Abstract
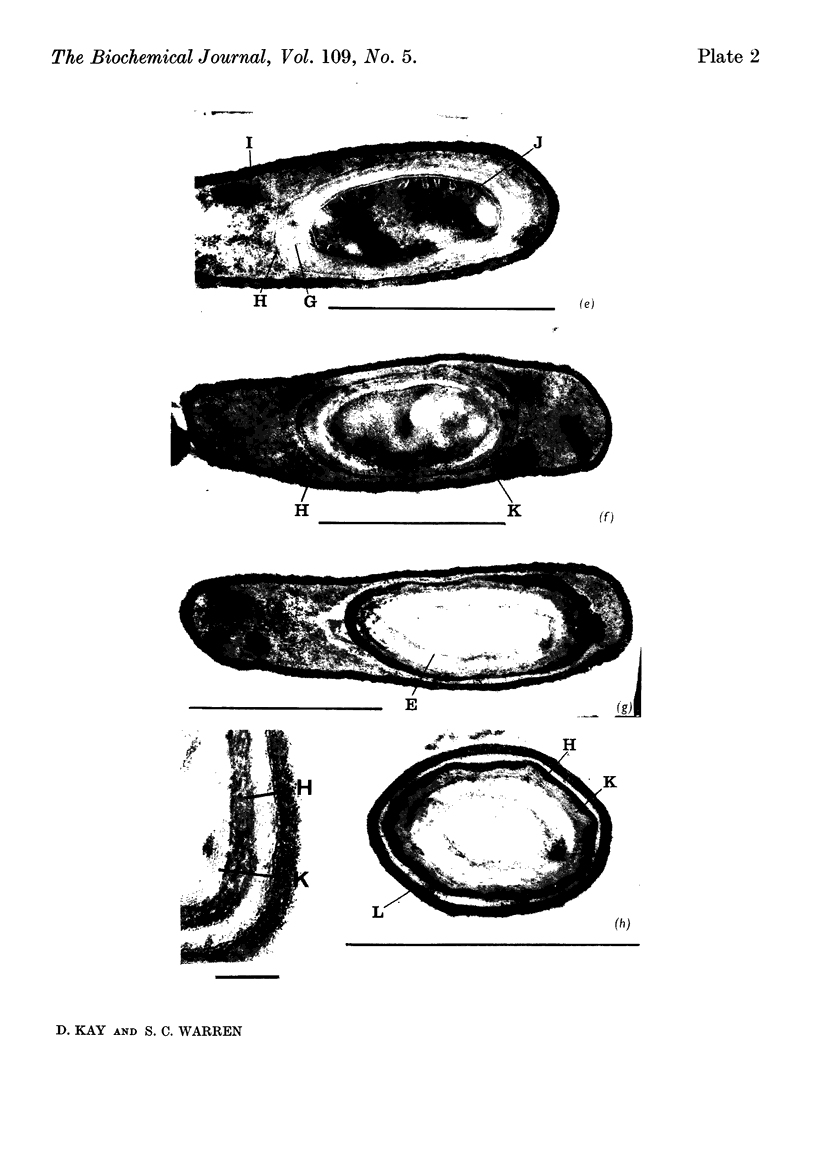
1. When Bacillus subtilis was grown in a medium in which sporulation occurred well-defined morphological changes were seen in thin sections of the cells. 2. Over a period of 7·5hr. beginning 2hr. after the initiation of sporulation the following major stages were observed: axial nuclear-filament formation, spore-septum formation, release of the fore-spore within the cell, development of the cortex around the fore-spore, the laying down of the spore coat and the completion of the corrugated spore coat before release of the spore from the mother cell. 3. The appearance of refractile bodies and 2,6-dipicolinic acid and the development of heat-resistance began between 5 and 6·5hr. after initiation of sporulation. 4. The appearance of 2,6-dipicolinic acid and the onset of refractility appeared to coincide with a diminution of electron density in the spore core and cortex. 5. Heat-resistance was associated with the terminal stage, the completion of the spore coat. 6. The spore coat was composed of an inner and an outer layer, each of which consisted of three or four electron-dense laminae. 7. Serial sections through cells at an early stage of sporulation showed that the membranes of each spore septum were always continuous with the membranes of a mesosome, which was itself in close contact with the bacterial or spore nucleoid. 8. These changes were correlated with biochemical events occurring during sporulation.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACH J. A., SADOFF H. L. Aerobic sporulating bacteria. I. Glucose dehydrogenase of Bacillus cereus. J Bacteriol. 1962 Apr;83:699–707. doi: 10.1128/jb.83.4.699-707.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK S. H., GERHARDT P. Permeability of bacterial spores. III. Permeation relative to germination. J Bacteriol. 1962 Feb;83:301–308. doi: 10.1128/jb.83.2.301-308.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADLEY D. E., WILLIAMS D. J. An electron microscope study of the spores of some species of the genus Bacillus using carbon replicas. J Gen Microbiol. 1957 Aug;17(1):75–79. doi: 10.1099/00221287-17-1-75. [DOI] [PubMed] [Google Scholar]
- DONNELLAN J. E., Jr, NAGS E. H., LEVINSON H. S. CHEMICALLY DEFINED, SYNTHETIC MEDIA FOR SPORULATION AND FOR GERMINATION AND GROWTH OF BACILLUS SUBTILIS. J Bacteriol. 1964 Feb;87:332–336. doi: 10.1128/jb.87.2.332-336.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnellan J. E., Jr, Setlow R. B. Thymine Photoproducts but not Thymine Dimers Found in Ultraviolet-Irradiated Bacterial Spores. Science. 1965 Jul 16;149(3681):308–310. doi: 10.1126/science.149.3681.308. [DOI] [PubMed] [Google Scholar]
- Ellar D. J., Lundgren D. G., Slepecky R. A. Fine structure of Bacillus megaterium during synchronous growth. J Bacteriol. 1967 Oct;94(4):1189–1205. doi: 10.1128/jb.94.4.1189-1205.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn E. D., Weisman R. A. I. Loss of lipids during preparation of amoebae for electron microscopy. Biochim Biophys Acta. 1966 Apr 4;116(2):309–316. doi: 10.1016/0005-2760(66)90013-0. [DOI] [PubMed] [Google Scholar]
- Mishiro Y., Ochi M. Effect of dipicolinate on the heat denaturation of proteins. Nature. 1966 Sep 10;211(5054):1190–1190. doi: 10.1038/2111190a0. [DOI] [PubMed] [Google Scholar]
- Neihof R., Thompson J. K., Deitz V. R. Sorption of water vapour and nitrogen gas by bacterial spores. Nature. 1967 Dec 30;216(5122):1304–1306. doi: 10.1038/2161304a0. [DOI] [PubMed] [Google Scholar]
- POWELL J. F. Isolation of dipicolinic acid (pyridine-2:6-dicarboxylic acid) from spores of Bacillus megatherium. Biochem J. 1953 May;54(2):210–211. doi: 10.1042/bj0540210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART B. T., HALVORSON H. O. Studies on the spores of aerobic bacteria. II. The properties of an extracted heat-stable enzyme. Arch Biochem Biophys. 1954 Mar;49(1):168–178. doi: 10.1016/0003-9861(54)90178-2. [DOI] [PubMed] [Google Scholar]
- Smith K. C., Yoshikawa H. Variation in the photochemical reactivity of thymine in the DNA of B. subtilis spores, vegetative cells and spores germinated in chloramphenicol. Photochem Photobiol. 1966 Oct;5(10):777–786. doi: 10.1111/j.1751-1097.1966.tb05773.x. [DOI] [PubMed] [Google Scholar]
- Warren S. C. Sporulation in Bacillus subtilis. Biochemical changes. Biochem J. 1968 Oct;109(5):811–818. doi: 10.1042/bj1090811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise J., Swanson A., Halvorson H. O. Dipicolinic acid-less mutants of Bacillus cereus. J Bacteriol. 1967 Dec;94(6):2075–2076. doi: 10.1128/jb.94.6.2075-2076.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]