Abstract
Aims—Insulin-like growth factor type I (IGF-I) antisense cellular gene therapy of tumours is based on the following data: rat glioma or hepatoma cells transfected with the vector encoding IGF-I antisense cDNA lose their tumorigenicity and induce a tumour specific immune response involving CD8+ T cells. Recently, using the IGF-I triple helix approach in studies of tumorigenicity, major histocompatibility complex class I (MHC-I) antigens were demonstrated in rat glioma transfected cells. This study used comparative IGF-I antisense and triple helix technologies in human primary glioma cells to determine the triple helix strategy that would be most appropriate for the treatment of glioblastoma.
Methods—The cells were transfected using the IGF-I triple helix expression vector, pMT-AG, derived from the pMT-EP vector. pMT-AG contains a cassette comprising a 23 bp DNA fragment transcribing a third RNA strand, which forms a triple helix structure within a target region of the human IGF-I gene. Using pMT-EP, vectors encoding MHC-I or B7 antisense cDNA were also constructed.
Results—IGF-I triple helix transfected glioma cells are characterised by immune and apoptotic phenomena that appear to be related. The expression of MHC-I and B7 in transfected cells (analysed by flow cytometry) was accompanied by programmed cell death (detected by dUTP fluorescein terminal transferase labelling of nicked DNA and electron microscopic techniques). Cotransfection of these cells with MHC-I and B7 antisense vectors suppressed the expression of MHC-I and B7, and was associated with a pronounced decrease in apoptosis.
Conclusion—When designing an IGF-I triple helix strategy for the treatment of human glioblastoma, the transfected tumour cells should have the following characteristics: the absence of IGF-I, thepresence of both MHC-I and B7 molecules, and signs of apoptosis.
Keywords: glioblastoma, insulin-like growth factor I triple helix, apoptosis, major histocompatibility complex class I and B7
Malignant glioma, the most common human brain tumour, is almost uniformly fatal. Median survival is less than one year. Different gene therapy strategies for the treatment of gliomas have been proposed.1–4 Our treatment strategy is based on the enhancement of the tumour “anti-gene” approach using antisense5, 6 or triple helix7, 8 anti-IGF-I (insulin-like growth factor I) technologies.9, 10
Alteration in the expression of growth factors and/or their receptors is associated with the growth and development of animal and human tumours, including tumours of the central nervous system.11 One such growth factor is insulin-like growth factor type I (IGF-I), a 70 amino acid polypeptide involved in tissue growth and differentiation.12–16 IGF-I and the related polypeptide IGF-II are expressed in many tissues, including brain, in which they are involved in the proliferation of neuronal and glial precursors.17 High concentrations of IGF-I and IGF-II are also found in some nervous system derived tumours, such as glioblastomas, astrocytomas, and meningiomas,18, 19 and tumour cell lines (IGF-I, C6 glioma20; IGF-II, SK-N-S neuroblastoma21).
Previous reports found that the rat C6 glioma cell line expresses high amounts of IGF-I.9, 22 Because C6 cells are also known to express both IGF-I and IGF-II receptors,22 it is possible that an autocrine loop involving IGF-I and its receptors might contribute to the tumorigenic phenotype. In principle, mutagenesis strategies offer more definitive and unambiguous approaches for the analysis of autocrine mechanisms in tumour cells. Antisense and triple helix strategies bypass the inherent limitations of functional studies, which are dependent upon naturally mutated or artificially mutagenised cells.5, 7, 23 Triple helix forming oligonucleotides (TFOs) block the activity of RNA polymerase by forming triple helicalstructures with complementary DNA. This approach promises to provide a new class of sequence specific, DNA binding agents that can target malignancies at the transcriptional level.8, 24–26 TFOs have also been used to form intermolecular triplex structures that block the binding of transcription factors and suppress the transcription of specific growth modulating genes, such as epidermal growth factor (EGF) or growth factor receptor,27 and oncogenic myc,28, 29 HER2,30, 31 Ha-ras,32 and Ki-ras.33
The production of IGF-I is downregulated in rat C6 glioma cells when they are transfected with a vector expressing IGF-I antisense cDNA or IGF-I TFOs. The transfected cells lose their tumorigenicity and elicit tumour specific immunity, which leads to a cure of established tumours.2, 10 The immune response was shown to be mediated mainly by T cells, as indicated by an abundant infiltration of the CD8+ T cell subset in tumour tissues.2, 34, 35 Here, we examine the possibility that the suppression of intracellular IGF-I in rat and human glioma cells, using triple helix technology, alters the cell phenotype and the production of different cytokines (as seen in MHC-I and B7 antisense transfected cells35–37), and that this process is also associated with increased programmed cell death (apoptosis).
Other studies have shown that the antisense strategy directed at the IGF receptor induces apoptosis in tumour cells.15, 22, 38 Recently, we reported the occurrence of apoptosis in IGF-I antisense transfected hepatoma cells.39 Apoptosis is a normal physiological process contributing to the maintenance of a balance between cell proliferation and cell death. The disturbance of this balance can be induced by various stimuli, such as cytokines or growth factors.40 Inhibition of apoptosis in malignant cells could occur by several mechanisms including overexpression of proteins such as bcl-2 or c-myc, abrogation of the function of the p53 gene, or stimulation of IGF-I or its receptor.40–44
During the process of developing antitumour IGF-I strategies for the neuroectodermal neoplasia (glioblastoma multiforme), we detected a relation between the antitumour immune response and apoptosis. We showed that IGF-I triple helix transfection induces the expression of both major histocompatibility complex class I (MHC-I) and B7 molecules. In addition, we found that when vectors encoding MHC-I and B7 antisense cDNA are also transfected into these cells both MHC-I and B7 expression can be reversed and a pronounced decrease in apoptosis occurs. These data define the immune and apoptotic characteristics of tumoral cells that would be appropriate for the treatment of glioma, and enable triple helix technology to be introduced as a new gene therapy approach for the treatment of human glioblastoma.
Materials and methods
CELL CULTURE
The human primary glioma cell line36, 37 was used. Cells were cultured in DMEM (GIBCO-BRL, Cergy-Pontoise, France) supplemented with 10% fetal calf serum (FCS), 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin, at 37°C and 5% CO2. Hygromycin B (Boehringer Mannheim, Mannheim, Germany), at a concentration of 0.5 mg/ml, was added 48 hours after transfection to select for transfected cells. In experiments using the rat C6 glioma cell line as a positive control,20 the concentration of hygromycin B was changed to 2 mg/ml after one week and maintained with each change of fresh medium during the next three to four months. In experiments with human primary gliomas, the concentration of hygromycin for cell culture was determined as described previously.37 The B-104 rat neuroblastoma cell line (obtained from the American Tissue Collection, Rockville, Maryland, USA) was used as a negative control.9
Primary cell cultures of human glioma were derived from tumours of five patients with glioblastoma during surgical resection in the Hôpital Val-de-Grâce, Paris and the University Hospital of Cleveland, Ohio, USA. Surgical sections approximately 3 × 3 mm and 1–2 cm in length were placed in DMEM containing a high glucose concentration, 100 U/ml penicillin, and 100 μg/ml streptomycin. Specimens were then transferred to phosphate buffered saline (PBS) containing no Ca2+ or Mg2+ and dissected into 1–2 mm fragments. The PBS was changed to PBS containing 0.3 mg/ml collagenase and the tissue was incubated for 20 minutes at 37°C with gentle agitation and centrifuged at 200 ×g for five minutes. The pellet was resuspended in DMEM containing 20% FCS supplemented with 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10 ng/ml EGF (Sigma, St Louis, USA). The cells were adjusted to a concentration of 2 × 106cells/well in six well plates and incubated at 37°C and 5% CO2 in culture medium containing 10 ng/ml EGF. After two days, dead cells were removed and the incubation was continued in DMEM containing 10% FCS, and no EGF, for another three days. The medium was then changed to DMEM without FCS and incubation was continued for another two days. After this first week, cells were maintained in DMEM containing 5% FCS in 10% CO2 at 37°C until stable transfection was established (approximately four weeks).
PLASMIDS
The episome based plasmid pMT-anti-IGF-I was constructed as described previously.9 The vector pMT-EP, under the control of the metallothionin (MT-I) inducible promoter, was used as its base. The cassette contains the Epstein-Barr virus origin of replication and the gene encoding nuclear antigen I, which together drive extrachromosomal replication. Downstream of the insertion site is a poly A termination signal followed by the hygromycin B and ampicillin resistance genes. For comparison, the same plasmid was also prepared containing either cytomegalovirus (CMV) or heat shock (HS) promoters.
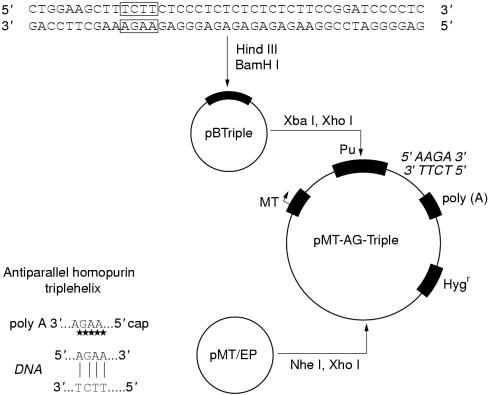
The vector expressing IGF-I triple helix (pMT-AG triple helix) was constructed as described previously10 (fig 1 ▶). This cassette consists of a 23 bp DNA fragment cloned into the pMT-EP vector, which transcribes a third RNA strand to form a triple helix structure within the target region of the human IGF-I gene, between its transcription and translation initiation sites. The pMT-EP vector with either the lac Z reporter gene or cDNA expressing IGF-II antisense RNA as insert was used in control experiments.34
Figure 1.
Construction of plasmid pMT-AG-TH encoding oligopurine variants of the triple helix third strand. Pu, oligopurine.
Vectors encoding MHC-I or B7 antisense cDNA were constructed in the laboratory of J Ilan (CWRU, Cleveland, Ohio, USA) using pMT-EP containing the neomycin (G418) resistance gene instead of the hygromycin B resistance gene, and the MHC-I or B7 insert in antisense orientation in place of the IGF-I gene sequence. The details of the vector expressing mouse and rat MHC-I antisense are as follow: the insertion is a 300 bp fragment, which contains an MHC-I a3 domain from the Kb locus, cloned in antisense orientation into the polylinker (GenBank accession number L 22338; MHC-I gene H-2T24, the range is 278 bp starting from nucleotide 595 and finishing at 872). In the vector expressing human MHC-I antisense, the insertion is a 300 bp fragment, which contains an MHC-I a3 domain, cloned in antisense orientation (GenBank accession number X 03071; HLA-A2 gene (exon 4), the range is 276 bp). In the vector expressing mouse and rat B7 antisense, the insertion is 960 bp long and is composed of fragments of B7-1 and B7-2, both in antisense orientation (GenBank accession numbers X 60958 and L 25606, respectively; the range is 353 bp for B7-1 and 560 bp for B7-2). In the vector expressing human B7-1 and B7-2 antisense, the insertion is 1500 bp in antisense orientation (GenBank accession number NM 005191; the range is 1549 bp).
TRANSFECTION
Cell cultures (60–80% confluent) were transfected in six well plates using 1 μg plasmid DNA/400 000 cells. The FuGENE 6 transfection reagent (Boehringer Mannheim) was used according to the supplier's instructions. To determine the efficiency of transfection, the process was carried out using the pMT-EP construct containing lac Z as a reporter gene. Cell cultures were washed in PBS and incubated at 37°C in the presence of the staining solution, which contained 5 mM K3Fe(CN)6, 2 mM MgCl2, 0.8 mg/ml X-gal in PBS. The selected IGF-I antisense or triple helix cell clones (expressing MHC-I and B7) were cotransfected with vectors encoding either MHC-I or B7 antisense cDNA in the presence of 0.4 mg/ml of G-418.
NORTHERN BLOTTING
The content of IGF-I antisense RNA was determined in 50% confluent cell cultures. Cells were deprived of serum and cultured overnight in DMEM containing 0.1% bovine serum albumin (BSA); 60 μM ZnS04 (Sigma) was then added for five hours to induce the MT-I promoter. The cells were then prepared for northern blotting as described previously.35 The labelling of rat and human IGF-I cDNA and chicken β actin cDNA and hybridisations were carried out according to the procedures described previously35; the 770 bp human IGF-I cDNA and 500 bp rat IGF-I cDNA used as probes were a gift from J Ilan (CWRU, Cleveland, USA). Northern blotting was also used to verify the expression of IGF-I in solid glioblastomas.
IMMUNOCYTOCHEMISTRY AND FLOW CYTOMETRY (FACS) ANALYSIS
After induction of the MT-I promoter, cells were fixed in 4% paraformaldehyde. Immunocytochemical localisation of the IGF-I protein was carried out by means of the immunoperoxidase technique (Vectastain ABC kit; Vector Laboratories, Burlingame, California, USA). Polyclonal antibodies against rat and human IGF-I were purchased from Valbiotech (Paris, France).
For FACS analysis, paraformaldehyde fixed cells were treated as described previously.36 Stained cells were analysed in a FACSCAN flow cytometer (Becton Dickinson, New Jersey, USA). The cells used for FACS analysis were separated into two groups: one group of cells was non-irradiated, whereas the other group of human or rat glioma cells was irradiated with 5000 rads of 60Co.
FLUORESCEIN CELL DEATH DETECTION
Apoptosis was determined by dUTP fluorescein terminal transferase labelling of nicked DNA (TUNEL apoptosis assay). The “in situ cell death detection kit, fluorescein” (Boehringer Mannheim) was used according to the supplier's instructions.
ELECTRON MICROSCOPY
Cells were fixed in 1% glutaraldehyde and 4% formaldehyde in 0.l M phosphate buffer (pH 7.2), rinsed for 40 minutes in 0.1 M phosphate buffer (×3), and then fixed again in phosphate buffered 1% OsO4 for one hour. After rinsing in distilled water for 20 minutes, the cells were stained with 2% uranyl acetate for 40 minutes at 60°C. They were then rinsed in distilled water (×3), dehydrated in a graded series of ethanol and 100% propylene oxide, and embedded in Spurr's resin. Sections (80 nm) were cut, placed on 200 mesh copper grids, and contrasted with lead citrate and uranyl acetate. Photomicrographs were taken using a Philips EM-2000 electron microscope operating at 80 kV.
Results
IMMUNOCYTOCHEMICAL AND MOLECULAR CHARACTERISATION OF TUMOUR CELLS
Human glioma cells established from five glioblastoma multiforme cancers demonstrated morphological characteristics similar to those described previously.36 The proportion of IGF-I positive cells in the human cell lines ranged from 50% to 70%. Each cell line was subcloned to obtain IGF-I positive clones; cell line HG1 was selected for further analysis. IGF-I antisense transfected cells (pMT-anti-IGF-I) as well as IGF-I triple helix transfected cells (pMT-AG triple helix) were immunocytochemically negative for IGF-I (fig 2 ▶). The frequency of transfection for the pMT-EP-lac Z vector (as determined by staining with X-gal at 48 hours after transfection) was between 5% and 15% for both rat and human glioma lines. No difference in transfection efficiency was observed for constructs containing the different promoters (MT, CMV, or HS).
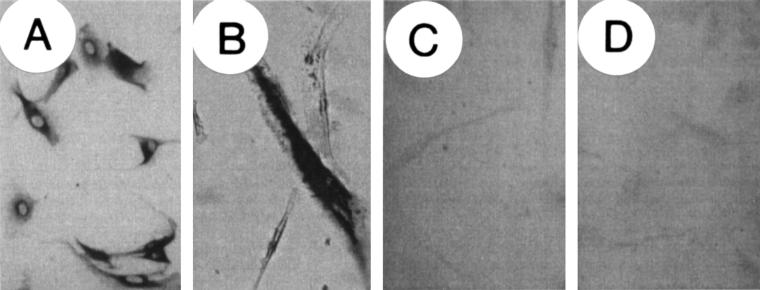
Figure 2.
Immunocytochemical localisation of insulin-like growth factor I (IGF-I) in non-transfected, parental, and in IGF-I antisense and triple helix transfected cells. Cells were stained for IGF-I by means of the immunoperoxidase technique using a polyclonal antibody against IGF-I. (A) parental (non-transfected) cells showing cytoplasmic staining for IGF-1; (B) cells transfected with a vector expressing β galactosidase showing cytoplasmic staining for X-gal; (C) cells transfected with pMT-anti-IGF-I; (D) cells transfected with pMT-AG triple helix. Note the change in morphology of antisense and triple helix transfected cells (C,D) compared with parental cells (A); the transfected cells do not express IGF-I .
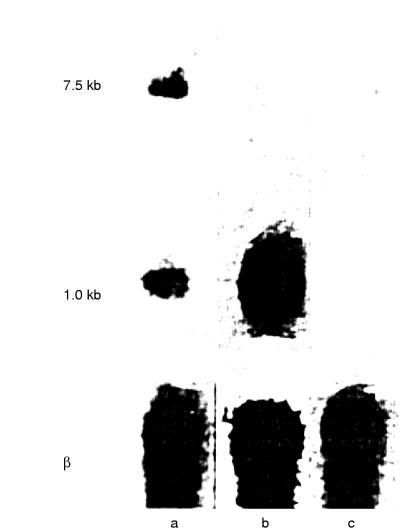
A comparison of the RNA of human glioma cells four weeks after transfection with that of parental cells is shown in the northern blot (fig 3 ▶). The RNA of non-transfected cells is distributed in 7.5 kb and l.0 kb bands corresponding to the different processing stages of endogenous human IGF-I RNA (lane a). The RNA of pMT-anti-IGF-I transfected cells shows only an abundant 1.0 kb band (lane b). As expected, RNA of pMT-AG triple helix transfected cells shows no band when human IGF-I cDNA is used as a probe (lane c). The northern blotting pattern of rat glioma cells has been published previously.9
Figure 3.
Northern blot analysis of transfected and non-transfected human glioma cells. Lane a, parental cells; lane b, pMT-anti-IGF-I transfected HG1 cells; lane c, pMT-AG triple helix transfected HG1 cells; β actin blots are included as a control (β).
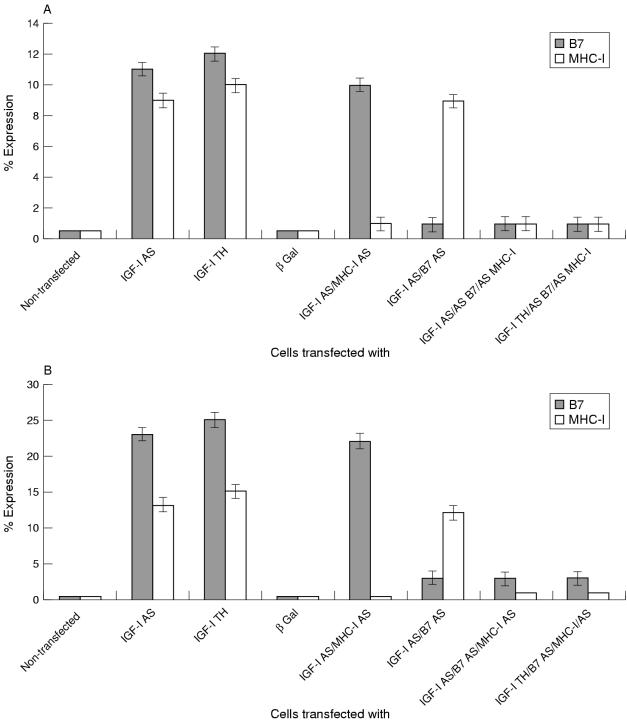
The expression of MHC-1 and B7 molecules on the surface of transfected and parental human glioma cells was determined by flow cytometry using monoclonal antibodies against the corresponding molecules. Transfection with either the pMT-anti-IGF-I or pMT-AG triple helix vector induced 13–15% and 23–25% increases in the expression of MHC-I and B7, respectively. Transfection with pMT-EP expressing the lac Z reporter gene or with vector expressing IGF-II in place of IGF-I antisense cDNA produced no increase in the expression of MHC-I or B7 molecules. When cotransfected with vectors encoding MHC-I or B7 antisense cDNA, the IGF-I antisense or triple helix transfected cells did not express MHC-I and B7 molecules above that seen in the parental cell lines (fig 4A ▶). The rat glioma cells treated in the same manner gave similar results (fig 4B ▶). The cells transfected with the vector expressing IGF-II in place of IGF-I antisense RNA or the vector expressing the lac Z reporter gene stained positively for IGF-I. We found no difference in MHC-I and B7 expression between irradiated and non-irradiated cells.
Figure 4.
Expression of major histocompatibility complex class I (MHC-I), B7, and insulin-like growth factor type I (IGF-I) in: (A) human glioma cells, (B) rat glioma cells. Cells were transfected and analysed by immunofluorescence (IGF-I) or flow cytometry (MHC-I and B7). The data are expressed as per cent change in value of fluorescence relative to fluorescence in control non-transfected cells and are an average of five experiments; the per cent increases in MHC-I and B7 are significant (p < 0.01). AS, antisense; TH, triple helix.
DETECTION OF APOPTOTIC CHANGES IN IGF-I DOWNREGULATED CELLS
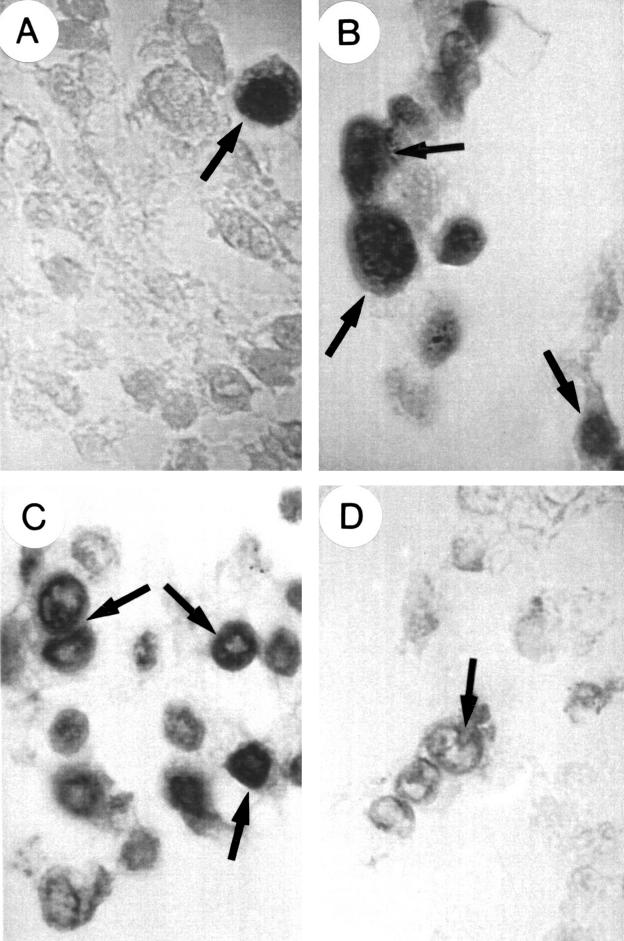
We detected apoptosis induced morphological changes in pMT-anti-IGF-I and pMT-AG triple helix transfected cells using the May-Grünwald-Giemsa technique and the TUNEL assay. Figure 5 ▶ shows individual apoptotic bodies and rosettes formed by smaller apoptotic elements surrounding the nucleus. Earlier changes indicative of apoptosis in transfected cells were demonstrated by the TUNEL assay. Incorporation of fluorescein conjugated dUTP was detected in approximately 60–70% of the IGF-I antisense and triple helix transfected cells. In cultures of both IGF-I antisense and triple helix transfected cells, approximately 40–50% of the cells became rounded and detached. These cells were all apoptotic. In contrast, evidence of apoptosis was observed in less than 5–10% of non-transfected cells. The IGF-I antisense or triple helix cell cultures cotransfected with either MHC-I or B7 antisense expressing vectors also showed 60–70% apoptotic cells, and those transfected with both anti-MHC-I and anti-B7 vectors showed lower numbers (20–30%) of apoptotic cells. These results were confirmed in seven separate experiments (p = 0.01). These results are in agreement with electron micrograph images of IGF-I antisense and triple helix transfected cells and with cells cotransfected with MHC-I and/or antisense vectors (fig 6 ▶). The apoptotic changes occurred within five to six hours of incubation of transfected cells in the presence of 60 μM ZnS04.
Figure 5.
Apoptosis detection in human glioma cells using the dUTP fluorescein terminal transferase labelling of nicked DNA technique (characteristic examples are marked by arrows). (A) Non-transfected cells; (B) insulin-like growth factor type I (IGF-I) antisense transfected cells; (C) IGF-I triple helix transfected cells; (D) IGF-I triple helix cells cotransfected with vectors encoding major histocompatibility complex class I (MHC-I) antisense and B7 antisense cDNA. Note the incorporation of labelled dUTP (dark staining with DAPI and fluorescein isothiocyanate) in apoptotic cells showing chromatin fragmentation: these cells are very rare in non-transfected cells (A), but are numerous in IGF-I antisense and triple helix transfected cells (B,C). On the contrary, apoptosis is infrequent in IGF-I triple helix cotransfected cells (D).
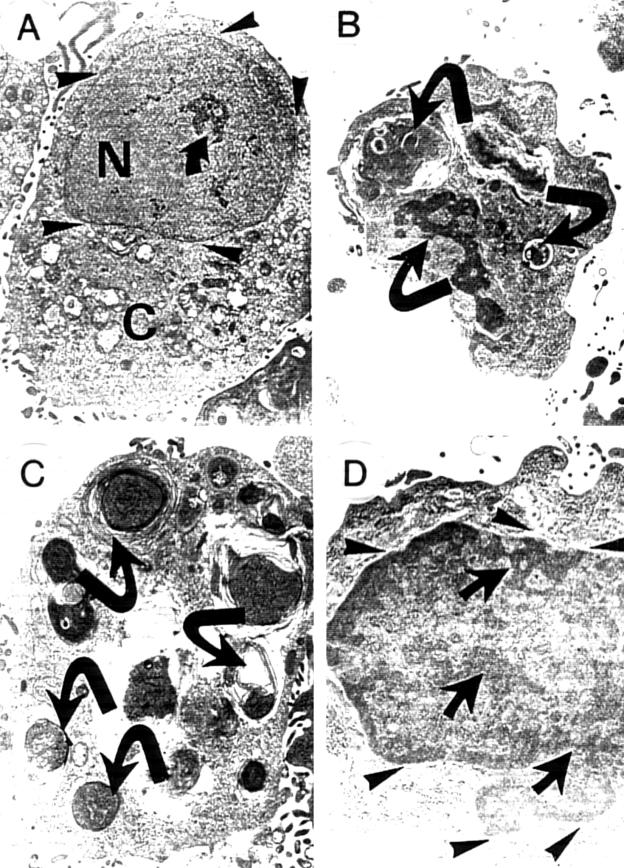
Figure 6.
Ultrastructural characteristics of human glioma cells: (A) parental, non-transfected cells; (B) insulin-like growth factor type I (IGF-I) antisense transfected cells; (C) IGF-I triple helix transfected cells; and (D) IGF-I triple helix cells cotransfected with vectors encoding major histocompatibility complex class I (MHC-I) antisense and B7 antisense cDNA. (A) the parental cell (N, nucleus; C, cytoplasm) with well developed nuclear envelope (arrowheads). The nucleus is uniform and well separated from the cytoplasm, and sometimes contains small islets of condensed electron dense chromatin (arrow); (B) and (C) transfected cells have the characteristic morphology of apoptotic cells, with nuclei that are distorted as a result of the formation of apoptotic bodies, and condensed electron dense chromatin forming, often with nuclear vesicularisation (arrows); the nuclear envelope is destroyed. (D) IGF-I triple helix cotransfected cells: the nuclear envelope is well conserved (arrowheads). There are few characteristics of apoptosis, although some condensed electron dense chromatin is present (arrows). Moreover, the proportion of apoptotic cells is much lower than is seen in the IGF-I triple helix cells. Original magnification, ×7000.
Discussion
Antisense strategies have been used successfully to suppress a growing number of genes in cultured glial cells. For example, the growth of transformed human astrocytes has been inhibited by antisense RNA to basic fibroblast growth factor,45 and the antisense inhibition of glial S100 protein has been used to alter cell proliferation and cytoskeletal organisation.46
Gene therapies that modify or suppress the expression of specific genes have been termed “anti-gene” strategies.8 For example, in the triple helix strategy, the inhibition of the initiation of gene transcription can be achieved with TFOs targeted upstream of the transcriptional start site within the region recognised by transcriptional activators.47 Genes such as the EGF receptor, or the c-myc, HER2 , H-ras, or c-ki-ras oncogenes have been blocked effectively by the formation of a triple helix structure with the respective TFO.31, 48, 49
The results of the triple helix strategy presented here show that, in cultured human primary glioma or rat C6 cells, an RNA strand containing a 23 nucleotide oligopurine sequence10 might be capable of forming a triple helix with an oligopurine–oligopyrimidine sequence of the IGF-I gene. Although we cannot exclude other mechanisms, triple helix formation remains the most plausible explanation for the inhibition of expression of the IGF-I gene.10 Downregulation of cellular IGF-I mRNA is accompanied by a decrease of cellular IGF-I. Transcription of the IGF-I gene is downregulated for four to six passages in rat or human glioma cells transfected with triple helix vector. In control experiments using the same vector carrying the lac Z gene in place of the TFO or IGF-I antisense DNA sequence, we found a 15% transgene frequency 48 hours after transfection (100% after three weeks) and strong cytoplasmic staining for X-gal in rat C6 cells. In human glioma cells, the transfection frequency was 4–5% after 48 hours (100% after four weeks), which is acceptable when dealing with primary cultures.
The induction of the IGF-I antisense or IGF-I triple helix structure in transfected cells was followed by changes in cell morphology, increases in apoptosis, and enhanced expression of MHC-I and B7. Transfected cells were long and narrow in shape and frequently string-like in appearance. This change was associated with a reduction in synthesis of the IGF-I protein. The change might be indicative of either a reversion of the malignant phenotype or the recovery of some antigenic potential of these cells.
However, the IGF-I antisense transfected cells, when cotransfected with vectors encoding MHC-I and/or and B7 antisense cDNA, maintained their previous IGF-I antisense morphology; the number of apoptotic cells in the cultures of the double cotransfected IGF-I antisense glioma cells decreased from 60–70% to 20–30%. This suggests that there could be an association between immunogenicity and apoptosis in IGF-I transfected cells. These results also indicate that both antigens, B7 and MHC-I, are necessary to render the IGF-I antisense or triple helix glioma cells immunogenic. The role of both B7 and MHC-I antigens in the induction of T cell immunity against tumours has been investigated extensively.50–54 As far the appearance of B7 in IGF-I antisense or triple helix transfected cells is concerned, the absence of IGF-I synthesis would be expected to lead to a higher activation of the IGF-I receptor (tyrosine kinase). This in turn could lead to the induction of the expression of the B7 antigen: enhancement of B7 costimulation through a cAMP mechanism linked to the tyrosine kinase activity of the CD28 receptor has been reported previously.55 Whether or not similar signalling occurs through the tyrosine kinase activity of the IGF-I receptor will need to be investigated.36, 55, 56 The IGF-I induced downregulation of MHC-I has been reported in rat thyroid cells.57 This is in agreement with our results, which find an inverse correlation between IGF-I and MHC-I protein expression in glioma cells.
In tumour cells, the absence of IGF-I, when induced by IGF-I antisense technology, is associated with massive apoptosis, as demonstrated previously in transfected hepatoma cells.40 In the work presented here, the occurrence of apoptotic bodies with distinctive morphological features (vacuolisation of cytoplasm and chromatin condensation) in the triple helix and antisense transfected clones suggests that IGF may be involved in modulating the apoptotic process of glioma cells. Nuclear fragmentation is probably the result of calcium dependent endonuclease activity in the linker region between nucleosomes.58, 59 Another characteristic of the apoptotic pathway is that integrity of the plasma membrane was preserved. Most functions of the membrane remained unchanged.59
IGF-I has been reported to block the apoptotic pathway in a variety of cell lines.60 Rats given injections of C6 glioma cells expressing antisense IGF-I receptor did not develop tumours and were protected from a subsequent challenge with wild-type C6 glioma cells for at least three months.61 However, pre-existing wild-type tumours were not totally eradicated.61 In another study, a qualitative relation between the expression of the IGF-I receptor and tumorigenesis in nude mice, which correlated with the extent of apoptosis, was shown.44 When the activity of the IGF-I receptor is decreased, glioma cells undergo massive apoptosis. Thus, it was concluded that activation of the IGF receptor by its ligand prevents programmed cell death. This effect was even more striking in vivo than in vitro.44 Another possible interpretation could be that an immune response occurring in the animals inhibits tumorigenesis: nude mice have a residual immune system comprising both natural killer cells and B cells.62
Using rat and mouse hepatocarcinoma cells expressing IGF-I,35, 39 it was shown that selected cell clones “disrupted” for IGF-I expression but expressing MHC-I can induce an immune response towards parental tumours (B7 is never expressed in IGF-I antisense transfected hepatoma cells). Using another model of cells expressing IGF-I, mouse teratocarcinoma PCC3 cells, we have shown that IGF-I antisense PCC3 cells express both B7 and MHC I molecules.34 Only selected PCC3 cell clones, which are disrupted for IGF-I expression using the antisense approach and express both immunogenic MHC-I and B7 molecules, can induce an immune response towards the parental tumour.21 In our study, we found that PCC3 cells transfected with vectors inducing IGF-I RNA–DNA structures (triple helix) stopped producing IGF-I, changed phenotype, expressed MHC-I and B7 molecules, and also showed increased apoptosis (at least 60% of cells). IGF-I triple helix transfected cells injected subcutaneously into syngeneic 129 mice lost tumorigenicity. In addition, these injected cells suppressed the established teratocarcinoma in vivo. The same triple helix cells when cotransfected in vitro with vectors encoding both MHC-I and B7 antisense cDNA stopped expressing MHC-I and B7; moreover, the number of apoptotic cells was significantly reduced. The injection of these double cotransfected cells into 129 Sv mice resulted in the development of the teratocarcinoma tumour. IGF-I triple helix teratocarcinoma cell clones, which did not express the MHC-I and B7 molecules or in which the expression of MHC-I and B7 was disrupted using MHC-I and B7 antisense vectors, were unable to suppress the growth of the corresponding established tumours in vivo.
Our results show that the inhibition of IGF-I upregulates B7 and MHC-I expression in transfected triple helix glioma cells. Increased expression of protease nexin I, which may reduce the tumorigenic potential of the C6 glioma cells, was also observed when the IGF-I triple helix cells or IGF-I receptor triple helix cells were injected into nude mice.10, 63 In contrast, in our work on mouse hepatoma cells transfected using the IGF-I triple helix approach, we found a decrease in cytokines such as interleukin 10, which is a strong immunosuppressor,64 and tumour necrosis factor α, which can act to stimulate tumour growth.65 Moreover, we have found increased concentrations of TAP 1 and 2 in these cells. Further investigations of the relation between the immune process (with regard to MHC-I or the HLA system)66 and the apoptotic process is under investigation. Recently, it was shown that dendritic cells, which are involved in the mechanisms of tumour immunogenicity by the activation of CD8+ T cells in the context of MHC-I, recognise apoptotic cells.67
The relation between the two phenomena—immunogenicity and apoptosis—is crucial to discussions on the mechanism of IGF-I triple helix gene therapy. Moreover, this point is of utmost importance in the selection of the cell clones to be used in gene therapy of glioblastoma; our observations show that IGF-I triple helix cells should express both MHC-I and B7 molecules, and be apoptotic (70% of cultured cells). Data obtained in the rat glioma model mimic those obtained for human glioma cells: the establishment in our laboratories of IGF-I triple helix human cell lines derived from patients with glioma constitutes the beginning of preclinical studies of cellular therapy for glioblastoma using IGF-I anti-gene technology.
Acknowledgments
We are especially grateful to Professors R Baserga (Thomas Jefferson University, Philadelphia, USA), M Fellous (Institut Pasteur, Paris, France), D Bout (University of Tours, Fance), and J-Y Delattre and P Evrard (INSERM, Paris, France) for useful discussion of experimental results. We thank Professor J Ilan (Case Western Reserve University, Cleveland, Ohio, USA) and Dr M Laburth (INSERM, Paris, France) for generously facilitating the molecular biology technology, and Dr LC Upegui-Gonzalez (University, Paris XI, France) and Professor C Bouchaud (CNRS and Inter University Center of Electron Microscopy, Paris VI and VII, France) for helpful suggestions concerning the measurement of apoptosis. We are also indebted to C Bedel and F Pernin for technical assistance. This work was supported by grants from ARC Villejuif (number 1084), LCC Indre-et-Loire (JT 2001), and KBN Univ. Krakow and Univ. Bydgoszcz (JT 2001).
References
- 1.Culver KW, Rarn Z, Wallbridge S, et al. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science 1992;256:1550–2. [DOI] [PubMed] [Google Scholar]
- 2.Trojan J, Johnson TR, Rudin SD, et al. Loss of tumorigenicity of rat glioblastoma directed by episome-based antisense cDNA transcription of insulin-like growth factor 1. Science 1993;259:94–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Resnicoff M, Sell C, Rubini M, et al. Rat glioblastoma cells expressing an anti-sense RNA to the insulin-like growth factor I (IGF-I) receptor are non tumorigenic and induce regression of wild-type tumors. Cancer Res 1994;54:2218–22. [PubMed] [Google Scholar]
- 4.Ridoux V, Robert JJ, Zhang X, et al. Adenoviral vectors as functional retrograde neuronal tracers. Brain Res 1994;648:171–5. [DOI] [PubMed] [Google Scholar]
- 5.Izant JG, Weintraub H. Constitutive and conditional suppression of exogenous and endogenous genes by antisense RNA. Science 1985;229:345–52. [DOI] [PubMed] [Google Scholar]
- 6.Green P J, Pines O, Inouye M. The role of antisense RNA in gene regulation. Annu Rev Biochem 1986;55:569–97. [DOI] [PubMed] [Google Scholar]
- 7.Maher LJ, Wold B, Dervan PB. Analysis of promoter specific repression by triple-helical DNA complexes in a eukaryotic cell-free transcription system. Antisense Res Dev 1991;1:77–281. [DOI] [PubMed] [Google Scholar]
- 8.Héléne C, Garestier T, Giovannangeli C, et al. Sequence-specific control of gene expression by anti gene and clamp oligonucleotides. Ciba Found Symp 1997;209:94–102 [DOI] [PubMed] [Google Scholar]
- 9.Trojan J, Blossey BK, Johnson TR, et al. Loss of tumorigenicity of rat glioblastoma directed by episome-based antisense cDNA transcription of insulin-like growth factor 1. Proc Natl Acad Sci U S A 1992;89:4874–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shevelev A, Burfeind P, Schulze E, et al. Potential triple helix mediated inhibition of IGF-I gene expression significantly reduces tumorigenicity of glioblastoma in an animal model. Cancer Gene Ther 1997;4:105–12. [PubMed] [Google Scholar]
- 11.Heldin C-H, Westermark B. Growth factors as transforming proteins. Eur J Biochem 1989;184:487–96. [DOI] [PubMed] [Google Scholar]
- 12.Daughaday WH, Hall K, Raben MS, et al. Somatomedin: proposed designation for sulphation factor. Nature 1972;235:107. [DOI] [PubMed] [Google Scholar]
- 13.Froesch C S, Schwander J, Zapf J. Actions of insulin-like growth factors. Annu Rev Physiol 1985;47:443–67. [DOI] [PubMed] [Google Scholar]
- 14.Han VKM, D'Ercole A, Lund PK. Cellular localization of somatomedin (insulin-like growth factor) message RNA in human fetus. Science 1987;236:193–7. [DOI] [PubMed] [Google Scholar]
- 15.Baserga R. Oncogenes and strategy of growth factors. Cell 1994;79:927–30. [DOI] [PubMed] [Google Scholar]
- 16.Rubin R, Baserga R. Biology of disease. Insulin-like growth factor I receptor. Its role in cell proliferation, apoptosis and tumorigenicity. Lab Invest 1995;73:311–31. [PubMed] [Google Scholar]
- 17.Ayer-le-Lievre C, Stahlbom P A, Sara VR. Expression of IGF-I and -II mRNA in the brain and craniofacial region of the rat fetus. Development 1991;111:105–15. [DOI] [PubMed] [Google Scholar]
- 18.Sandberg AC, Engberg C, Lake M, et al. The expression of insulin-like growth factor I and insulin-like growth factor II genes in the human fetal and adult brain and in glioma. Neurosci Lett 1998;93:114–19. [DOI] [PubMed] [Google Scholar]
- 19.Antoniades HN, Galanopoulis T, Nevile-Golden J, et al. Expression of insulin like growth factor I and II and their receptor mRNAs in primary human astrocytomas and meningiomas: in vivo studies using in situ hybridization and immunocytochemistry. Int J Cancer 1992;50:215–22. [DOI] [PubMed] [Google Scholar]
- 20.Kiess W, Lee L, Graham DE, et al. Rat C6 glial cells synthesize insulin-like growth factor I (IGF-I) and express IGF-I receptors and IGF-ll/mannose 6-phosphate receptors. Endocrinology 1989;124:1727–36. [DOI] [PubMed] [Google Scholar]
- 21.El-Badry OM, Helman LJ, Chatten J, et al. Insulin-like growth factor II-mediated proliferation of human neuroblastoma. J Clin Invest 1991;87:648–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baserga R, Sell C, Porcu P, et al. The role of IGF-I receptor in the growth and transformation of mammalian cells. Cell Prolif 1994;27:63–78. [DOI] [PubMed] [Google Scholar]
- 23.Héléne C. Control of oncogene expression by antisense nucleic acids. Eur J Cancer 1994;30A:1721–6. [DOI] [PubMed] [Google Scholar]
- 24.Maher LJ, III. Prospects for the therapeutic use of antigene oligonucleotides. Cancer Invest 1996;14:66–82. [DOI] [PubMed] [Google Scholar]
- 25.Chan PP, Glazer PM. Triplex DNA: fundamentals, advances and potential applications for gene therapy. J Mol Med 1997;75:267–82. [DOI] [PubMed] [Google Scholar]
- 26.Vasquez KM, Wilson JH. Triplex-directed modification of genes and gene activity. Trends Biochem Sci 1998;23:4–9. [DOI] [PubMed] [Google Scholar]
- 27.Durland R, Kessler D, Gunnell S, et al. Binding of triple helix forming oligonucleotides to sites in gene promoters. Biochemistry 1991;30:9246–55. [DOI] [PubMed] [Google Scholar]
- 28.Postel EH, Flint SJ, Kessler DJ, et al. Evidence that triplex-forming oligodeoxyribonucleotide binds to the c-myc promoter in HeLa cells, thereby reducing c-myc mRNA levels. Proc Natl Acad Sci U S A 1991;88:8227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas T, Faaland C, Gallo M, et al. Suppression of c-myc oncogene expression by a polyamine complexed triplex forming oligonucleotide in MCF-7 breast cancer cells. Nucleic Acids Res 1995;23:3594–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebbinghaus S, Gee J, Rodu B, et al. Triplex formation inhibits HER-2/neu transcription in vitro. J Clin Invest 1993;92:2433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porumb H, Gousset H, Letellier R, et al. Temporary ex vivo inhibition of the expression of the human oncogene HER2 (NEU) by a triple helix-forming oligonucleotide. Cancer Res 1996;56:515–22. [PubMed] [Google Scholar]
- 32.Mayfield C, Ebbinghaus S, Gee J, et al. Triplex formation by the human Ha-ras promoter inhibits Spl binding and in vitro transcription. J Biol Chem 1994;269:18232–8. [PubMed] [Google Scholar]
- 33.Alunni-Fabbroni M, Pirulli D, et al. (A,G)-oligonucleotides form extraordinary stable triple helices with a critical R. Y. sequence of the murine c-Ki-ras promoter and inhibit transcription in transfected NIH 3T3 cells. Biochemistry 1996;35:16361–9. [DOI] [PubMed] [Google Scholar]
- 34.Trojan J, Johnson TR, Rudin SD, et al. Gene therapy of murine teratocarcinoma: separate functions for insulin-like growth factor I and II in immunogenicity and differentiation. Proc Natl Acad Sci U S A 1994;91:6088–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lafarge-Frayssinet C, Sarasin A, Duc HT, et al. Gene therapy for hepatocarcinoma: antisense IGF-I transfer into a rat hepatoma cell line inhibits tumorigenesis into syngeneic animal. Cancer Gene Ther 1997;4:276–85. [PubMed] [Google Scholar]
- 36.Trojan J, Duc H, Upegui-Gonzalez L, et al. Presence of MHC-I and B-7 molecules in rat and human glioma cells expressing antisense IGF-1 mRNA. Neurosci Lett 1996;212:9–12. [DOI] [PubMed] [Google Scholar]
- 37.Anthony D, Pan Y, Wu S, et al. Ex vivo and in vivo IGF-I antisense RNA strategies for treatment of cancers in humans. Adv Exp Med Biol 1998;451:27–34. [DOI] [PubMed] [Google Scholar]
- 38.Baserga R. The insulin-like growth factor I receptor: a key to tumour growth? Cancer Res 1995;55:249–52. [PubMed] [Google Scholar]
- 39.Upegui-Gonzalez LC, Duc HT, Buisson Y, et al. Use of the IGF-I antisense strategy in the treatment of the hepatocarcinoma. Adv Exp Med Biol 1998;451:35–42. [DOI] [PubMed] [Google Scholar]
- 40.Ellouk-Achard S, Djenabi S, De Oliveira GA, et al. Induction of apoptosis in rat hepatocarcinoma cells by expression of IGF-I antisense c-DNA. J Hepatol 1998;29:807–18. [DOI] [PubMed] [Google Scholar]
- 41.Muta K, Krantz S. Apoptosis of human erythroid colony forming cells is decreased by stem cell factor and insulin-like growth factor I as well as erythropoïetin. J Cell Physiol 1993;156:264–71. [DOI] [PubMed] [Google Scholar]
- 42.Harrington EA, Bennett MR, Fanidi A, et al. c-myc-induced apoptosis in fibroblasts is inhibited by specific cytokines. EMBO J 1994;13:3286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sell C, Baserga R, Rubin R. Insulin-like growth factor I (IGF-I) and the IGF-I receptor prevent etoposide-induced apoptosis. Cancer Res 1995;55:303–6. [PubMed] [Google Scholar]
- 44.Resnicoff M, Abraham D, Yutanawiboonchai W, et al. The insulin-like growth factor I receptor protects tumour cells from apoptosis in vitro. Cancer Res 1995;55:2463–9. [PubMed] [Google Scholar]
- 45.Morrison R S. Suppression of basic fibroblast growth factor expression by antisense deoxyribonucleotides inhibits the growth of transformed human astrocytes. J Biol Chem 1991;266:728–34. [PubMed] [Google Scholar]
- 46.Selinfreund RH, Barger S W, Welsh M J, et al. Antisense inhibition of glial S100 beta production results in alterations in cell morphology, cytoskeletal organisation and cell proliferation. J Cell Biol 1990;11:2021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Couture LA, Stinchcomb DT. Anti-gene therapy: the use of ribozymes to inhibit gene function. Trends Genet 1996;12:510–15. [DOI] [PubMed] [Google Scholar]
- 48.Gee JE, Yen RL, Hung MC, et al. Triplex formation at the rat neurooncogene promoter. Gene 1994;149:109–14. [DOI] [PubMed] [Google Scholar]
- 49.Vignasvaren NL, Mayfield C, Rodu B, et al. Influence of GC and AT specific DNA minor groove binding drugs on intermolecular triplex formation in the human c-ki ras promoter. Biochemistry 1996;36:1106–14. [DOI] [PubMed] [Google Scholar]
- 50.Linsley PS, Clark EA, Ledbetter JA. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/13. Proc Natl Acad Sci U S A 1990;87:5031–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Freeman GB, Gray GS, Gimmi CD, et al. Structure, expression and T cell costimulatory activity of murine homologue of the human B lymphocyte activation antigen B7. J Exp Med 1991;174:625–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen L, Ashe S, Brady WA, et al. Costimulation of anti-tumour immunity by the B7 counter receptor for the T lymphocyte molecules CD28 and CTLA-4. Cell 1992;71:1093–102. [DOI] [PubMed] [Google Scholar]
- 53.Harding F, Mc Arthur JG, Gross JA, et al. CD28-mediated and signalling co-stimulates murine T cells and prevents induction of anergy in T cell clones. Nature 1992;356:607–9. [DOI] [PubMed] [Google Scholar]
- 54.Guo Y, Wu M, Chen H, et al. Effective tumour vaccine generated by fusion of hepatoma cells with activated B cells. Science 1994;263:518–20. [DOI] [PubMed] [Google Scholar]
- 55.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4 and B7/BBI in interleukin-2 production and immunotherapy. Cell 1992;71:1065–8. [DOI] [PubMed] [Google Scholar]
- 56.Satoh J, Lee YB, Kim SU. T-cell costimulatory molecules B7-1 (CD80) and B7-2 (CD86) are expressed in human microglia but not in astrocytes in culture. Brain Res 1995;704:95–6. [DOI] [PubMed] [Google Scholar]
- 57.Saji M, Moriarty J, Ban T, et al. Major histocompatibility complex class I gene expression in rat thyroid cells is regulated by hormones, methimazole and iodide as well as interferon. J Clin Endocrinol Metab 1992;75:871–8. [DOI] [PubMed] [Google Scholar]
- 58.Orrenius S, McConkey DJ, Bellomo G, et al. Role of calcium in toxic cell killing. Trends Pharmacol Sci 1989;7:281–5. [DOI] [PubMed] [Google Scholar]
- 59.Wyllie AH, Arends MJ, Morris RG, et al. The apoptosis endonuclease and its regulation. Semin Immunol 1992;4:389–98. [PubMed] [Google Scholar]
- 60.Rodriguez-Tarduchy G, Collins MKL, Garcia I, et al. Insulin-like growth factor-I inhibits apoptosis in IL-3-dependent hemopoietic cells. Eur J Immunol 1992;149:535–40. [PubMed] [Google Scholar]
- 61.Resnicoff M, Sell C, Rubini M, et al. Rat glioblastoma cells expressing an anti-sense RNA to the insulin-like growth factor I (IGF-I) receptor are non tumorigenic and induce regression of wild-type tumour. Cancer Res 1994; 54:2218–22. [PubMed] [Google Scholar]
- 62.Taghian A, Bucach W, Zietman A, et al. Quantitative comparison between the transplantability of human and murine tumors into the brain of NCr/Scd-nu/nu nude and severe combined immunodeficient mice. Cancer Res 1993;53:5018–21. [PubMed] [Google Scholar]
- 63.Rininsland F, Johnson T, Chernicky C, et al. Suppression of insulin-like growth factor type I receptor by a triple helix strategy inhibits IGF-I transcription and tumorigenic potential of rat C6 glioblastoma cells. Proc Natl Acad Sci USA 1997;94:5854–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gerard CM, Bruyns C, Delvaux A, et al. Loss of tumorigenicity and increased immunogenicity induced by interleukin-10 gene transfer in B16 melanoma cells. Hum Gene Ther 1996;7:23–31. [DOI] [PubMed] [Google Scholar]
- 65.Buck C, Digel W, Schoniger W, et al. Tumour necrosis factor alpha but not lymphotoxin, stimulates growth of tumour cells in hairy cell leukemia. Leukemia 1990;4:431. [PubMed] [Google Scholar]
- 66.Blanchet O, Bourge JF, Zinszner H, et al. Altered binding of regulatory factors to HLA class I enhancer sequence in human tumour cell lines lacking class I antigen expression. Proc Natl Acad Sci U S A 1992;89:3488–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matthew L, Saiter B, Bhardwag N. Dendritic cells acquire antigen from apoptotic cells and induce class I restricted CTL. Nature 1998;392:86–9. [DOI] [PubMed] [Google Scholar]