Abstract
Aims—Post-transplant lymphoproliferative disease (PTLD) is an important and serious complication in transplant patients. Recent studies have suggested that quantitative assessment of Epstein-Barr virus (EBV) infection in transplant patients might help to identify those at risk of developing PTLD. Therefore, tonsils from paediatric liver transplant recipients were studied for evidence of EBV infection.
Methods—Tonsils were studied by in situ hybridisation for the detection of the small EBV encoded nuclear RNAs (EBERs). The phenotype of EBV infected cells was determined by double labelling in situ hybridisation and immunohistochemistry. The expression of viral latent and lytic antigens was determined by immunohistochemistry. Tonsils from patients without known immune defects were studied as controls.
Results—Tonsils from transplant patients showed pronounced follicular hyperplasia and minor paracortical hyperplasia. In situ hybridisation revealed variable numbers of EBV infected B cells in the tonsils from transplant patients (range, 2–1000/0.5 cm2; mean, 434/0.5 cm2; median, 105/0.5 cm2). Lower numbers were detected in the control tonsils (range, 1–200/0.5 cm2; mean, 47/0.5 cm2; median, 9/0.5 cm2). The latent membrane protein 1 (LMP1) of EBV was not detected and there were only rare cells in two cases showing expression of the EBV encoded nuclear antigen 2 (EBNA2). There was no evidence of lytic infection. None of the patients developed PTLD within a follow up period of up to five years.
Conclusions—These data indicate that tonsillar enlargement in paediatric liver transplant patients does not necessarily imply a diagnosis of PTLD. Furthermore, the presence of increased numbers of EBV infected cells in tonsils from liver transplant recipients by itself does not indicate an increased risk of developing PTLD.
Keywords: Epstein-Barr virus, transplantation, lymphoproliferative disease, tonsil
Epstein-Barr virus (EBV) associated post-transplant lymphoproliferative disease (PTLD) is an important complication in transplant patients.1 The risk of developing PTLD is variable depending, among others, on the nature of the transplanted organ and on the level of iatrogenic immunosuppression.1 In general, patients undergoing primary EBV infection after transplantation and those treated with anti-T cell reagents are particularly at risk.1 The incidence of PTLD is higher in paediatric patients than in adults.2 The disease frequently involves Waldeyer's ring.3 PTLDs are usually of B cell lineage, and include a spectrum of disorders ranging from polyclonal polymorphic lymphoproliferations to monoclonal non-Hodgkin's lymphoma.1 The prognosis of PTLD remains poor and treatment is controversial.1
Suppression of EBV specific T cell immunity is thought to be the central factor in the pathogenesis of PTLD, allowing the outgrowth of an initially polyclonal B cell population. Through the acquisition of additional genetic alterations, such as c-myc translocations or p53 mutations, this is believed to give rise to fully malignant monoclonal lymphomas.4 In support of this model, reduction or withdrawal of the immunosuppressive treatment has been reported to result in a spontaneous remission of some cases of PTLD.5 In addition, successful prevention or treatment of PTLD has been demonstrated by the infusion of EBV specific cytotoxic T cells (CTLs).6 Alternatively, the application of B cell specific monoclonal antibodies has been used successfully for the treatment of PTLD.7 Early detection of PTLD or identification of patients at risk of developing PTLD is clearly important because therapeutic approaches aimed at modifying the EBV specific immunity are likely to be more successful in early stages of the disease. It has been suggested that PTLD in paediatric liver transplant recipients may frequently localise to the tonsils and may be diagnosed by tonsillar biopsy.8, 9 It has also been reported that the detection of EBV positive B cells in liver transplant biopsy specimens by in situ hybridisation (ISH) may indicate a risk of PTLD,10 but this has not been confirmed by others.11 Recent studies have focused on applying quantitative polymerase chain reaction (PCR) assays to this problem, and have suggested that increased EBV DNA detected in the peripheral blood of transplant patients might be useful for the detection and monitoring of PTLD.12–18 However, Babcock et al have shown that the number of EBV infected B cells might be raised in immunosuppressed transplant patients without PTLD.19 Therefore, we studied tonsillectomy specimens from liver transplant recipients for histopathological evidence of PTLD and for EBV infection.
Materials and methods
TISSUES
Of 115 paediatric orthotopic liver transplant (OLT) recipients who survived for more than two years after OLT, 32 (28%) developed appreciable tonsillar enlargement. Twenty six of these underwent tonsillectomy and 15 were included in our study. All transplants were carried out at the Birmingham Children's Hospital. The patient details are summarised in table 1 ▶. All patients underwent tonsillectomy for symptomatic tonsillar enlargement resulting in upper airways obstruction and failure to thrive. Formalin fixed, paraffin wax embedded tonsillectomy specimens were studied. There were 13 girls and two boys (age range at tonsillectomy, 3 to 14 years; mean 6.25; median, 6). Palatine tonsils were available from 14 patients and pharyngeal tonsils from four. From all patients between numbers 1 and 9 tissue blocks were available and all blocks were examined. Reasons for transplantation were extrahepatic biliary atresia (seven cases), intrahepatic biliary atresia (one case), Crigler-Najjar syndrome (one case), viral hepatitis (three cases), hepatoblastoma (one case), and cryptogenic cirrhosis (two cases). Immunosuppressive treatment included cyclosporin, azathioprine, and prednisolone. Prednisolone was withdrawn after three months and azathioprine after 12 months. Cytomegalovirus (CMV) negative recipients of CMV positive grafts received oral acyclovir for three months after transplantation. The time intervals between transplantation and tonsillectomy were from two to six years. Six children were already EBV seropositive before transplantation, and five of these showed evidence of EBV reactivation (defined as a fourfold increase in the IgG antibody titre against the viral capsid antigen of EBV) in the post-transplant period. Of the nine remaining patients, seven seroconverted in the post-transplant period and two remained seronegative. Seroconversion occurred within the first year after transplantation in most cases (range, 4 to 41 months; table 1 ▶). Eight children received acyclovir in the early post-transplant period. In addition, palatine tonsils were available from a 49 year old man who received a liver transplant for primary biliary cirrhosis. No serological information was available for this patient. Routine immunosuppression included corticosteroids, azathioprine, and cyclosporin A. At the time of last follow up (January 2000), 14 of the 15 paediatric transplant patients were alive without evidence of PTLD. One patient had developed chronic transplant rejection and died after re-transplantation. The single adult patient was alive and well without evidence of PTLD.
Table 1.
Detection of Epstein-Barr virus (EBV) in tonsils from paediatric liver transplant recipients: clinical summary and quantitative assessment of EBV infection
| Patient | Age at OLT (years) | Sex | EBV serology pre-OLT | EBV serology post-OLT (months post-OLT) | Acyclovir | Tonsillectomy (years post-OLT) | EBV+ cells (/0.5 cm2) | IgH PCR |
| 1 | 0.5 | F | Positive | React. (13) | Yes | 6 | 8 | P |
| 2 | 0.5 | F | Positive | – | No | 5 | 24 | O |
| 3 | 4 | F | Positive | React. (4) | Yes | 4 | 30 | P |
| 4 | 4 | M | Positive | React. (21) | Yes | 5 | 105 | ND |
| 5 | 7 | M | Positive | React. (16) | Yes | 5 | 3 | P |
| 6 | 1 | F | Positive | React. (14) | No | 3 | 700 | P |
| 7 | 1 | F | Negative | Negative | No | 5 | 0 | P |
| 8 | 2 | F | Negative | Seroconv. (4) | Yes | 4 | 83 | P |
| 9 | 2 | F | Negative | Seroconv. (15) | Yes | 6 | 1000 | O |
| 10 | 7 | F | Negative | Seroconv. (41) | No | 4 | 2 | P |
| 11 | 6 | F | Negative | Seroconv. (7) | No | 3 | 1500 | ND |
| 12 | 2 | F | Negative | Seroconv. (6) | No | 2 | 650 | O |
| 13 | 1 | F | Negative | Seroconv. (12) | Yes | 3 | 900 | P |
| 14 | 8 | F | Negative | Seroconv. (6) | No | 5 | 0 | ND |
| 15 | 4 | F | Negative | Negative | Yes | 3 | 0 | ND |
F, female; IgH: immunoglobulin heavy chain locus; M, male; ND, not done; O, oligoclonal; OLT, orthotopic liver transplantation; P, polyclonal; React., EBV reactivation; Seroconv., EBV seroconversion.
For control purposes, 15 tonsillectomy specimens from patients without known immune defects were studied (10 males and five females; age range, 1 to 29 years; mean, 12; median, 10). A tonsil from a patient with acute infectious mononucleosis served as positive control for ISH and immunohistochemistry studies.
IN SITU HYBRIDISATION
35S or digoxigenin labelled, single stranded RNA probes specific for the small EBV encoded RNA molecules, EBER1 and EBER2, were generated by in vitro transcription as described.11 ISH was carried out as reported previously.11 After hybridisation and stringent washing, immobilised 35S labelled probes were detected using autoradiography. Digoxigenin labelled probes were detected using a digoxin specific monoclonal antibody (Sigma, Deisenhofen, Germany) and immunohistochemistry as described below. For the quantitation of EBER expressing cells, sections subjected to ISH with 35S labelled probes were assessed and the number of labelled cells was counted in a 0.5 cm2 area, as described previously.20 Statistical analysis was done using the Mann-Whitney test with the SPSS software.
IMMUNOHISTOCHEMISTRY AND DOUBLE LABELLING
The monoclonal antibodies PE-2 (specific for the EBV encoded nuclear antigen 2 (EBNA2)), CS1-4 (recognising the EBV encoded latent membrane protein 1 (LMP1)), BZ-1 (directed against the EBV transactivator protein, BZLF1), and JCB117 (specific for the CD79a B cell antigen) were all from Dako, Glostrup, Denmark. Dewaxed and rehydrated paraffin wax embedded sections were subjected to antigen retrieval using microwave irradiation before incubation with the appropriately diluted primary monoclonal antibody. Bound primary antibodies were detected using an alkaline phosphatase labelled avidin–biotin complex method (Dako) and Fast red (Sigma) as chromogen. The phenotype of EBV infected cells was determined using double labelling immunohistochemistry (IH) and ISH as described previously.20
POLYMERASE CHAIN REACTION
PCR for the detection of rearrangements of the IgH locus was performed as described previously.21 Paraffin wax embedded sections were dewaxed and digested in proteinase K, followed by heat inactivation of the enzyme. Tubes were then centrifuged and 3 μl of the supernatant was used as template in PCR reactions. PCR was carried out in the presence of framework 3 and 4 consensus primers. Conditions were 25 cycles of 45 seconds at 94°C, 45 seconds at 55°C, and 110 seconds at 72°C. Subsequently, a semi-nested PCR was performed using 1 μl of the first PCR as template with an internal framework 4 consensus primer. Conditions were 20 cycles of 45 seconds at 94°C, 45 seconds at 55°C, and 110 seconds at 72°C. PCR products were analysed on a 10% polyacrylamide gel.
Results
Histological examination revealed pronounced follicular hyperplasia in all tonsils from transplant patients. In addition, there was a variable but usually minor degree of paracortical activation with extrafollicular blast proliferation. Thus, the changes observed in the tonsils from paediatric liver transplant recipients were very similar to those seen in hyperplastic tonsils from non-immunocompromised individuals. Changes suggestive of PTLD were not present in any of our cases and none of these patients developed clinically manifest PTLD during the four to five years of follow up after tonsillectomy. All control tonsils from non-immunocompromised patients showed pronounced follicular hyperplasia.
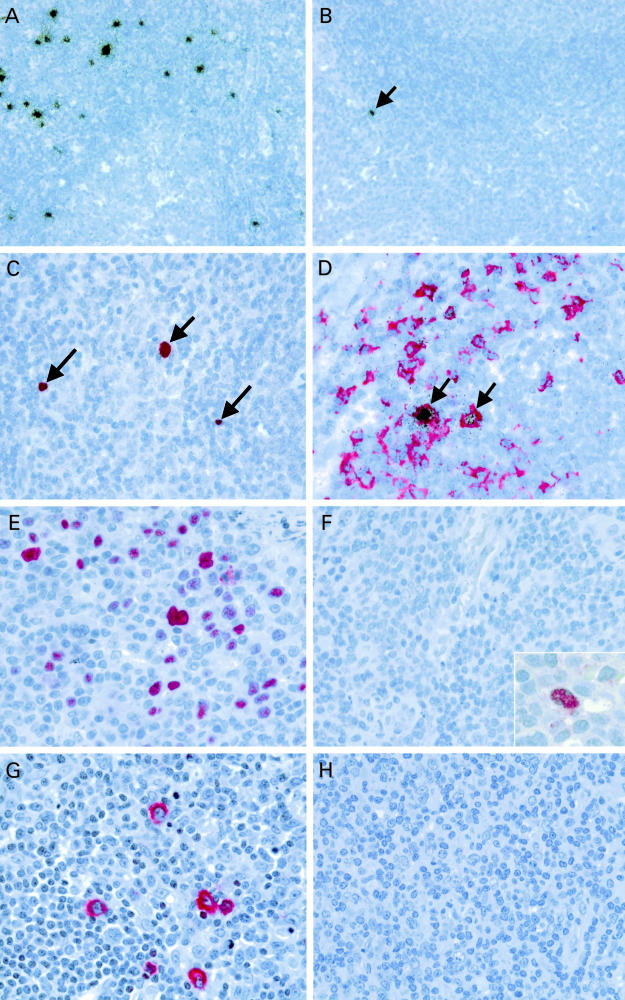
Using ISH, variable numbers of EBER expressing cells were detected in tonsils from 12 of 15 paediatric liver transplant patients, from the single adult liver transplant patient, and from nine of 15 non-immunocompromised control cases (fig 1A,B ▶; table 1 ▶). The EBV+ cells were mostly located in the extrafollicular areas (fig 1A,B ▶), with occasional scattered cells found within secondary follicles, as described previously.20 The EBV+ cells were mainly small lymphocytes but some larger blast cells were also seen (fig 1C ▶). Double labelling ISH and IH identified most of the EBV+ cells as CD79a positive B cells (fig 1D ▶). In one tonsil from a transplant patient and in two tonsils from the control group, isolated germinal centres with a diffuse expansion of EBV+ cells were noticed, similar to our previous observations on hyperplastic lymph nodes.20 The number of EBV+ cells in the extrafollicular regions was variable, ranging from 0 to 1500 EBV+ cells/0.5 cm2 (mean, 350; median, 27; table 1 ▶) in the transplant population and from 0 to 200 EBV+ cells/0.5 cm2 (mean, 29; median, 2) in the control group. There was very little variability between tissue blocks from different tonsillar sites in individuals patients (not shown). In cases with multiple tonsil blocks, average numbers of EBV+ cells/0.5 cm2 were calculated. The difference between transplant patients and controls was not significant (p = 0.056). Among the transplant patients, those who seroconverted after transplantation displayed higher numbers of EBV+ cells (mean, 590; median, 650; table 1 ▶) than those who were already EBV+ before transplantation (mean, 145; median, 27; table 1 ▶). This difference was not significant (p = 0.568). No EBV+ cells were detected in the tonsils from two paediatric liver transplant patients who remained seronegative after transplantation.
Figure 1.
(A,B) In situ hybridisation with 35S labelled small Epstein-Barr virus (EBV) encoded nuclear RNA (EBER) specific probes identifies numerous (A; case 9) and isolated (B; case 3, arrow) EBV positive cells in palatine tonsils from two paediatric liver transplant patients. (C) Using digoxigenin labelled EBER probes (red nuclear signal) the presence of EBV in small lymphocytes (long arrows) and in larger blast cells (short arrow; case 9) is demonstrated. (D) Double labelling immunohistochemistry (red signal) and in situ hybridisation (black grains) shows the presence of EBV in CD79a positive B cells (arrows). (E) Using immunohistochemistry, numerous EBN2A positive cells are detected in a tonsil from a patient with acute infectious mononucleosis, whereas (F) only isolated EBNA2 positive cells are seen in the tonsils from transplant patients (inset). (G) Numerous latent membrane protein 1 (LMP1) positive cells are present in a tonsil from a patient with infectious mononucleosis, whereas (H) LMP1 expression is not detected in the tonsils from transplant patients.
Using IH, numerous cells expressing the EBNA2 and LMP1 latent proteins of EBV (fig 1E,G ▶) were detected in a tonsil from a patient with infectious mononucleosis, as described previously.22 There were also isolated cells positive for the BZLF1 transactivator protein of EBV (not shown), suggesting entry into the lytic viral cycle. By contrast, only very rare EBNA2+ cells were detected in two tonsils from the liver transplant patients (fig 1F ▶). No cells expressing LMP1 (fig 1H ▶) or BZLF1 (not shown) were detected in these cases.
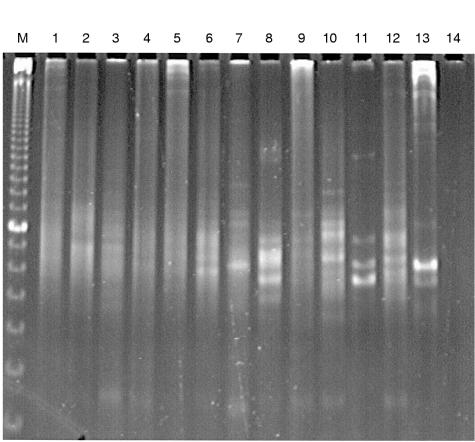
Eleven tonsils from liver transplant recipients containing EBV+ cells and one EBV− tonsil, in addition to 14 control tonsils, were subjected to PCR analysis of the IgH locus (fig 2 ▶). This revealed a polyclonal pattern in eight tonsils from transplant patients, with the four remaining tonsils showing an oligoclonal pattern with several distinct bands (fig 2 ▶). An oligoclonal rearrangement was detected in four tonsils with 24, 640, 650, and 1000 EBV+ cells/0.5 cm2, respectively, including the adult transplant recipient. Thirteen of 14 control tonsils yielded a polyclonal and one an oligoclonal pattern (not shown).
Figure 2.
PCR analysis of IgH gene rearrangement in tonsils from liver transplant recipients. M, 10 bp ladder (bar, 100 bp); lanes 1 to 11, patients 13, 8, 6, 3, 7, 10, 5, 9, 1, 2, 12, respectively (table 1 ▶); lane 12, adult liver transplant patient; lane 13, positive control (BJAB cell line); lane 14, negative control (H2O).
Discussion
We report the results of a study of tonsillectomy specimens from one adult and 15 paediatric liver transplant recipients. All tonsils showed reactive lymphoid hyperplasia but no histopathological evidence of PTLD. This diagnosis was also supported by the absence of clonal IgH gene rearrangements as detected by PCR. In comparison with non-immunosuppressed controls, increased numbers of EBV+ B cells were detectable in hyperplastic tonsils from paediatric liver transplant patients. However, this difference was not significant, probably because of the small size of the series. In the transplant patients who had already been EBV seropositive before transplantation, the numbers of EBV+ cells were similar to those observed in the immunocompetent controls. In contrast, higher numbers of EBV+ cells were detected in patients who experienced seroconversion after transplantation. Again, this finding was not significant probably because of the small number of cases, the large standard deviation, and the fact that two patients remained EBV seronegative after transplantation. The tendency for higher numbers of EBV+ cells seen in the transplant patients who were EBV seronegative before transplantation is in agreement with the known increased risk of such individuals to develop PTLD.23 Nevertheless, there was a striking heterogeneity even in this group, with three patients showing low numbers of EBV+ cells within the range of the immunocompetent controls. Moreover, none of our patients developed PTLD within a follow up period of up to five years, suggesting that the number of EBV positive cells detectable in tonsils is not an indicator of PTLD risk. The quantitation of EBV infected cells in the peripheral blood by in situ hybridisation has been proposed as a suitable way of identifying patients at risk of developing PTLD.24, 25 However, in agreement with our observations, Babcock et al have reported increased numbers of EBV+ B cells in the peripheral blood of transplant recipients without PTLD.19 Moreover, we have shown previously that the number of EBV infected cells in allograft biopsies from liver transplant recipients does not correlate with the risk of developing PTLD.11, 26 Thus, there is a growing body of evidence to suggest that the number of EBV positive cells detected in tissue or peripheral blood from transplant patients does not provide a reliable indicator of PTLD risk.
Several other groups have recently used quantitative techniques for the study of EBV infection in transplant patients. Quantitative PCR (qPCR) for the detection of EBV DNA in the peripheral blood can be used to monitor disease progress in patients with established PTLD.13, 15, 17, 18 Although regression of PTLD is accompanied by a reduction of viral load, a later rebound of EBV DNA load has been observed, which did not indicate PTLD recurrence.17 Using qPCR, Lucas et al have reported that five of seven patients with raised EBV values developed PTLD and two patients with PTLD had normal EBV values.14 These results suggest that qPCR alone is not sufficient to detect patients at risk of developing PTLD. However, these authors used the B95.8 cell line as a standard for the qPCR assay. A proportion of B95.8 cells spontaneously enters into virus replication making a quantitative assessment of these results difficult.27 To our knowledge, at present there is no study showing that EBV qPCR is useful for the detection of an increased PTLD risk in advance of the development of the disease.
Some of the transplant patients had extremely high numbers of EBV+ tonsillar B cells, occasionally reaching the figures otherwise only seen in infectious mononucleosis tonsils.20 Nevertheless, unlike in infectious mononucleosis, these cells did not show detectable expression of LMP1 and there were only exceptional cells expressing EBNA2. Thus, the EBV latency III pattern (EBNA2+, LMP1+) characteristically seen in virus driven lymphoproliferations was not detectable in these tonsils.28 This is further evidence to suggest that the increased numbers of EBV+ B cells in our cases is not related to the development of PTLD, which typically shows a type III latency.28 The lack of detectable expression of those latent viral proteins (EBNA2, LMP1) that drive EBV associated B cell proliferation (for example, in lymphoblastoid cell lines and in infectious mononucleosis) raises questions as to the mechanism accounting for the increased numbers of EBV+ cells in these patients. Long term studies of healthy virus carriers have shown that the virus load in the peripheral blood is variable between individuals but remains remarkably constant in each individual, suggesting that variable steady state levels are established after primary infection.29 Thus, the increased numbers of EBV+ cells in patients undergoing seroconversion after transplantation may indicate that these patients establish a steady state level different from immunocompetent individuals, but does not necessarily imply a failure of immunological control of EBV infection. This notion is supported by the absence of PTLD in these patients and by the lack of a type III latency, the presence of which in vivo usually suggests an impairment of EBV specific immunity.28
In summary, our data indicate that tonsillar enlargement in paediatric liver transplant recipients does not necessarily imply a diagnosis of PTLD, and that the presence of raised numbers of EBV infected cells in tonsils from liver transplant patients by itself is not a reliable indicator of an increased PTLD risk. Further studies are required to determine whether a switch from the restricted form of EBV latency (EBNA2−, LMP1−) detected in healthy virus carriers towards a latency III pattern (EBNA2+, LMP1+) would provide a more appropriate marker for identifying patients at risk of developing PTLD.
References
- 1.Nalesnik MA. Clinical and pathological features of post-transplant lymphoproliferative disorders (PTLD). Springer Semin Immunopathol 1998;20:325–42. [DOI] [PubMed] [Google Scholar]
- 2.Ho M, Jaffe R, Miller G, Breinig MK, et al. The frequency of Epstein-Barr virus infection and associated lymphoproliferative syndrome after transplantation and its manifestation in children. Transplantation 1988;45:719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lattyak BV, Rosenthal P, Mudge C, et al. Posttransplant lymphoproliferative disorder presenting in the head and neck. Laryngoscope 1998;108:1195–8. [DOI] [PubMed] [Google Scholar]
- 4.Knowles DM, Cesarman E, Chadburn A, et al. Correlative morphologic and molecular genetic analysis demonstrates three distinct categories of posttransplantation lymphoproliferative disorders. Blood 1995;85:552–65. [PubMed] [Google Scholar]
- 5.Starzl TE, Nalesnik MA, Porter KA, et al. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporin–steroid therapy. Lancet 1984;i:583–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rooney CM, Smith CA, Ng CYC, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood 1998;92:1549–55. [PubMed] [Google Scholar]
- 7.Benkerrou M, Jais J-P, Leblond V, et al. Anti-B-cell monoclonal antibody treatment of severe posttransplant B-lymphoproliferative disorder: prognostic factors and long-term outcome. Blood 1998;92:3137–47. [PubMed] [Google Scholar]
- 8.Broughton S, McClay JE, Murray A, et al. The effectiveness of tonsillectomy in diagnosing lymphoproliferative disease in pediatric patients after liver transplantation. Arch Otolaryngol Head Neck Surg 2000;126:1444–7. [DOI] [PubMed] [Google Scholar]
- 9.Lones MA, Mishalani S, Shintaku IP, et al. Changes in tonsils and adenoids in children with posttransplant lymphoproliferative disorder: report of three cases with early involvement of Waldeyer's ring. Hum Pathol 1995;26:525–30. [DOI] [PubMed] [Google Scholar]
- 10.Randhawa PS, Jaffe R, Demetris AJ, et al. Expression of Epstein-Barr virus-encoded small RNA (by the EBER-1 gene) in liver specimens from transplant recipients with post-transplantation lymphoproliferative disease. N Engl J Med 1992;327:1710–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niedobitek G, Mutimer DJ, Williams A, et al. Epstein-Barr virus infection and malignant lymphomas in liver transplant recipients. Int J Cancer 1997;73:514–20. [DOI] [PubMed] [Google Scholar]
- 12.Rowe DT, Qu L, Reyes J, et al. Use of quantitative competitive PCR to measure Epstein-Barr virus genome load in the peripheral blood of pediatric transplant patients with lymphoproliferative disorders. J Clin Microbiol 1997;35:1612–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mutimer DJ, Kaur N, Tang H, et al. Quantitation of Epstein-Barr virus (EBV) DNA in the blood of adult liver transplant recipients. Transplantation 2000;69:954–9. [DOI] [PubMed] [Google Scholar]
- 14.Lucas KG, Burton RL, Zimmerman SE, et al. Semiquantitative Epstein-Barr virus (EBV) polymerase chain reaction for the determination of patients at risk for EBV-induced lymphoproliferative disease after stem cell transplantation. Blood 1998;91:3654–61. [PubMed] [Google Scholar]
- 15.Kogan DL, Burroughs M, Emre S, et al. Prospective longitudinal analysis of quantitative Epstein-Barr virus polymerase chain reaction in pediatric liver transplant recipients. Transplantation 1999;67:1068–70. [DOI] [PubMed] [Google Scholar]
- 16.Kenagy DN, Schlesinger Y, Weck K, et al. Epstein-Barr virus DNA in peripheral blood leukocytes of patients with posttransplant lymphoproliferative disease. Transplantation 1995;60:547–54. [DOI] [PubMed] [Google Scholar]
- 17.Green M, Cacciarelli TV, Mazariegos GV, et al. Serial measurement of Epstein-Barr viral load in peripheral blood in pediatric liver transplant recipients during treatment for posttransplant lymphoproliferative disease. Transplantation 1998;66:1641–4. [DOI] [PubMed] [Google Scholar]
- 18.Cacciarelli TV, Reyes J, Mazariegos GV, et al. Natural history of Epstein-Barr virus load in peripheral blood of pediatric liver transplant recipients during treatment for posttransplant lymphoproliferative disorder. Transplant Proc 1998;31:488–9. [DOI] [PubMed] [Google Scholar]
- 19.Babcock GJ, Decker LL, Freeman RB, et al. Epstein-Barr virus-infected resting memory B cells, not proliferating lymphoblasts, accumulate in the peripheral blood of immunosuppressed patients. J Exp Med 1999;190:567–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niedobitek G, Herbst H, Young LS, et al. Patterns of Epstein-Barr virus infection in non-neoplastic lymphoid tissue. Blood 1992;79:2520–6. [PubMed] [Google Scholar]
- 21.Diss TC, Pan L, Wotherspoon AC, et al. Sources of DNA for detecting B cell monoclonality using PCR. J Clin Pathol 1994;47:493–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niedobitek G, Agathanggelou A, Herbst H, et al. Epstein-Barr virus (EBV) infection in infectious mononucleosis: virus latency, replication and phenotype of EBV-infected cells. J Pathol 1997;182:151–9. [DOI] [PubMed] [Google Scholar]
- 23.Ho M, Miller G, Atchison RW, et al. Epstein-Barr virus infections and DNA hybyridization studies in posttransplantation lymphoma and lymphoproliferative lesions: the role of primary infection. J Infect Dis 1985;152:876–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egawa H, Oh-ishi T, Arai T, et al. Application of in situ hybridization technique for quantitative assessment of ongoing symptomatic Epstein-Barr virus infection after living related liver transplantation. Clin Transplant 1998;12:116–22. [PubMed] [Google Scholar]
- 25.Nakazawa Y, Chisuwa H, Ikegami T, et al. Efficacy of quantitative analysis of Epstein-Barr virus-infected peripheral blood lymphocytes by in situ hybridization of EBER1 after living-related liver transplantation. Transplantation 1997;63:1363–6. [DOI] [PubMed] [Google Scholar]
- 26.Hubscher SG, Williams A, Davison SM, et al. Epstein-Barr virus in inflammatory diseases of the liver and liver allografts: an in situ hybridization study. Hepatology 1994;20:899–907. [DOI] [PubMed] [Google Scholar]
- 27.Miller G, Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A 1973;70:190–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rowe M, Niedobitek G, Young LS. Epstein-Barr virus gene expression in PTLD. Springer Semin Immunopathol 1998;20:389–403. [DOI] [PubMed] [Google Scholar]
- 29.Khan G, Miyashita EM, Yang B, et al. Is EBV persistence in vivo a model for B cell homeostasis? Immunity 1996;5:173–9. [DOI] [PubMed] [Google Scholar]