We recently reported a new method for the extraction of DNA from paraffin wax embedded bone marrow trephine biopsies.1 The DNA extracted from EDTA decalcified bone marrow trephine biopsies using this method was sufficiently intact to allow the amplification and sequencing of relatively long polymerase chain reaction (PCR) products, including the 600 bp t(11;14) MTCA PCR product. A shorter 294 bp PCR product could only be amplified from six of 10 formic acid decalcified bone marrow trephine biopsies reported in a previous study by Provan et al.2 These findings suggested a correlation between DNA degradation and formic acid decalcification, but required a comparative study for confirmation.J Clin Pathol: Mol Pathol 2000;53:336–337
We have subsequently extracted DNA from 11 formic acid decalcified bone marrow trephine biopsies using our method and determined the quality of DNA using agarose gel electrophoresis and PCR analysis, as in our initial study.
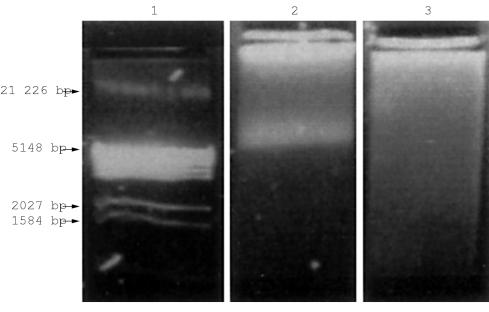
The mean DNA yield from the formic acid decalcified blocks was twice that of the EDTA decalcified samples: 9.4 μg and 4.3 μg, respectively. This reflected the fact that the formic acid blocks contained approximately twice as much bone marrow trephine biopsy material as a result of the differences in practice between the two centres involved in the study (Exeter, EDTA decalcification; Southampton, formic acid decalcification). However, when the formic acid decalcified DNA samples were analysed by agarose gel electrophoresis, no high molecular weight DNA was detected; only a smear of degraded DNA was seen. In contrast, analysis of the EDTA decalcified bone marrow trephine biopsy DNA samples showed DNA ranging from 5 to 21 kb in length (fig 1 ▶).
Figure 1.
Agarose gel electrophoresis of DNA extracted from EDTA and formic acid decalcified bone marrow trephine biopsies. Lane 1, DNA size standard; lane 2, DNA extracted from an EDTA decalcified bone marrow trephine biopsy; lane 3, DNA extracted from a formic acid decalcified bone marrow trephine biopsy.
It was not possible to amplify the 147 bp factor V PCR product from three of the formic acid decalcified DNA samples. The remaining eight samples generated very weak products compared with control DNA extracted from peripheral blood lymphocytes. This short PCR product was previously amplified successfully from all eight EDTA decalcified bone marrow trephine biopsy DNA samples.
The 482 bp BRCA 1 exon 11B product was only amplified successfully from one of the 11 formic acid decalcified samples. The intensity of the band seen on agarose gel electrophoresis was very weak compared with the control. Previously, all of our EDTA decalcified bone marrow trephine biopsy DNA samples were amplified successfully to generate this product.
Three formic acid decalcified samples yielded BRCA 1 exon 11A products (643 bp). However, the intensity of these products was so weak compared with the control that they were barely visible in the agarose gel. This relatively long PCR product had been amplified successfully using all EDTA decalcified bone marrow trephine biopsy DNA samples; five bands were of a similar intensity to the positive control, two were relatively weak, and one had to be diluted 1/20 to generate a band.1
This comparative study strongly suggests that formic acid decalcification of bone marrow trephine biopsies causes DNA degradation, rendering specimens decalcified by this method unsuitable for use as a source of archival DNA.
Consequently, in view of the increased requirement for the use of molecular techniques in the diagnosis and monitoring of patients with lymphoma and leukaemia, the use of formic acid as a bone marrow trephine biopsy decalcifying agent should be reviewed. Decalcification with EDTA has been used routinely in the histopathology department at the Royal Devon and Exeter NHS Trust for several years and, despite the minor delay involved in tissue processing, causes no impairment of the quality of immunohistochemical and tinctoral staining in bone marrow trephine biopsies compared with formic acid decalcification. Directly as a result of the outcome of this comparative study, Southampton University Hospitals NHS Trust has now converted from the use of formic acid to EDTA for decalcification of BMT.
References
- 1.Wickham CL, Boyce M, Joyner MV, et al. Amplification of PCR products in excess of 600 base pairs using DNA extracted from decalcified, paraffin wax embedded bone marrow trephine biopsies. J Clin Pathol: Mol Pathol 2000;53:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Provan AB, Hodges E, Smith AG, et al. Use of paraffin wax embedded bone marrow trephine biopsy specimens as a source of archival DNA. J Clin Pathol 1992;45:763–5. [DOI] [PMC free article] [PubMed] [Google Scholar]