Abstract
Aims—To study the expression of the endothelial and inducible isoforms of nitric oxide synthase (eNOS and iNOS, respectively) in human bladder carcinoma and schistosomal bladder disease, and to compare it with normal adult and fetal urothelium. Nitric oxide is thought to play a complex role in human carcinogenesis, but has only recently been investigated in bladder cancer.
Methods—Immunohistochemistry was performed on paraffin wax embedded sections of 33 human bladder carcinomas and five bladder carcinoma cell lines; in addition, seven schistosomal bladder cases and normal and fetal urothelium were investigated. In the cell lines enzymatic activity was examined by the NADPH diaphorase reaction.
Results—Immunoreactivity for eNOS was present in most cells of all 31 cases examined. Immunoreactivity for iNOS was less abundant and was seen in 23 of 25 cases. Similar findings were noted in schistosomal bladder cancer. In the normal bladder mucosa, eNOS immunoreactivity was found only in the superficial cell layer and iNOS was not expressed, whereas in the fetal urothelium immunoreactivity for both isoforms was seen in all cell layers. Enzymatic activity and immunoreactivity for eNOS and iNOS were evident in the five bladder carcinoma cell lines.
Conclusions—It is possible that NOS plays a role in the differentiation of the transitional epithelium in fetal life, has a biological function in the adult bladder mucosa, and is involved in bladder carcinogenesis. eNOS and iNOS immunoreactivity do not differ in schistosomal and non-schistosomal bladder carcinoma, but resemble the pattern of expression typical of fetal urothelium.
Keywords: bladder cancer, nitric oxide synthase, schistosoma
Nitric oxide (NO) is a small messenger molecule that was first discovered as a potent vasodilator, known as the endothelium derived relaxing factor, produced and released by vascular endothelial cells.1, 2 It is now well established that NO has several diverse biological functions, and is produced by many cell types other than endothelium.3, 4
The NO radical is generated by the action of the enzyme NO synthase (NOS). There are three distinct isoforms of this enzyme, encoded by three different genes. Two of the NOS isoforms are constitutive and calcium/calmodulin dependent—the endothelial and neuronal types (eNOS and nNOS, respectively); the third is inducible (iNOS), and is not dependent upon calcium/calmodulin for its enzymatic action.5, 6
Several reports on the possible role of NOS in neoplasia have been published recently. In colonic carcinomas and preneoplastic lesions a pronounced reduction in NOS activity, demonstrated by histochemistry, was found relative to normal colonic mucosa.7 In human colon cancer cell lines a diverse pattern of NOS gene expression, not always followed by NO generation, was found.8 In gynaecological cancer, constitutive NOS activity and immunoreactivity were inversely related to tumour differentiation.9 In breast cancer, the inducible and constitutive isoforms of NOS were found to be expressed in cellular elements other than the epithelial tumour cells.10, 11 This expression was correlated with tumour grade and metastatic potential.
We have previously shown prominent immunoreactivity for eNOS in trophoblastic cells of the normal placenta, an organ that manifests many characteristics of a neoplastic process, and in trophoblastic neoplasia.12 This prompted us to investigate eNOS in human bladder carcinoma, a type of cancer that we have studied in recent years.13, 14
Bladder carcinoma is the fourth most prevalent type of cancer in men in Western society. The main aetiological factors are exposure to industrial carcinogens and cigarette smoking.15 Another major aetiological factor is infestation by the parasite Schistosoma haematobium. This parasite is endemic in Africa and the Middle East. The adult worms reside in the venules of the urinary bladder where they lay the eggs. These elicit a pronounced inflammatory reaction and fibrosis in the bladder wall, and initiate hyperplasia of the bladder mucosa, followed by neoplastic transformation.15, 16
It has been postulated that NO produced by iNOS in the inflammatory process in schistosomal infection causes damage to genomic DNA at CpG rich sites, and may be the mechanism of induction of carcinogenesis in bladder carcinoma in infected individuals.17 Recently, iNOS expression has been documented in the inflammatory cells and in isolated tumour cells in bladder carcinoma,18 but the expression of NOS in schistosomal bladder cancer has not been studied. The inducible isoform of NOS was identified in the human urine pellet, and NOS activity was increased in urinary tract infections and decreased in interstitial cystitis.19 However, eNOS and its physiological function in the normal mucosa have not been investigated, and its role in bladder carcinogenesis has never been studied.
The purpose of our study was to screen a small series of bladder carcinomas for NOS isoform expression and to compare it with that seen in normal adult and fetal bladder mucosa as a first step to determine the possible role of these isoforms in bladder carcinogenesis. We have shown prominent immunoreactivity for eNOS and less intense immunoreactivity for iNOS in human bladder carcinoma and in bladder carcinoma cell lines. We have also demonstrated enzymatic activity by NADPH diaphorase. Similar findings were present in a small number of bladder carcinomas induced by schistosomiasis. The pattern of eNOS and iNOS immunoreactivity resembles that of the fetal bladder mucosa, and is different from normal adult urothelium.
Materials and methods
HUMAN BLADDER MUCOSA AND CANCER
Paraffin wax blocks of formalin fixed tissues were selected from the files of the department of pathology at the Hadassah University Hospitals. These included the following specimens: (1) Fetal bladder mucosa from five fetuses at the second trimester of pregnancy (22–26 weeks of gestation according to fetal measurements). These fetuses were aborted for therapeutic reasons (premature rupture of membranes, maternal drug abuse, and fetal malformations other than the genitourinary system). (2) Normal adult bladder mucosa from four patients. (3) First biopsies of 33 patients with transitional cell carcinomas of the urinary bladder. The cases were reviewed and graded blindly by one author (GP), a specialist in uropathology, according to the WHO grading system.20 (4) Biopsies from three patients with schistosoma induced bladder cancer and four patients with evidence of schistosomal disease without cancer.
The paraffin wax blocks were sectioned at 5 μm and mounted on SuperFrost Plus slides (Menzel-Glaser, Braunschweig, Germany). The slides were dried at 60°C for one hour and processed for immunohistochemical staining.
BLADDER CARCINOMA CELL LINES
Human bladder carcinoma cell lines (UM-UC-3, HT-1376, EJ 28, HT-1197, and T24) were obtained from the ATCC. They were maintained in DMEM-F 12 (1/1) medium (Gibco BRL, Paisley, UK) containing 10% fetal calf serum (inactivated at 55°C for 30 minutes), 25mM Hepes (pH 7.4) (Sigma, St Louis, Missouri, USA), penicillin (180 U/ml), streptomycin (100 μg/ml), and amphotericin B (0.2 μg/ml). The lines were plated at 4 × 104 cells/cm3 in polystyrene culture dishes (Nunc, Roskilde, Denmark). The cells were trypsinised every four days with 0.05% trypsin/EDTA solution (Biological Ind, Beit Haemek, Israel) for 15 minutes, and re-plated at the same initial density. For this experiment, 10 × 104 cells were seeded in each chamber on chamber slides (Nunc) in the same medium. After 48 hours the cells were washed in phosphate buffered saline (PBS) and fixed on the slides using a buffered mixture of acetone and formaldehyde (pH 6.6; 4°C) for 10 minutes. The cells were then washed again in PBS, and the slides were kept at 4°C for immunohistochemistry. Each cell line was grown in duplicate both for the study and negative control.
IMMUNOHISTOCHEMISTRY
Immunohistochemical detection of NOS isoforms on paraffin wax embedded sections was performed using rabbit polyclonal antiserum developed against a 20.4 kDa protein fragment corresponding to amino acids 1030–1209 of human eNOS, and a rabbit polyclonal antiserum developed against a 21 kDa fragment corresponding to residues 961–1144 of mouse iNOS, also active against human iNOS (Transduction Laboratories, Lexington, Kentucky, USA). Visualisation of staining for NOS was by means of the streptavidin–biotin immunoperoxidase technique using the Histostain SP kit (Zymed, San Francisco, California, USA), according to the manufacturer's instructions.
As a control, the same procedure was performed in the absence of the primary antibody. At least one control was undertaken in each experiment.
NADPH DIAPHORASE HISTOCHEMISTRY
Human bladder carcinoma cell lines UM-UC-3, HT-1376, EJ 28, HT-1197, and T24 were grown on chamber slides as described above. After 48 hours the cells were washed with PBS and fixed on the slides in 4% paraformaldehyde for two hours. The slides were then washed three times for 10 minutes with 20% sucrose in PBS azide and kept overnight in the same solution. The slides were then dried and kept at −70°C. The slides were brought to room temperature and washed three times for 10 minutes in 0.1M phosphate buffer at pH 7.4.21 Next, the slides were preincubated for 20 minutes in 0.1M Tris buffer (pH 7.4) containing 0.3% Triton X-100, followed by incubation with 0.3% Triton X-100, 60μM reduced β-NADPH (Sigma), and 25μM nitroblue tetrazolium (Sigma) diluted in Tris buffer for 30 minutes at 37°C. The slides were washed three times in 0.1M phosphate buffer (pH 8.0), dehydrated in alcohols in ascending concentrations, cleared in xylene, and mounted in mounting medium. As a negative control, the reaction was carried out omitting the NADPH from the reaction mixture. This reaction gives a blue product that is viewed in the absence of background staining.
MICROSCOPICAL EVALUATION AND STATISTICAL ANALYSIS
The slides were examined by conventional light microscopy. The amount and cell type exhibiting eNOS and iNOS immunoreactivity were recorded for each case. The amount of immunostaining was evaluated as +1 for immunostaining in more than 5% but less than one third of the cells, +2 for immunostaining in more than a third but less than two thirds of the cells, and +3 for immunostaining in more than two thirds of the tumour cells.
The data were analysed using non-parametric methods. The associations of eNOS and iNOS with the stage and grade of the tumour were tested both by the Jonckheere-Terpstra test and the one way Kruskal-Wallis analysis of variance. The first test is powerful when the alternative to the null hypothesis of equal distributions is ordered, whereas the second test is preferable for unordered alternatives. The p values reported are exact and not asymptotic. The correlation coefficients reported are Spearman's ρ values. The analyses were carried out using SPSS for Windows (version 9.0) and StarXact (version 4.0).
Results
NOS isoform immunoreactivity is localised in the cytoplasm of the cells expressing the protein. The staining is usually granular, but can also be diffuse. In normal bladder mucosa immunoreactivity for eNOS was noted in the most superficial cell layer, also designated umbrella cells, and was not detected in the deeper layers (fig 1A ▶). iNOS immunoreactivity was not seen. In the fetal bladder (20–26 weeks of gestation) immunoreactivity for eNOS and iNOS was noted in all the layers of the urinary bladder mucosa. A similar staining pattern was seen in the transitional epithelium of the ureters, renal pelvis and calyces, and the epithelium of the collecting ducts (which is also of mesonephric origin) (fig 1B, C ▶). At 23–24 weeks gestation, immunoreactivity for iNOS was stronger in the umbrella cells and at 26 weeks it was limited to the umbrella cells of the ureter, whereas staining for eNOS was diffuse, as in the earlier stage.
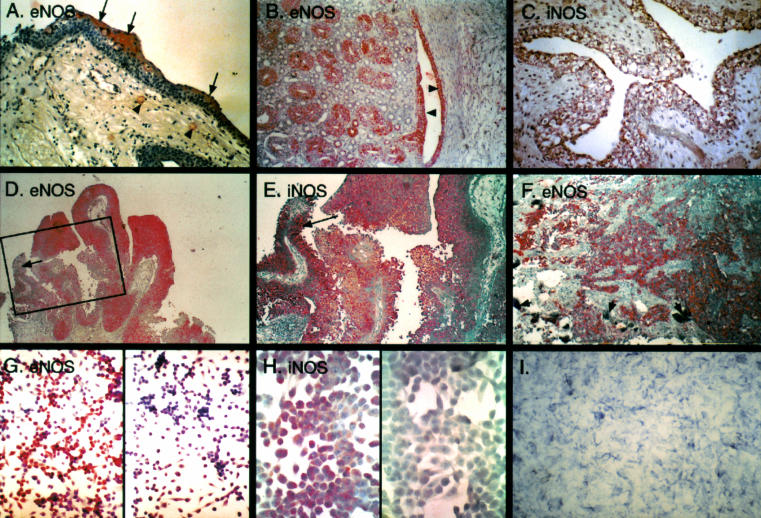
Figure 1.
Nitric oxide synthase (NOS) in bladder cancer. (A) Normal bladder mucosa. Immunoreactivity for endothelial NOS (eNOS) is confined to the umbrella cells comprising the superficial layer (arrows). Positive staining is also noted in capillaries in the submucosa (arrowheads). (B) eNOS immunoreactivity in the fetal urothelium. Diffuse positive staining is seen in the urothelium of the renal pelvis (arrowheads) and in the epithelium of the collecting tubules (on the left side of the picture). (C) Diffuse inducible NOS (iNOS) immunoreactivity of the fetal bladder urothelium. (D) Immunoreactivity for eNOS in papillary transitional cell carcinoma of the urinary bladder. Most of the cells are positively stained. (E) Positive immunoreactivity for iNOS in papillary transitional cell carcinoma of the urinary bladder. Compare with negative eNOS immunoreactivity in the inset in D, which represents the same area (arrows). (F) Prominent immunoreactivity for eNOS in invasive bladder cancer induced by schistosomal infection. Degenerated eggs of the parasite are seen in the bladder wall (short arrows). (G) eNOS immunoreactivity in the UM-UC-3 bladder carcinoma cell line. The negative control is on the right side. (H) iNOS immunoreactivity in the UM-UC-3 bladder carcinoma cell line. The negative control is on the left side. (I) Positive histochemical reaction with NADPH diaphorase demonstrating enzymatic activity of NOS (EJ28 bladder carcinoma cell line). The reaction gives a blue product in the absence of background staining.
First biopsies of 33 patients with non-schistosomal bladder carcinoma were included in our study. The patients' age ranged from 32 to 89 years, with a mean (SD) of 68 (12.6) years (median, 69). Grade I carcinoma was diagnosed in 15 patients, grade II in 10, and grade III in eight. In 22 patients, the tumour was non-invasive, in six the carcinoma invaded the submucosa, and five patients had muscle invasive tumours.
Immunoreactivity for eNOS was observed in all 31 bladder carcinomas examined for this isoform (14 grade I, 10 grade II, and seven grade III) (fig 1D ▶). In 20 of 31 biopsies, eNOS immunoreactivity was detected in more than two thirds of the tumour cells. Statistical analysis did not reveal a significant difference between the different grades and stages with regard to the strength of the staining.
iNOS immunoreactivity was evident in 23 of 25 biopsies examined (12 grade I, nine grade II, and four grade III) (fig 1E ▶). However, immunoreactivity was detected in more than two thirds of the tumour cells only in four of these biopsies, whereas in almost half of the cases (11 of 23) it was evident in less than one third of the cells. As for eNOS, no significant difference between grades and stages was found with regard to the amount of staining.
Israel is geographically close to an endemic region of S heamatobium, and therefore we have a small number of bladder schistosomiasis cases. In our three cases of schistosoma induced bladder carcinoma, prominent immunoreactivity for eNOS was demonstrated in most cancer cells (fig 1F ▶), whereas moderate immunoreactivity for iNOS was observed in two of the three cases. This was similar to our findings in the conventional bladder carcinomas.
In the four bladder biopsies, diagnosed as schistosomal disease without neoplastic transformation, immunoreactivity for eNOS confined to the umbrella cells (as in the normal mucosa) was seen in one case. In another case, the superficial cell layer was missing and no staining was seen; weak positive staining in all cell layers of the mucosa was seen in one of the remaining two cases with urothelial hyperplasia. Immunoreactivity for iNOS was observed in only one case with mucosal hyperplasia.
It should be emphasised that in many cases the staining for eNOS and iNOS was present in different regions of the biopsy, indicating that there was no crossreactivity between the antibodies used (compare inset of fig 1D ▶ with fig 1E ▶).
The results were similar for bladder carcinoma cell lines. Moderate to prominent immunoreactivity for eNOS was evident for all cell lines examined (fig 1G ▶), whereas iNOS was less prominent (fig 1H ▶). The enzymatic activity of NOS, as demonstrated by the NADPH diaphorase histochemical stain, was evident in all cell lines examined (fig 1I ▶), but did not directly parallel the immunoreactivity (table 1 ▶). No coloured product was seen when the reaction was carried out omitting the NADPH from the reaction mixture.
Table 1.
Nitric oxide synthase (NOS) immunoreactivity and enzymatic activity in five bladder carcinoma cell lines
| UM-UC-3 | EJ28 | T24 | HT-1197 | HT-1376 | |
| Endothelial NOS | ++ | ++ | ++ | ++ | + |
| Inducible NOS | +/++ | +/− | + | +/− | + |
| NADPH diaphorase | + | +++ | ++ | ++ | ++/+++ |
Discussion
Our findings indicate that NO has a biological function in the normal bladder mucosa, may play a role in bladder carcinogenesis, and is also linked to the development and differentiation of the transitional epithelium in fetal life. Further investigation is required to elucidate the role of NO in these biological systems.
The mature transitional epithelium that lines the urinary tract is noteworthy for the presence of a superficial layer of umbrella cells, which is in direct contact with the contents of the bladder. These cells are unique because they exhibit strikingly low permeability to fluxes of water, urea, and ammonia, in contrast to other cell membranes.22 Another special characteristic of the umbrella cells is their ability to distend. This last feature is the result of the presence of modified areas of plasma membrane, known as plaques, which fold inward as fusiform vesicles in an empty bladder and return to the surface when the bladder is distended.23
The production of NO by the urothelium has been established,24 but the production has not been anatomically localised to a specific cell type. In our study, we show for the first time that eNOS is localised to the umbrella cells of the normal adult urothelium. Decreased iNOS activity and concentrations of cGMP were found in the urine pellet in interstitial cystitis.19 However, our findings may indicate that disruption of the urinary bladder permeability barrier in non-infectious cystitis may be attributed to sloughing of the superficial layer of the urothelium and damage to the apical membrane present in this condition.25 Whether the production of NO in the umbrella cells is linked to their osmotic barrier function or to their special property of bladder distension (or both) needs further investigation.
The biological role of NO in the fetal urothelium is even more enigmatic. The functions of eNOS during the fetal and neonatal periods have been investigated mainly with regard to the circulation of the placenta in normal and pathological conditions26 and haemodynamics of fetal organs such as the lungs27 and kidneys.28 Our preliminary findings suggest that eNOS also has some (yet to be determined) functions in the mucosae of the urinary and digestive tracts (M Sochina and I Ariel, 1998, unpublished results). In the human fetus, differentiation to umbrella cells is first recognised by electron microscopy at 21 weeks of gestation.29 Our results suggest that both iNOS and eNOS are distributed in early stages in all layers of the fetal urothelium. At 26 weeks of gestation, iNOS becomes confined to the superficial cell layer, and presumably at a later stage towards maturation iNOS is no longer expressed and eNOS is expressed only in the umbrella cells.
The role of NO in carcinogenesis is complex. NO has both facilitatory and inhibitory effects on tumour growth.30 The production of NO in cancer may be induced in macrophages that have antitumour activity through the inhibition of mitochondrial respiration and DNA synthesis in tumour cells.31 The production of NO in tumour cells may induce apoptosis and thus be detrimental to their survival.32
In contrast, there is a growing body of evidence to suggest a conductive role for NO in tumour progression and metastasis. NO is converted to peroxynitrite in the presence of the superoxide anion. This is a highly potent toxic molecule that causes DNA damage and is therefore potentially carcinogenic.33 Tumour grade and metastatic potential were found to be positively correlated with NO production by tumour cells or other cellular elements in the tumour in gynaecological, breast, and lung cancer.9–11, 34
The different spectrum of DNA mutations in bladder cancer induced by schistosomiasis in comparison with non-schistosomal bladder carcinoma has been attributed to DNA damage caused by NO released by the enzyme iNOS from macrophages in the inflammatory infiltrate reactive to the parasite eggs.17 However, this hypothesis was not tested. iNOS expression was demonstrated recently not only by the inflammatory cells in bladder carcinoma, but also by some tumour cells.19 Our study demonstrated for the first time that eNOS is expressed abundantly in bladder cancer cells and that iNOS is also expressed, although to a lesser extent. Moreover, we have shown NO production by the NADPH diaphorase reaction in bladder carcinoma cell lines. Warren et al hypothesised that iNOS production by the inflammatory cells has a major role in schistosomal bladder carcinogenesis.17 We speculate that the different spectrum of p53 mutations in schistosomal bladder cancer might be induced at the initial stages of disease by high amounts of NO produced by macrophages; alternatively, transformed urothelial cells might be induced by monocytes to secret high amounts of NO.35 This issue can be solved only by following the dynamics of NOS expression through the early stages of bladder carcinogenesis in animal models.
NO production in fully developed bladder cancer might play other roles, whether inhibitory or facilitatory, in tumour progression. Tumour cells express many genes that are also expressed by normal cells during embryogenesis and fetal development. This common gene expression manifests phenotypically by characteristics such as reduced state of differentiation, rapid proliferation rate, and the ability to migrate. In this regard, eNOS expression in bladder carcinoma, simulating the pattern of fetal urothelium, may be viewed as an oncofetal characteristic of this type of tumour. Once the biological function of NO in the fetal bladder mucosa is elucidated we will gain a better understanding of its role in bladder carcinogenesis.
Acknowledgments
This work was supported by a trilateral grant (Germany–Israel–The Palestinian Authority) from the DFG, Germany. The authors thank Dr M Baras for professional statistical analysis and Mrs M Sappir for excellent technical assistance. We are grateful to Dr GS Buzard from the NCI for his invaluable comments and constructive discussions during the performance of this study.
References
- 1.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980;288:373–6. [DOI] [PubMed] [Google Scholar]
- 2.Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987;327:524–6. [DOI] [PubMed] [Google Scholar]
- 3.Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 1991;43:109–42. [PubMed] [Google Scholar]
- 4.Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem 1994;63:175–95. [DOI] [PubMed] [Google Scholar]
- 5.Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J 1994;298:249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffith OW, Stuehr DJ. Nitric oxide synthases: properties and catalytic mechanism. Annu Rev Physiol 1995;57:707–36. [DOI] [PubMed] [Google Scholar]
- 7.Chhatwal VJS, Ngoi SS, Chan STF, et al. Aberrant expression of nitric oxide synthase in human polyps, neoplastic colonic mucosa and surrounding peri tumoral normal mucosa. Carcinogenesis 1994;15:2081–5. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins DC, Charles IG, Baylis SA, et al. Human colon cancer cell lines show a diverse pattern of nitric oxide synthase gene expression and nitric oxide generation. Br J Cancer 1994;70:847–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomsen LL, Lawton FG, Knowles RG, et al. Nitric oxide synthase in human gynecological cancer. Cancer Res 1994;54:1352–4. [PubMed] [Google Scholar]
- 10.Thomsen LL, Miles DW, Happerfield L, et al. Nitric oxide synthase activity in human breast cancer. Br J Cancer 1995;72:41–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duenas-Gonzales A, Isales CM, Abad-Hernandez MM, et al. Expression of inducible nitric oxide synthase in breast cancer correlates with metastatic disease. Mod Pathol 1997;10:645–9. [PubMed] [Google Scholar]
- 12.Ariel I, Hochberg A, Shochina M. Endothelial nitric oxide synthase in early gestation and trophoblastic disease. J Clin Pathol 1998;51:427–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ariel I, Lustig O, Schneider T, et al. The imprinted H19 gene as a tumor marker in bladder carcinoma. Urology 1995;45:335–8. [DOI] [PubMed] [Google Scholar]
- 14.Elkin M, Ayesh S, Schneider T, et al. The dynamics of the imprinted H19 gene expression in the mouse model of bladder carcinoma induced by N-butyl-N-(4 hydroxybutyl) nitrosamine. Carcinogenesis 1998;19:2095–9. [DOI] [PubMed] [Google Scholar]
- 15.Wynder EL, Goldsmith R. The epidemiology of bladder cancer: a second look. Cancer 1977;40:1246–68. [DOI] [PubMed] [Google Scholar]
- 16.Gelfand M, Weinberg RW, Castle WM. Relation between carcinoma of the bladder and infestation with Schistosoma haematobium. Lancet 1967;i:1249–51. [DOI] [PubMed] [Google Scholar]
- 17.Warren W, Biggs PJ, El-Baz M, et al. Mutations in the p53 gene in schistosomal bladder cancer: a study of 92 tumours from Egyptian patients and comparison between mutational spectra from schistosomal and non-schistosomal urothelial tumours. Carcinogenesis 1995;16:1181–9. [DOI] [PubMed] [Google Scholar]
- 18.Swana HA, Smith SD, Perrotta PL, et al. Inducible nitric oxide synthase with transitional cell carcinoma of the bladder. J Urol 1999;161:630–4. [PubMed] [Google Scholar]
- 19.Smith SD, Wheeler MA, Foster HE, et al. Urinary nitric oxide synthase activity and cyclic GMP levels are decreased with interstitial cystitis and increased with urinary tract infections. J Urol 1996;155:1432–5. [PubMed] [Google Scholar]
- 20.Mostofi FH, Sobin LH, Torloni H. Histological typing of urinary bladder tumors. International classification of tumors, No. 10. Geneva: World Health Organisation, 1973.
- 21.Hanani M, Louzon V, Udassin R, et al. Nitric oxide-containing nerves in bowel segments of patients with Hirschsprung's disease. J Pediatr Surg 1995;30:818–22. [DOI] [PubMed] [Google Scholar]
- 22.Zeidel ML. Low permeabilities of apical membranes of barrier epithelia: what makes water-tight membranes water-tight? Am J Physiol 1996;271:F243–5. [DOI] [PubMed] [Google Scholar]
- 23.Ross MH, Reith EJ, Romrell LJ. The urinary system. In: Histology. A text and atlas, 2nd ed. Baltimore: Williams & Wilkins, 1989:545–8.
- 24.Birder LA, Apodaca G, De Groat WC, et al. Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Physiol 1998;275:F226–9. [DOI] [PubMed] [Google Scholar]
- 25.Lavelle JP, Apodaca G, Meyers SA, et al. Disruption of the guinea pig urinary bladder barrier in noninfectious cystitis. Am J Physiol 1998;274:F205–14. [DOI] [PubMed] [Google Scholar]
- 26.Mohamed S, Ghabour MD, Annie LW, et al. Immunohistochemical characterization of placental nitric oxide synthase expression in preeclampsia. Am J Obstet Gynecol 1995;173:687–94. [DOI] [PubMed] [Google Scholar]
- 27.Kinsella JP, Ivy DD, Abman SH. Ontogeny of NO activity and response to inhaled NO in the developing ovine pulmonary circulation. Am J Physiol 1994;267:H1955–61. [DOI] [PubMed] [Google Scholar]
- 28.Bogaert GA, Kogan BA, Mevorach RA. Effects of endothelium-derived nitric oxide on renal hemodynamics and function in the sheep fetus. Pediatr Res 1993;34:755–61. [DOI] [PubMed] [Google Scholar]
- 29.Newman J, Antonakopoulos GN. The fine structure of the human fetal urinary bladder. Development and maturation. A light, transmission and scanning electron microscopic study. J Anat 1989;166:135–50. [PMC free article] [PubMed] [Google Scholar]
- 30.Lala PK. Significance of nitric oxide in carcinogenesis, tumor progression and cancer therapy. Cancer Metastasis Rev 1998;17:1–6. [DOI] [PubMed] [Google Scholar]
- 31.Stuehr DJ, Nathan CF. Nitric oxide: a macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med 1989; 169:1543–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie K, Huang S, Dong Z, et al. Transfection with inducible nitric oxide synthase gene suppresses tumorigenicity and abrogates metastasis in K-1735 murine melanoma cells. J Exp Med 1995;181:1333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beckman JS, Koppenol WH. Nitric oxide, superoxide and peroxynitrite: the good the bad and the ugly. Am J Physiol 1996;271:C1424–37. [DOI] [PubMed] [Google Scholar]
- 34.Edwards P, Cendan JC, Topping DB, et al. Tumor cell nitric oxide inhibits cell growth in vitro, but stimulates tumorigenesis and experimental lung metastasis in vivo. J Surg Res 1996;63:49–52. [DOI] [PubMed] [Google Scholar]
- 35.Konur A, Krouse SW, Rehli M, et al. Human monocytes induce carcinoma cell line to secret high amounts of nitric oxide. J Immunol 1996;157:2109–15. [PubMed] [Google Scholar]