Abstract
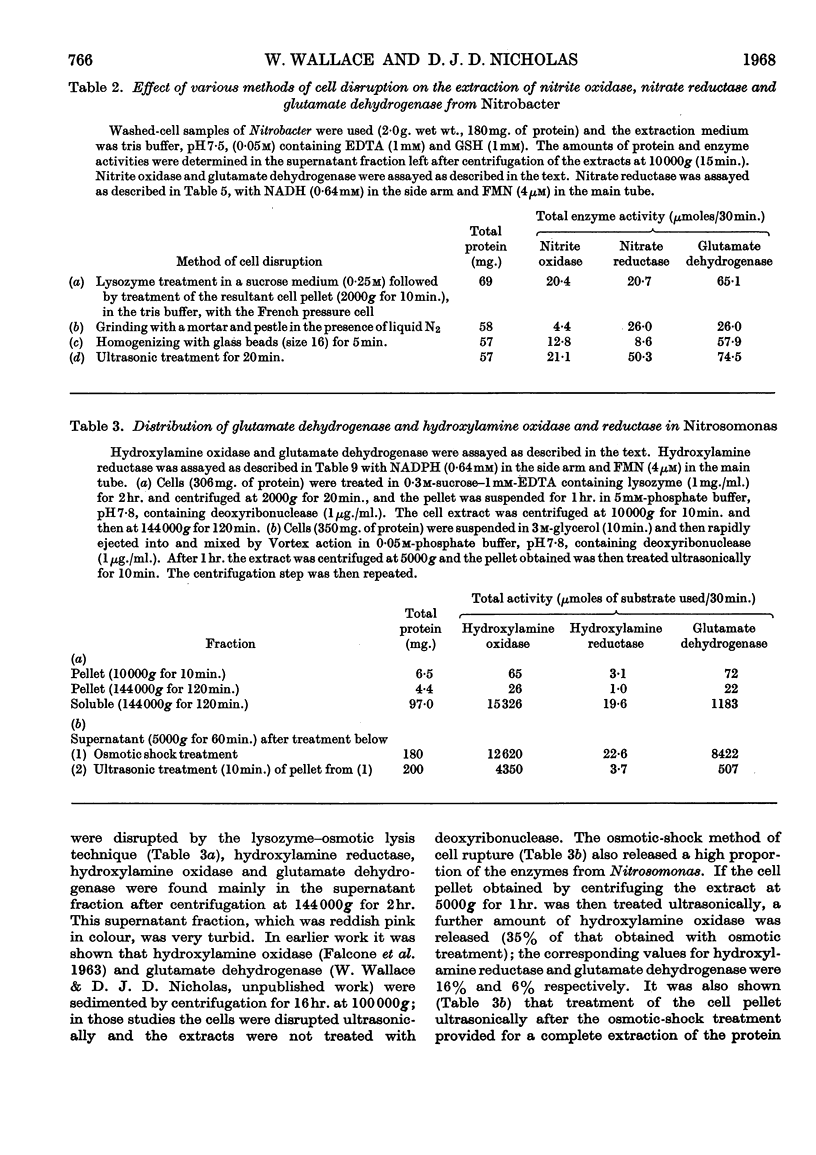
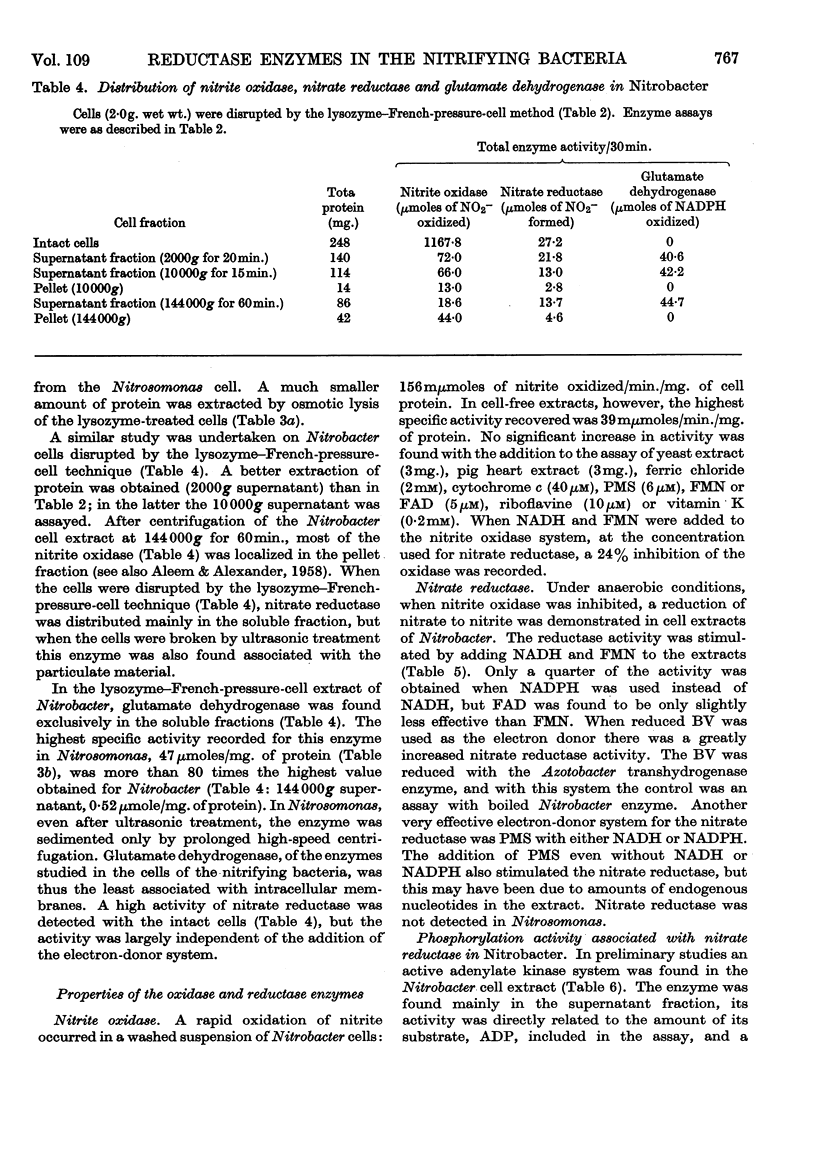
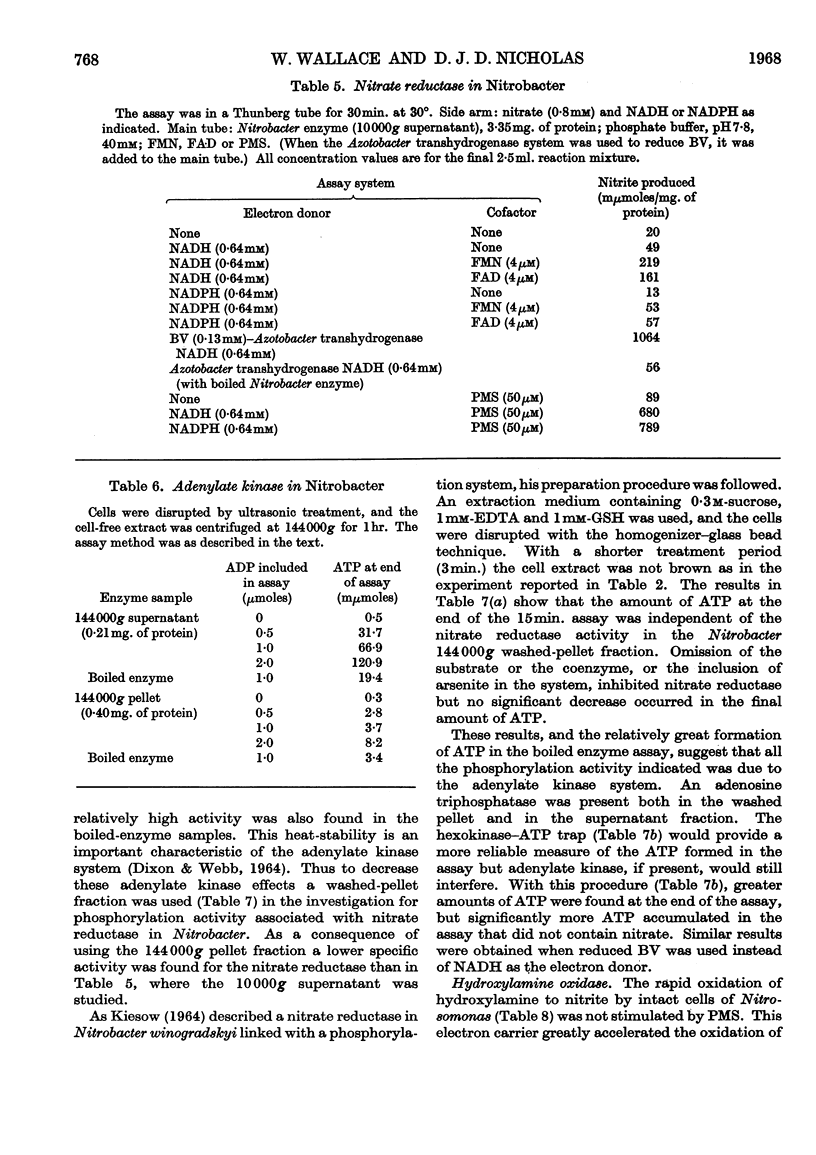
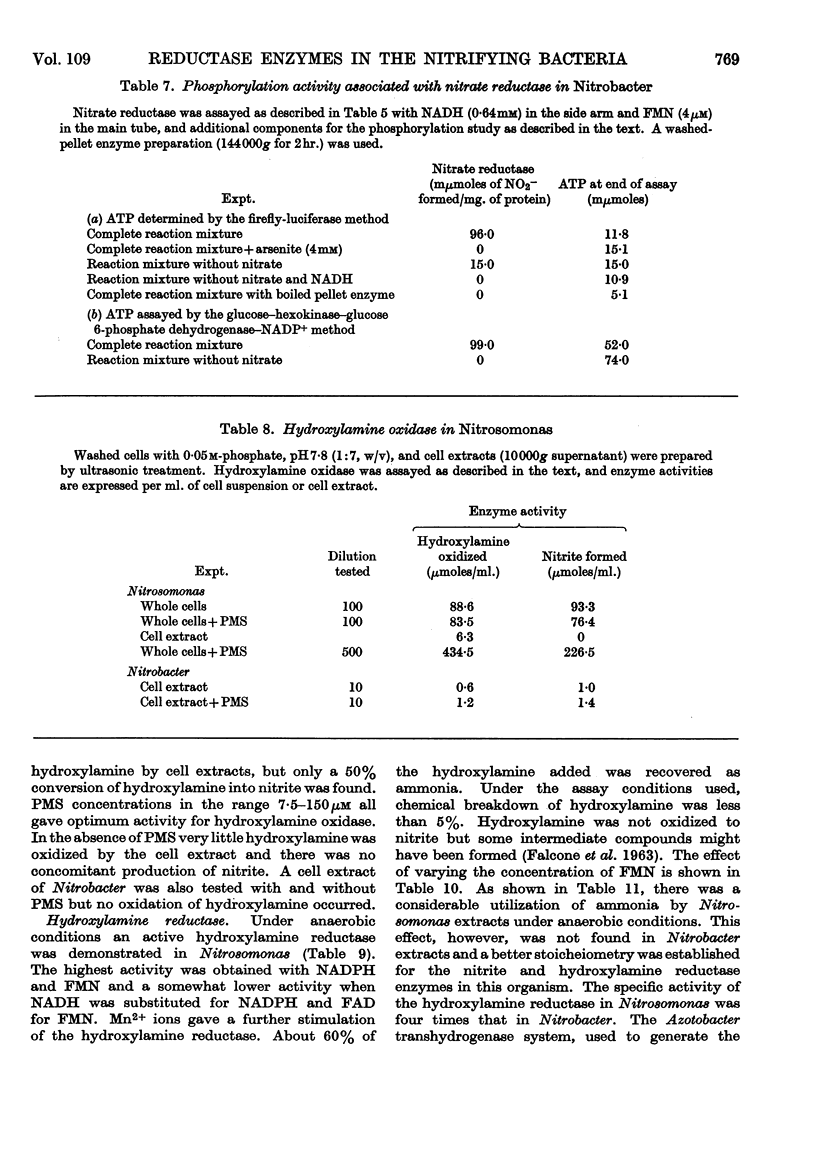
The reductase enzymes in Nitrosomonas and Nitrobacter were studied under anaerobic conditions when the oxidase enzymes were inactive. The most effective electron-donor systems for nitrate reductase in Nitrobacter were reduced benzyl viologen alone, phenazine methosulphate with either NADH or NADPH, and FMN or FAD with NADH. Nitrite and hydroxylamine reductases were found in both nitrifying bacteria, and optimum activity for each enzyme was obtained with NADH or NADPH with either FMN or FAD. The product of both these enzymes was identified as ammonia. In extracts of Nitrosomonas the ammonia was further utilized by an NADPH-specific glutamate dehydrogenase. 15N-labelled nitrite, hydroxylamine and ammonia were rapidly incorporated into cell protein by Nitrosomonas, and Nitrobacter in addition incorporated [15N]nitrate. Relatively gentle methods of cell disruption were compared with ultrasonic treatment, to enable a more exact study to be undertaken of the intracellular distribution of the oxidase and reductase enzymes. The functional relationship of these opposing enzyme systems in the nitrifying bacteria is considered.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEEM M. I., ALEXANDER M. Cell-free nitrification by Nitrobacter. J Bacteriol. 1958 Nov;76(5):510–514. doi: 10.1128/jb.76.5.510-514.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALEEM M. I., LEES H. ADENOSINE TRIPHOSPHATE-DEPENDENT REDUCTION OF NICOTINAMIDE ADENINE DINUCLEOTIDE BY FERRO-CYTOCHROME C IN CHEMOAUTOTROPHIC BACTERIA. Nature. 1963 Nov 23;200:759–761. doi: 10.1038/200759a0. [DOI] [PubMed] [Google Scholar]
- Aleem M. I., Nason A. PHOSPHORYLATION COUPLED TO NITRITE OXIDATION BY PARTICLES FROM THE CHEMOAUTOTROPH, NITROBACTER AGILIS. Proc Natl Acad Sci U S A. 1960 Jun;46(6):763–769. doi: 10.1073/pnas.46.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleem M. I. Path of carbon and assimilatory power in chemosynthetic bacteria. I. Nitrobacter agilis. Biochim Biophys Acta. 1965 Aug 24;107(1):14–28. doi: 10.1016/0304-4165(65)90384-3. [DOI] [PubMed] [Google Scholar]
- Brownell P. F., Nicholas D. J. Some Effects of Sodium on Nitrate Assimilation and N(2) Fixation in Anabaena cylindrica. Plant Physiol. 1967 Jul;42(7):915–921. doi: 10.1104/pp.42.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALCONE A. B., SHUG A. L., NICHOLAS D. J. SOME PROPERTIES OF A HYDROXYLAMINE OXIDASE FROM NITROSOMONAS EUROPAEA. Biochim Biophys Acta. 1963 Oct 1;77:199–208. doi: 10.1016/0006-3002(63)90493-1. [DOI] [PubMed] [Google Scholar]
- Hooper A. B., Hansen J., Bell R. Characterization of glutamate dehydrogenase from the ammonia-oxidizing chemoautotroph Nitrosomonas europaea. J Biol Chem. 1967 Jan 25;242(2):288–296. [PubMed] [Google Scholar]
- KIESOW L. ON THE ASSIMILATION OF ENERGY FROM INORGANIC SOURCES IN AUTOTROPHIC FORMS OF LIFE. Proc Natl Acad Sci U S A. 1964 Oct;52:980–988. doi: 10.1073/pnas.52.4.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp J. D., Atkinson D. E. Nitrite reductase of Escherichia coli specific for reduced nicotinamide adenine dinucleotide. J Bacteriol. 1966 Sep;92(3):628–634. doi: 10.1128/jb.92.3.628-634.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAZZARINI R. A., ATKINSON D. E. A triphosphopyridine nucleotide-specific nitrite reductase from Escherichia coli. J Biol Chem. 1961 Dec;236:3330–3335. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MEDINA A., NICHOLAS D. J. Interference by reduced pyridine nucleotides in the diazotization of nitrite. Biochim Biophys Acta. 1957 Feb;23(2):440–442. doi: 10.1016/0006-3002(57)90355-4. [DOI] [PubMed] [Google Scholar]
- MURRAY R. G., WATSON S. W. STRUCTURE OF NITROSOCYSTIS OCEANUS AND COMPARISON WITH NITROSOMONAS AND NITROBACTER. J Bacteriol. 1965 Jun;89:1594–1609. doi: 10.1128/jb.89.6.1594-1609.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHOLAS D. J., MABEY G. L. Some properties of glutamic dehydrogenase from Neurospora crassa. J Gen Microbiol. 1960 Feb;22:184–190. doi: 10.1099/00221287-22-1-184. [DOI] [PubMed] [Google Scholar]
- NICHOLAS D. J., RAO P. S. THE INCORPORATION OF LABELLED CO2 INTO CELLS AND EXTRACTS OF NITROSOMONAS EUROPAEA. Biochim Biophys Acta. 1964 Feb 10;82:394–397. doi: 10.1016/0304-4165(64)90311-3. [DOI] [PubMed] [Google Scholar]
- NICHOLAS D. J. THE METABOLISM OF INORGANIC NITROGEN AND ITS COMPOUNDS IN MICRO-ORGANISMS. Biol Rev Camb Philos Soc. 1963 Nov;38:530–568. doi: 10.1111/j.1469-185x.1963.tb00792.x. [DOI] [PubMed] [Google Scholar]
- Naik M. S., Nicholas D. J. NADH2-benzyl viologen reductase from Azotobacter vinelandii. Biochim Biophys Acta. 1966 Apr 12;118(1):195–197. doi: 10.1016/s0926-6593(66)80157-1. [DOI] [PubMed] [Google Scholar]
- Naik M. S., Nicholas D. J. Phosphorylation associated with nitrate and nitrite reduction in Micrococcus denitrificans and Pseudomonas denitrificans. Biochim Biophys Acta. 1966 Mar 7;113(3):490–497. doi: 10.1016/s0926-6593(66)80007-3. [DOI] [PubMed] [Google Scholar]
- Pangborn J., Marr A. G., Robrish S. A. LOCALIZATION OF RESPIRATORY ENZYMES IN INTRACYTOPLASMIC MEMBRANES OF AZOTOBACTER AGILIS. J Bacteriol. 1962 Oct;84(4):669–678. doi: 10.1128/jb.84.4.669-678.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAIAH A., NICHOLAS D. J. THE SYNTHESIS OF ATP AND THE INCORPORATION OF 32P BY CELL-FREE PREPARATIONS FROM NITROSOMONAS EUROPAEA. Biochim Biophys Acta. 1964 Jun 8;86:459–465. doi: 10.1016/0304-4165(64)90085-6. [DOI] [PubMed] [Google Scholar]
- REPASKE R. Lysis of gram-negative bacteria by lysozyme. Biochim Biophys Acta. 1956 Oct;22(1):189–191. doi: 10.1016/0006-3002(56)90240-2. [DOI] [PubMed] [Google Scholar]
- ROUSSOS G. G., NASON A. Pyridine nucleotide-nitrite and-hydroxylamine enzymes from soybean leaves. J Biol Chem. 1960 Oct;235:2997–3007. [PubMed] [Google Scholar]
- Rees M., Nason A. A P-450-like cytochrome and a soluble terminal oxidase identified as cytochrome o from Nitrosomonas europaea. Biochem Biophys Res Commun. 1965 Nov 8;21(3):248–256. doi: 10.1016/0006-291x(65)90279-2. [DOI] [PubMed] [Google Scholar]
- SPENCER D., TAKAHASHI H., NASON A. Relationship of nitrite and hydroxylamine reductases to nitrate assimilation and nitrogen fixation in Azotobacter agile. J Bacteriol. 1957 Apr;73(4):553–562. doi: 10.1128/jb.73.4.553-562.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAAT P. A., NASON A. CHARACTERIZATION OF A NITRATE REDUCTASE FROM THE CHEMOAUTOTROPH NITROBACTER AGILIS. J Biol Chem. 1965 Mar;240:1412–1426. [PubMed] [Google Scholar]
- Smith A. J., London J., Stanier R. Y. Biochemical basis of obligate autotrophy in blue-green algae and thiobacilli. J Bacteriol. 1967 Oct;94(4):972–983. doi: 10.1128/jb.94.4.972-983.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER G. C., NICHOLAS D. J. Hydroxylamine reductase from Pseudomonas aeruginosa. Biochim Biophys Acta. 1961 May 13;49:361–368. doi: 10.1016/0006-3002(61)90135-4. [DOI] [PubMed] [Google Scholar]


