Abstract
Background/Aims—The occurrence of myeloid leukaemia in patients with systemic mastocytosis is a well recognised phenomenon. However, the pathophysiological basis of such a coevolution has not been clarified. Recent data have shown that the c-kit mutation Asp 816 to Val is detectable in neoplastic mast cells in most patients with systemic mastocytosis, including those who have associated haematological disorders. The aim of this study was to study clonal disease evolution by analysing bone marrow cells from a patient with systemic mastocytosis and associated chronic myelomonocytic leukaemia (CMML) for the presence of this mutation.
Methods—The DNA of microdissected bone marrow cells from a patient with systemic mastocytosis and associated CMML was analysed for the presence of the c-kit mutation Asp 816 to Val by means of HinfI digestion and direct sequencing of semi-nested polymerase chain reaction (PCR) products.
Results—The two neoplasms could easily be identified and discriminated in paraffin wax embedded bone marrow sections by tryptase and chloroacetate esterase staining. A total number of 10 tryptase positive systemic mastocytosis infiltrates and 10 tryptase negative CMML infiltrates were removed by microdissection. As assessed by HinfI digestion and direct sequencing of semi-nested PCR products, the c-kit mutation Asp 816 to Val was detected in five of seven systemic mastocytosis infiltrates and four of six CMML infiltrates. By contrast, no c-kit mutation Asp 816 to Val was found in bone marrow infiltrates in patients with CMML without associated systemic mastocytosis (n = 20).
Conclusion—These data support a monoclonal evolution of systemic mastocytosis and concurrent CMML in the patient studied.
Keywords: mast cells, microdissection, c-kit point mutation
Mastocytosis is a term used to denote a heterogeneous group of disorders characterised by an abnormal proliferation and accumulation of mast cells in various tissues and organs. Cutaneous and systemic variants of the disease must be separated.1–4 Cutaneous mastocytosis usually develops during childhood and typically presents as urticaria pigmentosa.5,6 Systemic mastocytosis occurs at any age, predominantly in adults, and is characterised by mast cell infiltrates in at least one extracutaneous organ (bone marrow in most cases), with or without urticaria pigmentosa.1 In contrast to urticaria pigmentosa in childhood, systemic mastocytosis in adults does not regress spontaneously.6 Patients with systemic mastocytosis with skin involvement show an indolent course, whereas those lacking skin involvement follow an aggressive or even malignant course.1,7,8 In addition, patients with systemic mastocytosis without skin involvement have a certain risk of developing an associated (usually myeloid) haematological malignancy.7,9–13
The c-kit proto-oncogene encodes a transmembrane tyrosine kinase receptor that binds mast cell growth factor, also known as stem cell factor.14,15 c-kit is expressed on mast cells16 as well as haemopoietic stem cells,17–19 melanocytes,20 and germ cells.21 Activated c-kit mediates signals for proliferation and maturation in these cells.22 23 Likewise, the c-kit ligand, stem cell factor, induces differentiation of mast cells from CD34 positive progenitor cells. Moreover, mice with defects in the stem cell factor receptor c-kit or the c-kit ligand stem cell factor are mast cell deficient.24
Recent data have shown that c-kit may be mutated in patients with mastocytosis.25 In fact, distinct “gain of function” point mutations in the catalytic domain of c-kit cause autophosphorylation of the receptor and stem cell factor independent growth of mast cells.25–27 Such activating c-kit mutations have been detected recently in patients with mastocytosis.28–34 One of these mutations (Asp 816 to Val) has been identified in patients with mast cell leukaemia, patients with systemic mastocytosis (with and without skin involvement; with and without associated haematological malignancy), and in patients with urticaria pigmentosa.25,28–30,32–35
Approximately 20–25% of patients with systemic mastocytosis develop an associated haematological malignancy.2,36 Most of these associated haematological malignancies involve a myeloid progenitor cell, and in a large proportion of cases a myeloid leukaemia is diagnosed. Such leukaemias can resemble chronic myeloid leukaemia (CML), chronic myelomonocytic leukaemia (CMML), or acute myeloid leukaemia (AML).10–13,37 However, the exact origin and molecular biological properties of the leukaemic cells and their relation to the mast cell lineage have not yet been evaluated in detail. Published results vary with regard to whether systemic mastocytosis and the associated myeloid leukaemia represent two separate clonal disorders, or whether they derive from a common progenitor cell, indicating a monoclonal disease process.33,34 In our study, we used the technique of microdissection to examine isolated systemic mastocytosis and leukaemic bone marrow infiltrates for the presence of the c-kit mutation Asp 816 to Val (as a marker for clonality) in a patient with systemic mastocytosis and associated CMML.
Case report and methods
CASE REPORT
A 68 year old white man first presented in 1993. At that time, seropositive rheumatoid arthritis was diagnosed; the patient suffered from night sweats, weight loss, and normochromic anaemia. No splenomegaly or lymphadenopathy was found. The haemoglobin concentration was 106 g/litre, and the platelet count was 146 000/μl; in other respects the blood picture was normal. The patient was treated with methotrexate, non-steroidal anti-inflammatory drugs, and low dose prednisone. In response to this treatment, the arthritis improved. However, the anaemia did not resolve. A bone marrow examination was performed and disclosed pronounced hypercellularity together with focal infiltration by atypical mast cells consistent with the diagnosis “mastocytosis”. Staging investigations revealed additional mast cell infiltrates in the mucosa of the small and large bowel. The skin appeared normal without urticaria pigmentosa like infiltrates. In addition to his previous treatment, the patient received antihistamines to prevent symptoms resulting from mast cell degranulation (mediator syndrome). During the next few years the patient developed transfusion dependent anaemia, monocytosis (> 1000/μl), and hepatosplenomegaly. The diagnosis “systemic mastocytosis with associated CMML” was established. In June 1997, leukaemic progression occurred. At that time, the white blood cell count was 19 100/μl, haemoglobin 76 g/litre, and platelet count 162 000/μl. The differential count showed a pronounced left shift, with 11% blasts and 6% monocytes. Bone marrow examinations revealed a hypercellular marrow with prominent myelopoiesis, a high proportion of (immature) monocytic cells, many atypical mast cells, and 10% blasts. In addition, hyperfibrinolysis was found. Cytoreductive treatment (hydroxyurea) was initiated. In November 1997, he developed pneumonia (Pseudomonas aeruginosa), which was treated successfully with antibiotics. However, despite continuous supportive treatment, the clinical situation worsened and the patient died in January 1998.
TISSUE SAMPLING AND PROCESSING
Bone marrow biopsy specimens (and aspirates) were obtained from the iliac crest. Bone marrow was taken from our patient with systemic mastocytosis associated with CMML, as well as from 20 patients with CMML without concurrent mastocytosis. Biopsy specimens were fixed in 5% neutral buffered formalin, decalcified in EDTA, and embedded in paraffin wax.
STAINING PROCEDURES AND MICRODISSECTION
Sections (8 μm thick) were cut from the paraffin wax embedded bone marrow biopsy specimens and stained by means of the naphthol AS-D chloroacetate esterase reaction (CAE) and the antibody AA1 (Dako Diagnostika, Hamburg, Germany) directed against tryptase, a mast cell specific marker. Immunohistochemical staining for mast cell tryptase was performed as described previously.38 Compact, tryptase positive mast cell infiltrates (fig 1C and D ▶) and CAE positive, tryptase negative CMML infiltrates were microdissected separately using a hydraulic micromanipulator (Narishige, Tokyo, Japan). A total of 10 mastocytosis infiltrates and 10 areas representing CMML were microdissected from our patient with systemic mastocytosis and CMML. Microdissected areas were of an estimated size of 100–200 μm in diameter and contained about 50–150 cells. Microdissection was carried out according to published techniques.39 For extraction and analysis of total genomic DNA, intact bone marrow sections (no microdissection performed) were used from the patient with systemic mastocytosis and CMML as well as from controls (CMML without systemic mastocytosis; n = 20).
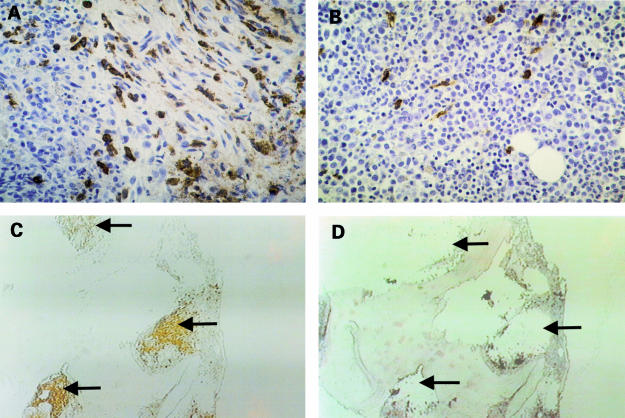
Figure 1.
Systemic mastocytosis with associated chronic myelomonocytic leukaemia (CMML). (A) Focal compact aggregate of medium sized, slightly pleomorphic, spindle shaped cells; note the pronounced collagen fibrosis associated with the infiltrate. Tumour cells express tryptase strongly and thus can be clearly identified as mast cells. (B) Extremely hypercellular bone marrow with diffuse involvement by CMML. Tryptase staining only of loosely scattered mast cells. Note that focal mast cell aggregates are absent. (C) Overview of the bone marrow trephine with tryptase stained mast cell infiltrates (arrows). (D) Bone marrow trephine after microdissection of three mast cell infiltrates (arrows). Tryptase was detected using the AA1 antibody.
DNA EXTRACTION
Microdissected mastocytosis infiltrates and areas representing CMML were transferred into PCR tubes containing proteinase K (1 μg/μl) in a volume of 10 μl proteinase K buffer (50 mM Tris, 1 mM EDTA, 0.5% Tween 20, pH 8.5) and digested at 55°C for one hour. Proteinase K was inactivated by heating to 95°C for 10 minutes. A total of 90 μl of PCR mixture was added to each PCR tube. For extraction of total genomic DNA from intact bone marrow biopsy specimens (systemic mastocytosis and CMML, n = 1; CMML without systemic mastocytosis, n = 20), five 8 μm thick sections were cut from each paraffin wax block. Sections were dewaxed according to standard protocols. In brief, sections were vortexed in 1 ml 100% xylene for one minute and centrifuged at 16 000 ×g for five minutes. The procedure was repeated three times. Then, samples were washed twice in pure ethanol and vacuum dried. Proteinase K digestion was carried out in a final volume of 200 μl buffer (50 mM Tris, 1 mM EDTA, 0.5% Tween 20, pH 8.5) containing 0.2 mM proteinase K at 55°C (two hours). Total DNA was extracted with phenol/chloroform/isoamyl alcohol (25/24/1 vol/vol/vol) and precipitated with 100% ethanol/8 M LiCl, as described elsewhere.40 An aliquot of 200 ng of total extracted DNA was used for PCR amplification of the c-kit gene.
PCR PROCEDURE
All PCRs were performed as semi-nested hot start PCRs in a final volume of 100 μl containing 50 mM KCl, 10 mM Tris/HCl (pH 8.3), 200 μM of each dNTP, 1.5 mM MgCl2, 1 U thermostable DNA polymerase (AmpliTaq DNA polymerase; PE Applied Biosystems, Weiterstadt, Germany), and 15 pmol of each primer. Aliquots of 2 μl of the PCR product were used as template for semi-nested PCR. Each PCR cycle consisted of 30 seconds denaturation at 94°C, 30 seconds annealing at 56°C, and 45 seconds extension at 72°C. The first cycle was preceded by four minutes denaturation at 94°C. The last cycle was extended by a four minute elongation at 72°C. Both PCR and nested PCR were run for 40 cycles. Semi-nested PCR for amplification of the region containing c-kit Asp 816 to Val point mutation was performed using the following primers: c-kit sense 1 (5′-CAC AGA GAC TTG GCA GCC AG-3′) and c-kit antisense (5′-CAG GAT TTA CAT TAT GAA AGT CAC GG-3′) in the first round of amplification, and primers c-kit sense 2 (5′-ATC CTC CTT ACT CAT GGT CGG ATC-3′) and c-kit antisense for the second round of amplification.
SEQUENCING OF PCR PRODUCTS
For direct sequencing, semi-nested PCR products were extracted from 2% agarose gels with a gel extraction kit (Qiagen, Hilden, Germany) according to the manufacturers' instructions. PCR products were then sequenced in both directions with primers c-kit sense 2 and c-kit antisense by the dye-deoxy terminator method using a 377 ABI Prism Sequencer (PE Applied Biosystems).
ANALYSIS BY HINFI DIGESTION
To analyse A to T substitution at nucleotide (nt) 7176 of c-kit DNA,14 semi-nested PCR products were digested with the endonuclease HinfI. Restriction products were analysed by electrophoresis on 6% polyacrylamide gels. The c-kit mutation Asp 816 to Val creates a second restriction site within products of semi-nested PCR using primers c-kit sense 2 and c-kit antisense (fig 2A ▶). Predicted sizes for the wild-type sequence are 121 bp and 68 bp, and for the mutated sequence 121 bp, 68 bp, 54 bp, and 14 bp (table 1 ▶).
Figure 2.
Molecular biological detection of of c-kit Asp 816 to Val point mutation. (A) Adenine/thymidine substitution at nucleotide (nt) 7176 of c-kit DNA (nt 2468 of c-kit mRNA) causing asparagine/valine substitution at residue 816 of the c-kit protein and creating a new restriction site for endonuclease HinfI. Note the pre-existing HinfI site at nt 7187. G!ANTC, HinfI recognition site; !, cutting position of the restriction endonuclease HinfI. (B) Electrographs obtained by direct sequencing of semi-nested PCR products. Sites of the point mutation are indicated by arrows. Note that the reverse PCR primer has been used for DyeTerminator cycle sequencing: complementary sequences are indicated on top of each electrograph; 3′ end, left; 5′ end, right. Nucleotide positions are indicated on top. The upper panel corresponds to fig 3 ▶, lane 2: a single peak indicates the mutated allele. The middle panel corresponds to fig 3 ▶, lane 3: a double peak indicates the heterozygous genotype. The lower panel corresponds to fig 3 ▶, lane 4: a single peak indicates the wild-type allele. Mut; mutation; loh, loss of heterozygosity; wt, wild-type.
Table 1.
HinfI digestion of semi-nested PCR products
| Mutated | ||||
| No digestion | Heterozygous | Homozygous/LOH? | Wild-type | |
| 189 bp | + | – | – | – |
| 121 bp | – | + | + | + |
| 68 bp | – | + | – | + |
| 54 bp | – | + | + | – |
| 14 bp* | – | + | + | – |
*Not visible on a polyacrylamide gel.
LOH, loss of heterozygosity.
Results
HISTOLOGY AND IMMUNOHISTOCHEMICAL STAINING
In the patient with systemic mastocytosis associated with CMML, the antibody against mast cell tryptase produced a clear diagnostic staining result. In fact, focal compact infiltrates of bone marrow mast cells were made visible by immunohistochemistry using antitryptase antibody (fig 1A ▶). In addition, diffusely scattered bone marrow mast cells were labelled (fig 1B ▶). In areas of CMML, these mast cells accounted for approximately 5–10%. By contrast, this antibody did not react with leukaemic cells. Thus, this staining reaction served as a useful basis for microdissection. Both neoplastic mast cells (a subset) and leukaemic cells (CMML) appeared to be strongly CAE positive. In control cases (CMML; n = 20), no focal infiltrates of mast cells were seen in tryptase staining experiments, thereby excluding the presence of concomitant systemic mastocytosis.
DETECTION OF C-KIT MUTATION ASP 816 TO VAL IN TOTAL BONE MARROW SECTIONS
Genomic DNA was obtained from intact bone marrow sections from our patient with systemic mastocytosis and CMML as well as from 20 control cases (CMML without systemic mastocytosis). The analysis of semi-nested PCR products by HinfI digestion and polyacrylamide gel electrophoresis as well as direct sequencing revealed the presence of the c-kit mutation Asp 816 to Val in the bone marrow from our patient with systemic mastocytosis and CMML. By contrast, this mutation was not detectable in any of the cases with CMML without concomitant mastocytosis.
DETECTION OF C-KIT MUTATION ASP 816 TO VAL IN MICRODISSECTED BONE MARROW CELLS
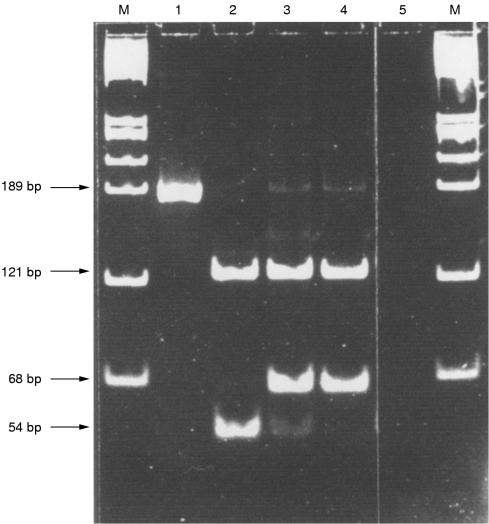
A total number of 10 mastocytosis infiltrates and 10 areas of CMML were microdissected from bone marrow sections from our patient with systemic mastocytosis and concomitant CMML and analysed for the presence of the c-kit mutation Asp 816 to Val. Using a semi-nested hot start PCR protocol, amplification products were obtained from seven of 10 mastocytosis infiltrates and six of 10 CMML infiltrates (table 2 ▶). The analysis of PCR products by HinfI digestion and direct sequencing revealed the presence of the c-kit Asp 816 to Val point mutation in five of seven microdissected compact mast cell infiltrates and in four of six microdissected areas of CMML (table 2 ▶). In all samples from which amplification products were obtained, results from HinfI digestion and polyacrylamide gel electrophoresis were consistent with the results from direct sequencing (data not shown). In one of five microdissected compact mast cell infiltrates in which the c-kit Asp 816 to Val point mutation was detected, both HinfI digestion and direct sequencing of semi-nested PCR products repeatedly demonstrated the presence of a homozygous mutated genotype (figs 2B and 3 ▶ ▶). In all other samples in which the c-kit Asp 816 to Val point mutation was detected, the prevalent genotype was heterozygous as demonstrated both by HinfI digestion and direct sequencing. Table 2 ▶ shows a summary of results obtained with microdissected bone marrow mast cell infiltrates and areas representing CMML.
Table 2.
Analysis of paraffin wax embedded bone marrow biopsies for c-kit mutation Asp 816 to Val
| Material | n | DNA amplification | c-kit mutation |
| Patient | |||
| Total biopsy sections | 1 | 1/1 | 1/1 |
| MC infiltrates | 10 | 7/10 | 5*/7 |
| CMML infiltrates | 10 | 6/10 | 4/10 |
| Controls | |||
| Total biopsy sections | 20 | 20/20 | 0/20 |
c-kit mutation was determined by HinfI digestion and sequencing.
The controls comprised CMML without concomittant mastocytosis.
*1/5 with loss of heterozygosity (?).
MC, mast cells; WT, wild-type; CMML, chronic myelomonocytic leukaemia.
Figure 3.
Molecular biological detection of of c-kit Asp 816 to Val point mutation. Semi-nested PCR products from control DNA (lane 1) and three different microdissected mast cell infiltrates (lanes 2–4). In lane 1, the undigested full length PCR product is shown. Lanes 2–4 contain HinfI digested PCR products (table 2 ▶). In lane 2 only the mutated allele is present, most probably indicating loss of heterozygosity (LOH). Signals from both the wild-type and mutated allele are present in lane 3 (heterozygous genotype). Lane 4 contains the wild-type sequence (homozygous genotype). In lane 5 only water was amplified as a negative control. M, HaeIII digested φX DNA.
Discussion
The development of myeloid leukaemia in patients with systemic mastocytosis is a well recognised phenomenon. However, little is known about the pathophysiology of such leukaemias and the molecular biological basis of their coevolution in systemic mastocytosis. In our study, we have examined the DNA of microdissected bone marrow cells for the presence of the c-kit mutation Asp 816 to Val in a patient with systemic mastocytosis and associated CMML. The c-kit mutation Asp 816 to Val was present in microdissected compact bone marrow mast cell infiltrates as well as microdissected areas of CMML in this patient.
Several recent studies have shown that the c-kit mutation Asp 816 to Val is detectable in most patients with systemic mastocytosis, but not in other myeloid malignancies.28,29,32,41 The results of our study are in line with this notion. In particular, the c-kit mutation Asp 816 to Val was detected in bone marrow cells in the patient with systemic mastocytosis and associated CMML, but not in patients with CMML without mastocytosis. The presence of the c-kit mutation Asp 816 to Val in various microdissected bone marrow mast cell infiltrates is also in line with the assumption that at least certain subtypes of mastocytosis result from clonal neoplastic processes.28,42,43
In cases of systemic mastocytosis and associated myeloid leukaemia, two recent studies have investigated the question of whether or not the c-kit mutation Asp 816 to Val is present in both malignancies.33,34 However, different concomitant leukaemias (AML and CMML) were investigated and differing results were obtained. In the study by Nagata et al, peripheral blood mononuclear cells in a patient with systemic mastocytosis and associated CMML were analysed and found to contain the c-kit mutation Asp 816 to Val.33 In the other study, the c-kit mutation Asp 816 to Val was detected in bone marrow aspirates containing mast cells but not in leukaemic blasts in a patient with systemic mastocytosis and concurrent AML.34 In our study, we were able to analyse neoplastic mast cells and leukaemic CMML cells as separated (microdissected) cell populations for the presence of the c-kit mutation Asp 816 to Val. In particular, the neoplastic mast cell infiltrates were made visible and could be separated from the CMML infiltrates by using tryptase stained bone marrow sections and microdissection.38,39 In the next step, DNA was amplified to analyse the mutation. However, the suitability of nucleic acids for PCR amplification is often reduced by routine pretreatment (such as formalin fixation, paraffin wax embedding, and EDTA decalcification) of bone marrow biopsy specimens. In our study, specific c-kit amplification products were obtained in 13 of 20 of microdissected mast cell and CMML infiltrates, respectively, underlining the sensitivity of the semi-nested PCR assay.44
The detection of the c-kit mutation Asp 816 to Val in areas of CMML in our patient is a remarkable finding. This observation strongly suggests a monoclonal process and the evolution of systemic mastocytosis and CMML from a common progenitor. This hypothesis would also be in line with the notion that the c-kit mutation Asp 816 to Val is variably found in diverse types of blood and bone marrow leucocytes, including myelomonocytic cells, B cells, and T cells, in patients with systemic mastocytosis.30 35 However, it cannot be completely ruled out that the detection of the mutation in areas representing CMML was the result of contaminating neoplastic mast cells. In this regard, it is noteworthy that strongly tryptase positive bone marrow mast cells were not only detectable in compact focal infiltrates but also as diffusely scattered cells within leukaemic areas of the bone marrow. Whether the CMML cells definitely carry the c-kit mutation in patients with systemic mastocytosis and associated CMML can only be clarified by the analysis of single leukaemic cells, isolated by microdissection.
In summary, we have described the detection of the c-kit Asp 816 to Val point mutation both in microdissected mastocytosis infiltrates and leukaemic areas in the bone marrow of a patient with systemic mastocytosis and concurrent CMML. We put forward the hypothesis that both the systemic mastocytosis and the associated CMML in this patient are derived from a common progenitor cell.
References
- 1.Lennert K, Parwaresch MR. Mast cells and mast cell neoplasia: a review. Histopathology 1979;3:349–65. [DOI] [PubMed] [Google Scholar]
- 2.Horny H-P, Parwaresch MR, Lennert K. Bone marrow findings in systemic mastocytosis. Hum Pathol 1985;16:808–14. [DOI] [PubMed] [Google Scholar]
- 3.Stein DH. Mastocytosis: a review. Pediatr Dermatol 1986;3:365–75. [DOI] [PubMed] [Google Scholar]
- 4.Metcalfe DD. Classification and diagnosis of mastocytosis: current status. J Invest Dermatol 1991;96:2S–4S. [PubMed] [Google Scholar]
- 5.Johnson WC, Helwig EB. Solitary mastocytosis (urticaria pigmentosa). Arch Dermatol 1961;84:806–15. [DOI] [PubMed] [Google Scholar]
- 6.Caplan RM. The natural course of urticaria pigmentosa: analysis and follow up of 112 cases. Arch Dermatol 1963;87:147–57. [DOI] [PubMed] [Google Scholar]
- 7.Horny H-P, Parwaresch MR, Lennert K. [Clinical picture and prognosis of generalized mastocytosis.] Klinisches Bild und Prognose generalisierter Mastozytosen. Klin Wochenschr 1983;61:785–93. [DOI] [PubMed] [Google Scholar]
- 8.Parwaresch MR, Horny H-P, Lennert K. Tissue mast cells in health and disease. Pathol Res Pract 1985;179:439–61. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence JB, Friedman BS, Travis WD, et al. Hematologic manifestations of systemic mast cell disease: a prospective study of laboratory and morphologic features and their relation to prognosis. Am J Med 1991;91:612–24. [DOI] [PubMed] [Google Scholar]
- 10.Travis WD, Li CY, Bergstralh EJ, et al. Systemic mast cell disease. Analysis of 58 cases and literature review. Medicine Baltimore 1988;67:345–68. [PubMed] [Google Scholar]
- 11.Horny H-P, Ruck M, Wehrmann M, et al. Blood findings in generalized mastocytosis: evidence of frequent simultaneous occurrence of myeloproliferative disorders. Br J Haematol 1990;76:186–93. [DOI] [PubMed] [Google Scholar]
- 12.Lindner PS, Pardanani B, Angadi C, et al. Acute nonlymphocytic leukemia in systemic mastocytosis with biclonal gammopathy. J Allergy Clin Immunol 1992;90:410–12. [DOI] [PubMed] [Google Scholar]
- 13.Wong KF, Chan JK, Chan JC, et al. Concurrent acute myeloid leukemia and systemic mastocytosis. Am J Hematol 1991;38:243–4. [DOI] [PubMed] [Google Scholar]
- 14.Yarden Y, Kuang WJ, Yang FT, et al. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J 1987;6:3341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu FH, Ray P, Brown K, et al. Primary structure of c-kit: relationship with the CSF-1/PDGF receptor kinase family—oncogenic activation of v-kit involves deletion of extracellular domain and C terminus. EMBO J 1988;7:1003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashman LK, Cambareri AC, To LB, et al. Expression of the YB5.B8 antigen (c-kit proto-oncogene product) in normal human bone marrow. Blood 1991;78:30–7. [PubMed] [Google Scholar]
- 17.Papayannopoulou T, Brice M, Broudy VC, et al. Isolation of c-kit receptor-expressing cells from bone marrow, peripheral blood, and fetal liver: functional properties and composite antigenic profile. Blood 1991;78:1403–12. [PubMed] [Google Scholar]
- 18.Reisbach G, Bartke I , Kempkes B, et al. Characterization of hemopoietic cell populations from human cord blood expressing c-kit. Exp Hematol 1993;21:74–9. [PubMed] [Google Scholar]
- 19.Ogawa M, Matsuzaki Y, Nishikawa S, et al. Expression and function of c-kit in hemopoietic progenitor cells. J Exp Med 1991;174:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orr UA, Avivi A, Zimmer Y, et al. Developmental expression of c-kit, a proto-oncogene encoded by the W locus. Development 1990;109:911–23. [DOI] [PubMed] [Google Scholar]
- 21.Manova K, Nocka K, Besmer P, et al. Gonadal expression of c-kit encoded at the W locus of the mouse. Development 1990;110:1057–69. [DOI] [PubMed] [Google Scholar]
- 22.Koch CA, Anderson D, Moran MF, et al. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science 1991;252:668–74. [DOI] [PubMed] [Google Scholar]
- 23.Pawson T. Protein modules and signalling networks. Nature 1995;373:573–80. [DOI] [PubMed] [Google Scholar]
- 24.Kitamura Y, Nakayama H, Fujita J. Mechanisms of mast cell deficiency in mutant mice of W/Wv and Sl/Sld genotype. In: Galli SJ, Austen KF, eds. Mast cell and basophil differentiation and function in health and disease. New York: Raven Press, 1989:12–15.
- 25.Furitsu T, Tsujimura T, Tono T, et al. Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of c-kit product. J Clin Invest 1993;92:1736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butterfield JH, Weiler D, Dewald G, et al. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res 1988;12:345–55. [DOI] [PubMed] [Google Scholar]
- 27.Kitayama H, Kanakura Y, Furitsu T, et al. Constitutively activating mutations of c-kit receptor tyrosine kinase confer factor-independent growth and tumorigenicity of factor-dependent hematopoietic cell lines. Blood 1995;85:790–8. [PubMed] [Google Scholar]
- 28.Longley BJ, Tyrrell L, Lu SZ, et al. Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat Genet 1996;12:312–14. [DOI] [PubMed] [Google Scholar]
- 29.Worobec AS, Semere T , Nagata H, et al. Clinical correlates of the presence of the Asp816Val c-kit mutation in the peripheral blood mononuclear cells of patients with mastocytosis. Cancer 1998;83:2120–9. [DOI] [PubMed] [Google Scholar]
- 30.Afonja O, Amorosi E, Ashman L, et al. Multilineage involvement and erythropoietin-“independent” erythroid progenitor cells in a patient with systemic mastocytosis. Ann Hematol 1998;77:183–6. [DOI] [PubMed] [Google Scholar]
- 31.Pignon JM, Giraudier S, Duquesnoy P, et al. A new c-kit mutation in a case of aggressive mast cell disease. Br J Haematol 1997;96:374–6. [DOI] [PubMed] [Google Scholar]
- 32.Longley BJJ, Metcalfe DD, Tharp M, et al. Activating and dominant inactivating c-KIT catalytic domain mutations in distinct clinical forms of human mastocytosis. Proc Natl Acad Sci U S A 1999;96:1609–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagata H, Worobec AS , Oh CK, et al. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci U S A 1995;92:10560–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sperr WR, Walchshofer S, Horny H-P, et al. Systemic mastocytosis associated with acute myeloid leukaemia: report of two cases and detection of the c-kit mutation Asp-816 to Val. Br J Haematol 1998;103:740–9. [DOI] [PubMed] [Google Scholar]
- 35.Akin C, Kirshenbaum AS, Semere T, et al. Analysis of the surface expression of c-kit and occurrence of the c-kit Asp816Val activating mutation in T cells, B cells, and myelomonocytic cells in patients with mastocytosis. Exp Hematol 2000;28:140–7. [DOI] [PubMed] [Google Scholar]
- 36.Brunning RD, McKenna RW, Rosai J, et al. Systemic mastocytosis. Extracutaneous manifestations. Am J Surg Pathol 1983;7:425–38. [DOI] [PubMed] [Google Scholar]
- 37.Travis WD, Li CY, Yam LT, et al. Significance of systemic mast cell disease with associated hematologic disorders. Cancer 1988;62:965–72. [DOI] [PubMed] [Google Scholar]
- 38.Horny H-P, Sillaber C , Menke D, et al. Diagnostic value of immunostaining for tryptase in patients with mastocytosis. Am J Surg Pathol 1998;22:1132–40. [DOI] [PubMed] [Google Scholar]
- 39.Marafioti T, Hummel M, Anagnostopoulos I, et al. Origin of nodular lymphocyte-predominant Hodgkin's disease from a clonal expansion of highly mutated germinal-center B cells. N Engl J Med 1997;337:453–8. [DOI] [PubMed] [Google Scholar]
- 40.Sotlar K, Selinka H-C , Menton M, et al. Detection of human papillomavirus type 16 E6/E7 oncogene transcripts in dysplastic and nondysplastic cervical scrapes by nested RT-PCR. Gynecol Oncol 1998;69:114–21. [DOI] [PubMed] [Google Scholar]
- 41.Bowen DT, Padua RA, Burnett AK, et al. Two new polymorphisms but no mutations of the KIT gene in patients with myelodysplasia at positions corresponding to human FMS and murine W locus mutational hot spots. Leukemia 1993;7:1883–5. [PubMed] [Google Scholar]
- 42.Galli SJ. New concepts about the mast cell. N Engl J Med 1993;328:257–65. [DOI] [PubMed] [Google Scholar]
- 43.Kröber SM, Horny H-P, Ruck P, et al. Mastocytosis: reactive or neoplastic? J Clin Pathol 1997;50:525–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roehrl MH, Becker KF, Becker I, et al. Efficiency of single-cell polymerase chain reaction from stained histologic slides and integrity of DNA in archival tissue. Diagn Mol Pathol 1997;6:292–7. [DOI] [PubMed] [Google Scholar]