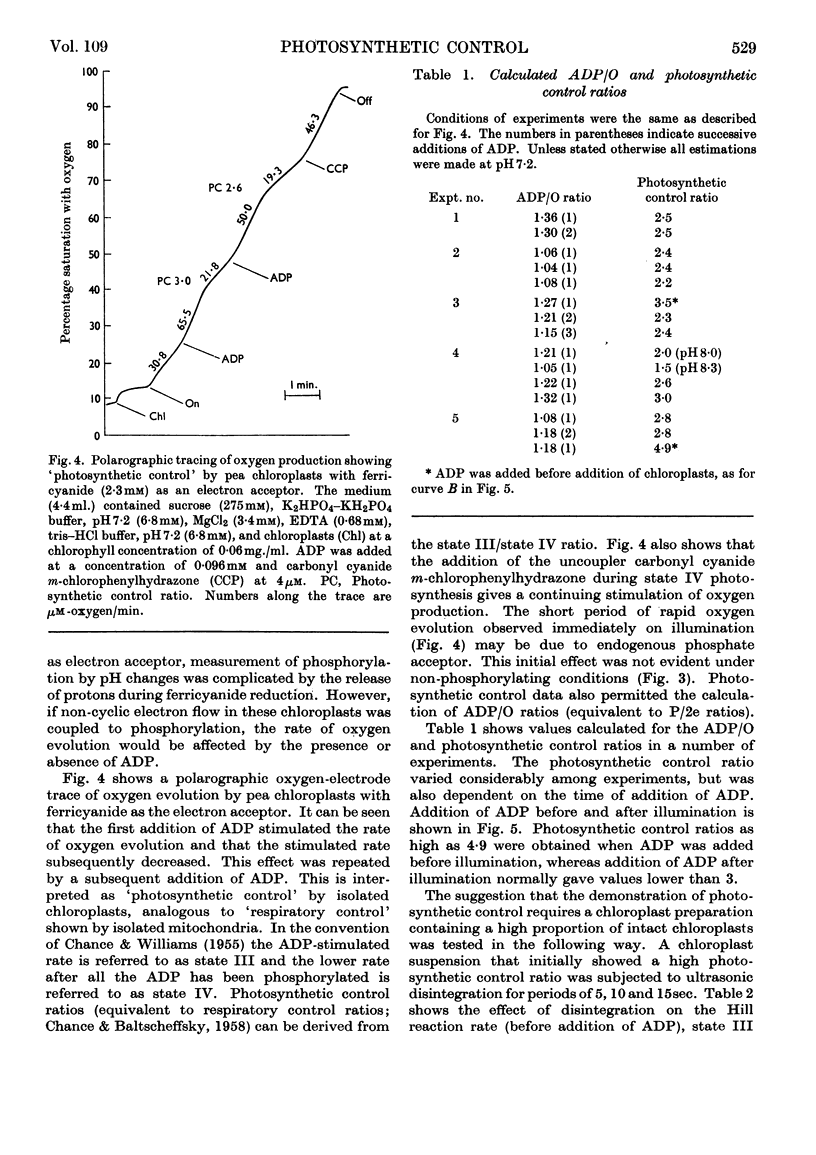
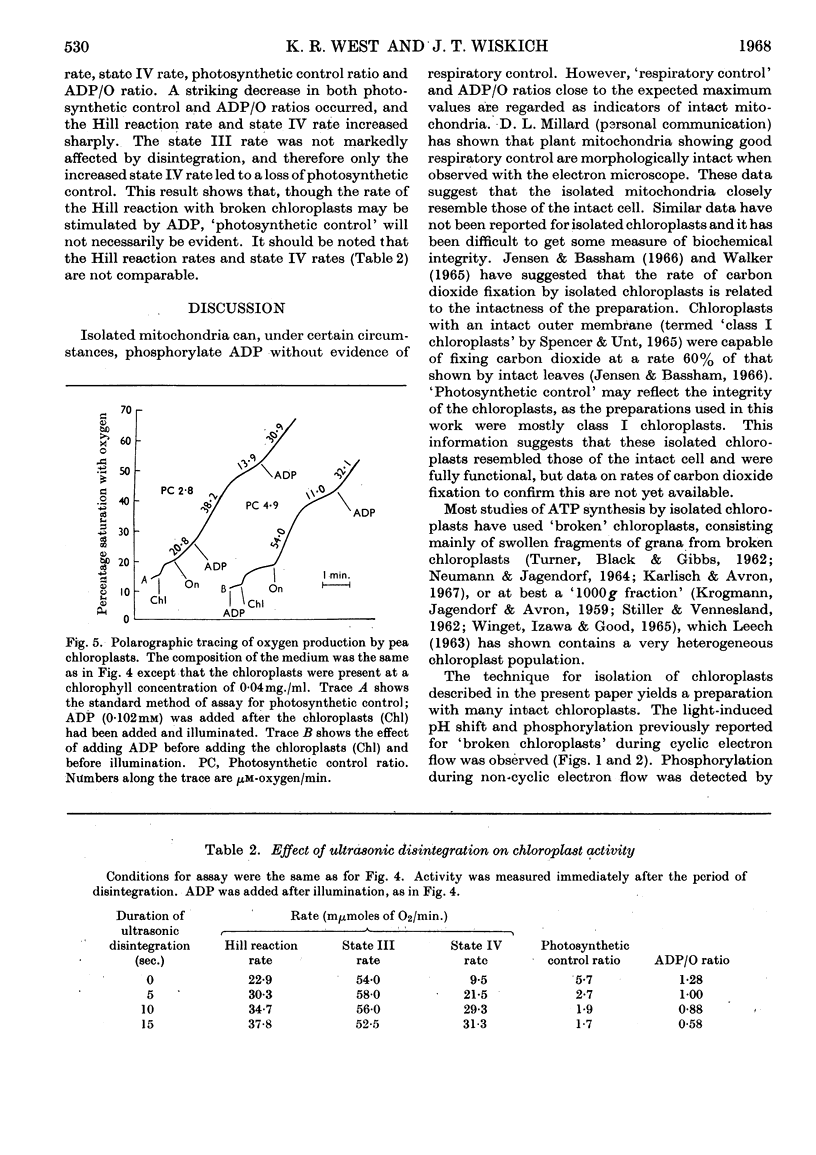
Isolated pea chloroplasts undergo both cyclic and non-cyclic electron flow. Both processes are coupled to photophosphorylation. During non-cyclic flow the rate of oxygen production showed ADP-governed `photosynthetic control' analogous to respiratory control of isolated mitochondria. Measurements of ADP/O and photosynthetic control ratios yielded values of 1–1·3 and 2–5·7 respectively. `Photosynthetic control' was shown to be dependent on the intactness of the chloroplasts.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M., JAGENDORF A. T. Interactions of catalytic cofactors for photosynthetic phosphorylation with Hill reaction oxidants. J Biol Chem. 1959 May;234(5):1315–1320. [PubMed] [Google Scholar]
- AVRON M., KROGMANN D. W., JAGENDORF A. T. The relation of photosynthetic phosphorylation to the Hill reaction. Biochim Biophys Acta. 1958 Oct;30(1):144–153. doi: 10.1016/0006-3002(58)90251-8. [DOI] [PubMed] [Google Scholar]
- Arnon D. I., Whatley F. R., Allen M. B. Assimilatory Power in Photosynthesis: Photosynthetic phosphorylation by isolated chloroplasts is coupled with TPN reduction. Science. 1958 May 2;127(3305):1026–1034. doi: 10.1126/science.127.3305.1026. [DOI] [PubMed] [Google Scholar]
- CHANCE B., BALTSCHEFFSKY M. Spectroscopic effects of adenosine diphosphate upon the respiratory pigments of rat-heart-muscle sarcosomes. Biochem J. 1958 Feb;68(2):283–295. doi: 10.1042/bj0680283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- DAVENPORT H. E. A protein from leaves catalysing the reduction of metmyoglobin and triphosphopyridine nucleotide by illuminated chloroplasts. Biochem J. 1960 Dec;77:471–477. doi: 10.1042/bj0770471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOOD N. E. Re-assessment of the stoichiometry of photo-phosphorylation. Nature. 1960 Nov 19;188:661–662. doi: 10.1038/188661a0. [DOI] [PubMed] [Google Scholar]
- Hill R. The biochemists' green mansions: the photosynthetic electron-transport chain in plants. Essays Biochem. 1965;1:121–151. [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogmann D. W., Jagendorf A. T., Avron M. Uncouplers of Spinach Chloroplast Photosynthetic Phosphorylation. Plant Physiol. 1959 May;34(3):272–277. doi: 10.1104/pp.34.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEECH R. M. Photosynthetic phosphorylation in mitochondria free chloroplast suspensions from leaves of Vicia faba L. Biochim Biophys Acta. 1963 May 14;71:253–265. doi: 10.1016/0006-3002(63)91080-1. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- NEUMANN J., JAGENDORF A. T. LIGHT-INDUCED PH CHANGES RELATED PHOSPHORYLATION BY CHLOROPLASTS. Arch Biochem Biophys. 1964 Jul;107:109–119. doi: 10.1016/0003-9861(64)90276-0. [DOI] [PubMed] [Google Scholar]
- STILLER M., VENNESLAND B. Photophosphorylation accompanying the Hill reaction with ferricyanide. Biochim Biophys Acta. 1962 Jul 16;60:562–579. doi: 10.1016/0006-3002(62)90875-2. [DOI] [PubMed] [Google Scholar]
- TURNER J. F., BLACK C. C., GIBBS M. Studies on photosynthetic processes. I. The effect of light intensity on triphosphopyridine nucleotide reduction, adenosine triphosphate formation, and carbon dioxide assimilation in spinach chloroplasts. J Biol Chem. 1962 Feb;237:577–579. [PubMed] [Google Scholar]
- Walker D. A. Correlation between Photosynthetic Activity and Membrane Integrity in Isolated Pea Chloroplasts. Plant Physiol. 1965 Nov;40(6):1157–1161. doi: 10.1104/pp.40.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winget G. D., Izawa S., Good N. E. The stoichiometry of photophosphorylation. Biochem Biophys Res Commun. 1965 Dec 9;21(5):438–443. doi: 10.1016/0006-291x(65)90401-8. [DOI] [PubMed] [Google Scholar]
- Wiskich J. T., Bonner W. D. Preparation and Properties of Sweet Potato Mitochondria. Plant Physiol. 1963 Sep;38(5):594–604. doi: 10.1104/pp.38.5.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskich J. T., Young R. E., Biale J. B. Metabolic Processes in Cytoplasmic Particles of the Avocado Fruit. VI. Controlled Oxidations and Coupled Phosphorylations. Plant Physiol. 1964 May;39(3):312–322. doi: 10.1104/pp.39.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]


