Abstract
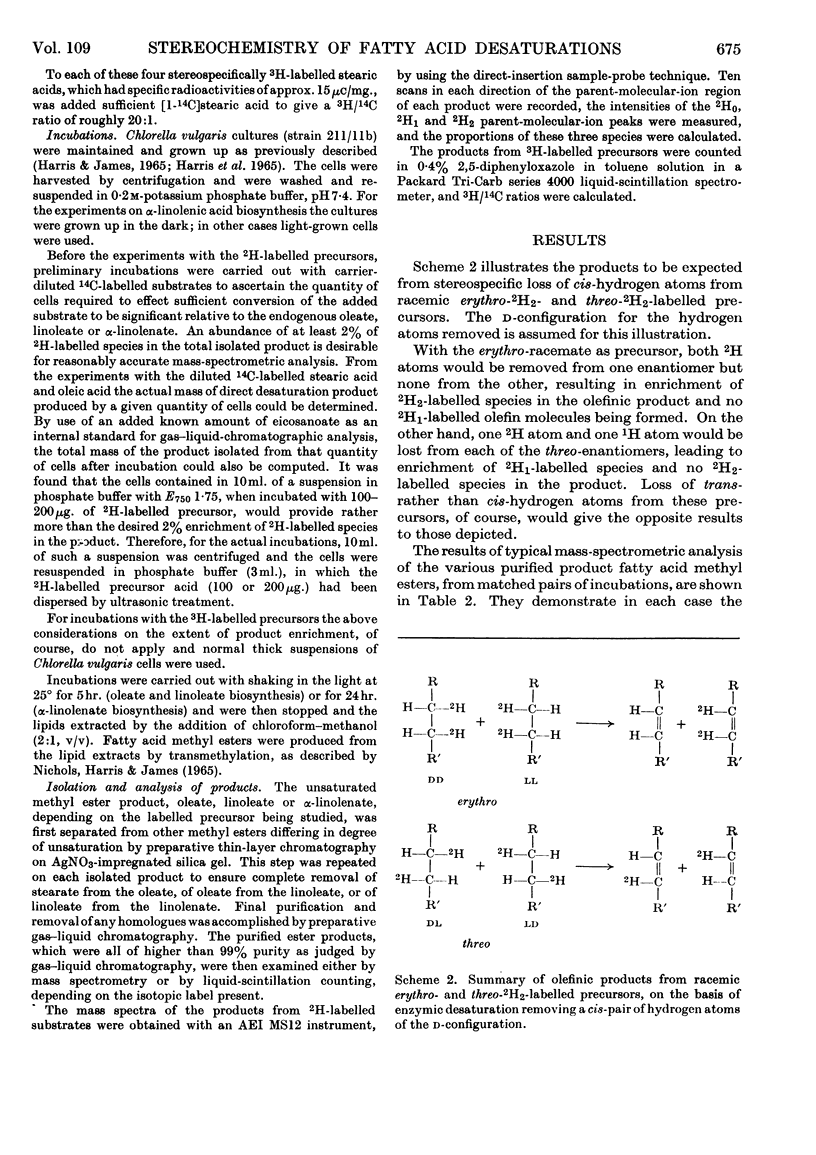
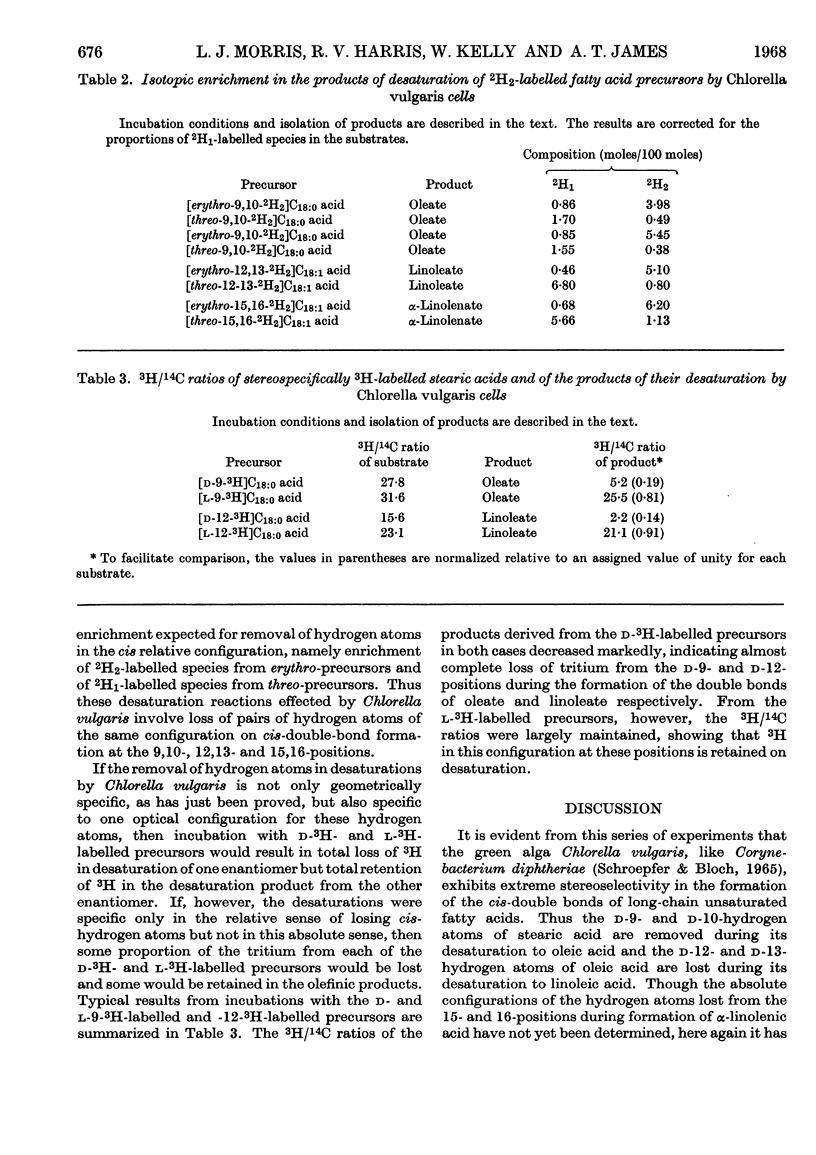
1. A study was made of the stereospecificity of hydrogen removal in the sequential desaturations performed by intact cells of Chlorella vulgaris in the biosynthesis of oleic acid, linoleic acid and α-linolenic acid. 2. By use of erythro- and threo-9,10-2H2-, -12,13-2H2- and -15,16-2H2-labelled precursors, it was demonstrated that the pair of hydrogen atoms removed from each of these positions had the cis relative configuration. 3. That the hydrogen atoms removed in oleic acid and linoleic acid formation were of the d absolute configuration was proved by use of d- and l-9-3H-and -12-3H-labelled precursors. 4. The presence of a substantial kinetic isotope effect of deuterium at both positions of the putative double bond was indicated, suggesting that the mechanism of desaturation involves simultaneous concerted removal of the pair of hydrogen atoms.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Harris R. V., James A. T. Linoleic and alpha-linolenic acid biosynthesis in plant leaves and green alga. Biochim Biophys Acta. 1965 Dec 2;106(3):456–464. doi: 10.1016/0005-2760(65)90062-7. [DOI] [PubMed] [Google Scholar]
- Harris R. V., James A. T. The fatty acid metabolism of Chlorella vulgaris. Biochim Biophys Acta. 1965 Dec 2;106(3):465–473. doi: 10.1016/0005-2760(65)90063-9. [DOI] [PubMed] [Google Scholar]
- Morris L. J., Harris R. V., Kelly W., James A. T. The stereospecificity of desaturations of long-chain fatty acids in Chlorella vulgaris. Biochem Biophys Res Commun. 1967 Sep 27;28(6):904–908. doi: 10.1016/0006-291x(67)90064-2. [DOI] [PubMed] [Google Scholar]
- Nichols B. W., Harris R. V., James A. T. The lipid metabolism of blue-green algae. Biochem Biophys Res Commun. 1965 Jul 26;20(3):256–262. doi: 10.1016/0006-291x(65)90356-6. [DOI] [PubMed] [Google Scholar]


