Abstract
Aims—Although the aetiology of medulloblastoma remains elusive, several lines of evidence suggest an association with the human neurotropic polyomavirus JC and its oncoprotein T antigen. The tumour forming properties of JC virus T antigen are the result, at least in part, of its ability to bind and inactivate tumour suppressor/cell cycle regulatory proteins, such as p53 and the retinoblastoma family of proteins.
Methods—To examine potential relations between these factors, immunohistochemistry was used to determine associations between the T antigen and the expression of p53 and the retinoblastoma proteins pRb, p107, and Rb2/p130 in eight medulloblastomas.
Results—Only the three medulloblastomas with T antigen expression also showed nuclear positivity with antibodies to p53. Although immunohistochemistry detected nuclear labelling for pRb in five of the cases, the three that were positive for T antigen showed the highest pRb labelling. The retinoblastoma related proteins p107 and Rb2/p130 were also immunopositive in most T antigen positive medulloblastomas. Double label immunohistochemistry also demonstrated p53 and pRb positivity in the same cells that were T antigen positive.
Conclusions—These correlations suggest that associations between T antigen and p53 and/or T antigen and pRb occur in some of these tumours. These data provide indirect evidence that JC virus, acting through T antigen, might be involved in the formation and progression of medulloblastoma.
Keywords: medulloblastoma, T antigen, p53, retinoblastoma protein
Brain tumours are the most common solid neoplasm of childhood.1 Most central nervous system (CNS) malignancies in children are primitive neuroectodermal tumours (PNETs), which account for 6–8% of all CNS tumours.2 These tumours, which include cerebral neuroblastomas, ependymoblastomas, pineoblastomas, and medulloblastomas, have been grouped together because of the young age of the patients in which they occur and their common histological features.3,4 Of the primitive neuroectodermal tumours, medulloblastomas are the most common, accounting for 20% of all paediatric intracranial neoplasms.5
Although the aetiology of these tumours remains elusive, several lines of evidence suggest an association between the human neurotropic polyomavirus JC (JCV) in a large number of these tumours. JCV is known to cause PNETs in experimental models. More than 80% of neonatal hamsters inoculated intracerebrally, intraperitoneally, or subcutaneously with strains of JCV isolated from progressive multifocal leucoencephalopathy lesions develop a wide range of tumours, including medulloblastomas, neuroblastomas, and pineocytomas.6,7 Although the mechanism of JCV induced neurotumorigenesis is not entirely clear, several lines of evidence point to the involvement of the viral early protein T antigen in this process. JCV T antigen is found in human and hamster glial cells transformed with JCV DNA.8 Transgenic mice that constitutively produce T antigen under the control of JCV early promoters/enhancers have been shown to develop adrenal neuroblastomas, primitive appearing mesenteric tumours, and medulloblastomas.9,10
In an attempt to delineate the possible contribution of polyoma viruses to the formation of medulloblastomas in humans, we recently investigated a series of these tumours for the presence of JCV DNA and T antigen.11,12 In those cases, most tumours contained JCV sequences. One fourth also expressed T antigen when assayed immunocytochemically. Because the oncogenic properties of JCV T antigen result, at least in part, from its ability to bind and inactivate tumour suppressor/cell cycle regulatory proteins, such as p53,13–17 a correlation may exist between T antigen and p53 expression in medulloblastomas. In support of this concept, increased p53 protein values have been reported in these tumours,18 despite a low frequency of mutations in the p53 gene.19–22
JCV T antigen is also known to associate with other tumour suppressors such as the retinoblastoma (Rb) family of proteins.14,23 Although the contribution of T antigen–retinoblastoma gene product (pRb) interactions to JCV induced malignancies remains to be elucidated, one could speculate that this association would result in release of pRb from E2F, its normal complex partner. E2F is a transcription factor that by stimulating S-phase specific genes accelerates G1/S phase entry, resulting in a positive effect on cell proliferation.24,25 Because both p53 and pRb operate at least in part through common cell regulatory pathways, it is likely that a T–pRb interaction would also indirectly affect p53 related mechanisms of tumorigenesis.26
To examine these potential relations in human tumour resection samples, we used immunocytochemistry to determine the contribution of T antigen to the expression of p53 and the Rb proteins in medulloblastomas. Using this approach, we have shown correlations between T antigen and p53 and between T antigen and the Rb proteins. These findings support the hypothesis that JCV, acting through T antigen, is involved in medulloblastoma formation and/or progression.
Materials and methods
TISSUE SAMPLES
Eight medulloblastoma samples were obtained from the archives of two Philadelphia institutions: Hahnemann University Hospital and Saint Christopher's Hospital for Children. All samples were formalin fixed and paraffin wax embedded. Formalin fixed and paraffin wax embedded control tissues obtained from the Hahnemann University Hospital archives included 10 samples of normal postmortem cerebellum spanning the ages from birth to 70 years, and two normal adult lung specimens. Additional formalin fixed paraffin wax embedded controls included JCV induced PNETs in Syrian hamster brain.
Sections (4 μm thick) of the medulloblastoma specimens were stained with haematoxylin and eosin to confirm tissue diagnoses. Of the tumour samples, four were available in large enough quantities to allow DNA extraction and polymerase chain reaction (PCR) analysis for JCV DNA. This was performed with methods reported previously12 to ascertain whether sequences were from the N-terminal portion of the early genome, the C-terminal portion of the early genome, or the VP1 portion of the late genome.
IMMUNOHISTOCHEMICAL ANALYSIS
Immunohistochemistry was performed by dewaxing the sections in xylene, followed by rehydration and non-enzymatic antigen retrieval in 0.01M sodium citrate buffer (pH 6.0) at 95°C for 40 minutes. After cooling to room temperature (approximately 20 minutes), slides were rinsed and endogenous peroxide was quenched by incubation in MeOH/3%H202 for 20 minutes. This was followed by a wash in phosphate buffered saline (PBS) and blocking in PBS/0.1% bovine serum albumin plus 5% normal horse serum for two hours at room temperature. Slides were then incubated overnight with primary antibodies. To detect T antigen, a mouse monoclonal antibody to the SV40 T antigen that crossreacts with JCV T antigen (pAb416; Oncogene Science, Cambridge, Massachusetts, USA) was used at a 1/100 dilution. p53 was detected with a mouse monoclonal antibody that recognises both wild-type and mutated forms of the protein (D0-7; Dako, Carpinteria, California, USA; 1/100 dilution). pRb was detected with a mouse monoclonal antibody to the human protein (14001A; Pharmigen; 1/100 dilution) and its family members p107 and Rb2/p130 were detected with rabbit polyclonal antibodies (provided by A Giordano, p107 and Rb2/p130; both 1/1000 dilution). Positive controls for T antigen were paraffin wax embedded tumours from Syrian hamsters intracerebrally inoculated with JCV. For p53, paraffin wax embedded sections of human astrocytomas were used. Human adult lung sections including bronchial epithelium served as positive controls for pRb, p107, and Rb2/p130.27 Negative controls consisted of tissues incubated in buffer without primary antibody. Primary antibodies were detected with biotinylated antimouse or antirabbit secondary antibodies, avidin–biotin peroxidase complex, and diaminobenzidine chromagen, according to manufacturer's instructions (Vectastain Elite ABC Peroxidase Kit; Vector Laboratories Burlingame, California, USA). After a light counterstain with haematoxylin, sections were dehydrated and coverslipped with Permount.
Double label immunohistochemistry for T antigen–p53 and T antigen–pRb colocalisations was performed by sequential applications of the primary antibodies to the same tissue section, which were both revealed by ABC systems but with different chromagens. After incubation of the first primary antibody, secondary antibody and ABC incubations were performed as described above, followed by development with DAB. The tissue was then taken back to buffer and blocking reagent, incubated in the second primary antibody overnight, followed by secondary antibody and ABC. The second chromagen used was Vector red, which was used according to the manufacturer's directions.
Each slide was examined independently by two microscopists (SC and LDV) for tumour type by haematoxylin and eosin morphology and the presence or absence of immunohistochemical stain product. In all tumours that stained positively, the percentage of immunopositive cells was calculated by counting the total number of tumour nuclei and the number of immunopositive nuclei in five random, non-contiguous fields at ×1000 magnification (an average of 73 nuclei/field). As listed in table 1 ▶, these raw data were used to calculate an average percentage of positive cells and the SD of that percentage for each positive immunostain. In the case of double stains, this analysis was extended to include the percentage of double positive nuclei in each section. This was compared with the “expected” percentage of double positive nuclei calculated as the product of single positive nuclei. Thus, in the case of T–p53 double staining, the expected percentage of T–p53 positive nuclei would be the product of the percentage of T positive and the percentage of p53 positive nuclei. This expected percentage would represent a chance process, whereas observed percentages clearly different from expected would represent a process not determined by chance. The double stains for T antigen and pRb were analysed similarly.
Table 1.
Patient data and PCR for JC virus (JCV) in eight medulloblastomas
| Case | Patient age/sex | Histology | Tumour location | JCV PCR |
| 1 | 5 years/M | Neuroblastic medulloblastoma | Posterior fossa | N-terminal + |
| C-terminal + | ||||
| VP1 + | ||||
| 2 | 12 years/M | Neuroblastic medulloblastoma | Left cerebellar hemisphere | N-terminal + |
| C-terminal − | ||||
| VP1 + | ||||
| 3 | 15 years/M | Desmoplastic medulloblastoma | Left cerebellar hemisphere | N-terminal + |
| C-terminal − | ||||
| VP1 − | ||||
| 4 | 9 years/F | Desmoplastic medulloblastoma | Posterior fossa | N-terminal − |
| C-terminal + | ||||
| VP1 + | ||||
| 5 | 10 years/M | Classic medulloblastoma | Cerebellum | — |
| 6 | 11 years/M | Classic medulloblastoma | Cerebellum | — |
| 7 | 4 years/M | Classic medulloblastoma | Cerebellum | — |
| 8 | 10 years/M | Classic medulloblastoma | Posterior fossa | — |
For statistical analysis, the single label immunohistochemistry results for T antigen positive versus T antigen negative groups were compared by the one tailed continuity corrected χ2 method (because of the small number of samples), with the p value for significance set at ≤ 0.05. In cases where this test did not return significance, the normal χ2 test and the t test were also applied. In the case of double label immunohistochemistry, the paired t test was used to compare observed with expected percentages in each microscopic field analysed.
Results
The 10 specimens of cerebellum obtained from normal paediatric and adult necropsies failed to show positive staining for T antigen or p53. Antibodies to pRb, p107, and Rb2/p130 produced nuclear labelling in most of the Purkinje cells and rare granule cells. In the three specimens of the youngest children whose cerebellum contained an external granule cell layer, these cells also displayed prominent nuclear labelling for pRb, p107, and Rb2/p130.
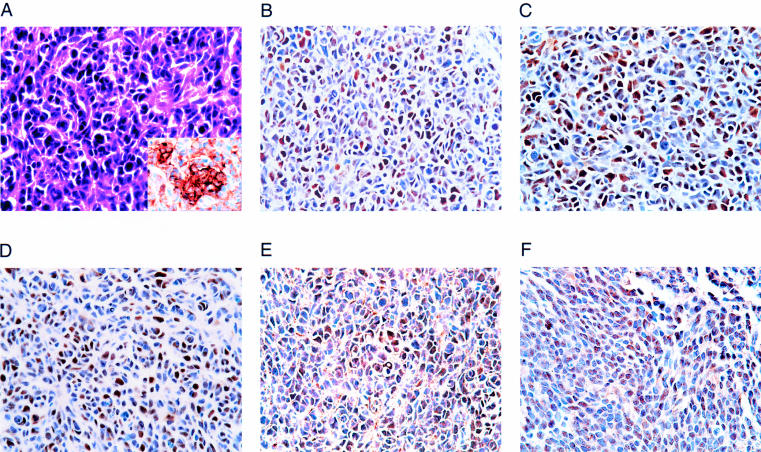
The clinical details of the eight tumours analysed are presented in table 1 ▶. They all occurred in the cerebellums of children and were medulloblastomas. Histologically, the three major subtypes—classic, neuroblastic, and desmoplastic medulloblastomas—were represented. Figure 1A ▶ shows case 1 stained with haematoxylin and eosin and immunohistochemically for synaptophysin.
Figure 1.
Labelling of a medulloblastoma with antibodies to T antigen, p53, pRb, p107, and Rb2/p130. (A) Haematoxylin and eosin stained sections of case 1 show the characteristic morphology of a medulloblastoma with a high nuclear to cytoplasmic ratio, dense nuclear chromatin, and cytoplasmic extensions with occasional rosette formation. Synaptophysin immunostaining further defines the neural phenotype of the tumour cells (inset). (B) T antigen staining shows nuclear positivity in approximately one third of the cells. (C) p53 nuclear positivity is found in tumour nuclei. Nuclear positivity for (D) pRb and its related proteins (E) p107, and (F) Rb2/p130. All original magnifications, ×40.
Of the four tumours analysed by PCR, all showed JCV sequences (table 1 ▶). Case 1 was positive for all sequences analysed. Cases 2 and 4 were positive for two of the three sequences (one early and one late each). Case 3 was positive only for the N-terminal portion of the early genome. It is difficult to analyse these data because all tumours analysed showed some portion of JCV genome. Nonetheless, it is useful to note that the one tumour that was positive for all three regions of JCV (case 1) expressed T antigen in the greatest percentage of nuclei (see below)
Table 2 ▶ shows data from the single label immunohistochemical analysis of the tumours. Three of the eight tumours were positive for T antigen and five were negative. The percentage of T antigen positive nuclei in these three cases ranged from 6.17% (case 3) to 41.26% (case 1). Figure 1B ▶ shows a characteristic T antigen positive field from case 1.
Table 2.
Single label immunohistochemistry
| Case | T antigen | p53 | pRb | p107 | Rb2/p130 |
| No. positive/no. counted (%; SD) | No. positive/no. counted (%; SD) | No. positive/no. counted (%; SD) | No. positive/no. counted (%; SD) | No. positive/no. counted (%; SD) | |
| 1 | 135/325 (41.26%; 3.91) | 74/433 (17.30%; 4.86) | 125/492 (25.66%; 4.30) | 158/375 (42.01%; 5.21) | 105/492 (1.38%; 3.46) |
| 2 | 105/474 (22.41%; 5.77) | 207/439 (47.40%; 4.87) | 199/421 (46.97%; 4.72) | 64/342 (18.56%; 6.70) | 49/374 (13.07%; 11.75) |
| 3 | 112/311 (6.17%; 8.42) | 63/316 (19.62%; 5.03) | 147/358 (40.75%; 5.78) | — | 22/273 (8.08%; 2.23) |
| 4 | — | — | 46/421 (10.62%; 3.46) | — | — |
| 5 | — | — | 23/489 (4.56%; 1.83) | — | — |
| 6 | — | — | 14/318 (4.60%: 2.01) | — | — |
| 7 | — | — | — | — | — |
| 8 | — | — | — | — | — |
The sections stained with the antibody to p53 were positive in all three tumours that were T antigen positive (cases 1–3), ranging from 17.30% to 47.40% of nuclei. None of the tumours that were T antigen negative (cases 4–8) showed p53 positivity. A positive immunoproduct for p53 is shown in fig 1C ▶ (case 1). Using the continuity corrected χ2 test, there is a clear difference in the p53 positivity of T antigen positive versus negative groups (p = 0.038, two tailed test; p = 0.019, one tailed test).
The antibody to pRb stained tumour nuclei in six of the eight cases, with positive nuclei ranging from 4.56% (case 5) to 46.97% (case 2). A region from case 1 similar to that illustrated in fig 1A ▶ is shown in fig 1D ▶ stained for pRb. With both the continuity corrected and standard χ2 methods, there is no significant difference in pRb positivity between the groups of T antigen positive and negative tumours. However, pRb positivity in the T positive cases ranged from 25.66% to 46.97%, whereas T negative cases ranged from 4.56% to 10.62%. Applying the t test instead and counting the negative stained cases as 0, p = 0.025 (two tailed test) and 0.0125 (one tailed test).
Staining with p107 was found in cases 1 and 2, which demonstrated nuclear immunopositivity in 42.01% and 18.56% of cells, respectively. Case 1 is used to illustrate p107 immunostaining (fig 1E ▶). These results are not significant with the continuity corrected χ2 test, but return a p = 0.035 (two tailed test) and p = 0.0125 (one tailed test) with the standard method.
In the case of Rb2/p130, all the T antigen positive tumours again demonstrated nuclear immunoreactivity (8.08–13.07%), whereas the T antigen negative tumours did not. Once again, positive immunostaining is demonstrated for case 1 (fig 1F ▶). Using the continuity corrected χ2, these results are the same as those for p53 (p = 0.038, two tailed test; p = 0.019, one tailed test) and are significant.
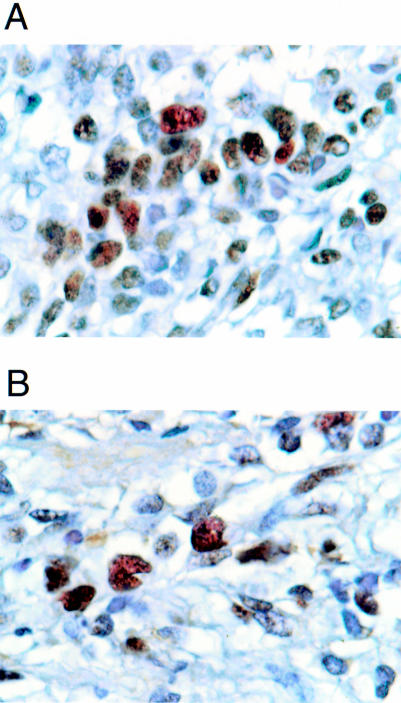
In several of the T antigen positive tumours, it was noted that the same populations of cells appeared to exhibit positivity for T antigen, p53, and pRb. This observation was pursued further with double label immunohistochemistry to determine whether the same cells expressed T antigen, p53, and pRb. The results are illustrated in fig 2A,B ▶. In table 3 ▶, the percentage of cells that were double immunopositive for T antigen–p53 and T antigen–pRb are compared with the percentages predicted from the positivity for T antigen, p53, and pRb alone. This is shown for each case overall and, in addition, in each of the fields counted.
Figure 2.
Double label immunohistochemistry of tumour nuclei with antibodies to T antigen, p53, and pRb. (A) In case 1, nuclei with immunopositivity for T antigen (brown with DAB as chromagen) are also immunopositive for p53 (Vector red chromagen). (B) T antigen positive nuclei of case 1 (DAB chromagen) are also positive for pRb (Vector red chromagen). All original magnifications, ×100.
Table 3.
Double label immunohistochemistry
| T–p53 positive | Predicted | T–pRb positive | Predicted | |
| No. p53 positive/no. T positive (%; SD) | No. p53 positive also T positive (% T–pRb positive; SD) | No. pRb positive/no. T positive (%; SD) | No. p53 positive also T positive (% T–pRb positive; SD) | |
| Case 1 overall | 56/173 (33.29%; 6.86) | 34/173 (19.78%; 5.30) | 34/66 (52.42%; 6.70) | 16/66 (24.66%; 11.52) |
| Field 1 | 13/36 | 9/36 | 8/16 | 4/16 |
| Field 2 | 9/23 | 5/23 | 7/12 | 2/12 |
| Field 3 | 10/39 | 6/39 | 7/16 | 3/16 |
| Field 4 | 11/42 | 10/42 | 6/12 | 3/12 |
| Field 5 | 13/33 | 4/33 | 6/10 | 4/10 |
| Case 2 overall | 29/59 (49.19%; 6.70) | 28/59 (48.83%; 8.20) | 22/39 (58.00%; 13.04) | 17/39 (43.21%; 5.44) |
| Field 1 | 4/7 | 3/7 | 3/6 | 3/6 |
| Field 2 | 4/10 | 4/10 | 6/10 | 4/10 |
| Field 3 | 8/15 | 9/15 | 4/5 | 2/5 |
| Field 4 | 8/16 | 7/16 | 4/8 | 4/8 |
| Field 5 | 5/11 | 6/11 | 5/10 | 4/10 |
| Case 3 overall | 13/22 (60.10%; 7.02) | 4/22 (18.05%; 4.38) | 33/37 (89.03%; 10.08) | 12/37 (29.04%; 8.64) |
| Field 1 | 4/7 | 2/7 | 6/6 | 2/6 |
| Field 2 | 2/3 | 1/3 | 9/11 | 5/11 |
| Field 3 | 3/5 | 1/5 | 5/6 | 2/6 |
| Field 4 | 2/4 | 1/4 | 4/5 | 1/5 |
| Field 5 | 2/3 | 1/3 | 9/9 | 3/9 |
In the case of p53 double staining, the field counts in cases 1 and 3 are fairly consistent, with all five counted fields showing greater positive percentages than predicted. These are significant by the paired t test (p < 0.05 for case 1 and p < 0.01 for case 3). Case 2 shows almost no pattern of positive staining because two cases demonstrate greater positive percentages than predicted, two are less than predicted, and one is equal to that predicted. This result is not significant by the paired t test.
All the fields of cases 1 and 3 counted showed a greater number of T antigen–pRb positive cells than predicted (p values < 0.01 by the paired t test). In Case 2, the pattern is not quite as consistent (four of five are in the hypothesised direction) and the result is not significant.
Discussion
We used immunohistochemistry in a series of medulloblastomas to demonstrate that p53 overexpression occurs in the same tumours as the expression of the polyoma oncoprotein T antigen. Similarly, the expression of the retinoblastoma gene product pRb and the related proteins p107 and Rb2/p130 is greatest in the T antigen positive tumours. We also used double label immunohistochemistry to demonstrate T antigen–p53 and T antigen–pRb colocalisation in the nuclei of three of these tumours, and used a quantitative approach to show that the colocalisation in two of these tumours was not coincidental.
Brain tumours account for 15% of all childhood neoplasms. They are the most common solid neoplasm of childhood and most malignant CNS tumours in children are primitive neuroectodermal tumours. Medulloblastomas, the primitive neuroectodermal tumours that occur in the cerebellum, are the most common, accounting for 20% of all paediatric intracranial neoplasms. The peak age of occurrence is 7 years and 70% of tumours present before the age of 16.1,5 Despite the potential for poor outcome with these lesions, the five year survival for children with medulloblastoma currently stands at 50–70%. The improvement in survival seen in the past three decades is largely attributable to improvements in imaging and surgery, which allow complete or near complete tumour resections in a larger proportion of patients than was previously possible. This has been supplemented by improvements in radiotherapy and chemotherapy.21 Despite the clinical importance of this tumour, the aetiology of most medulloblastomas remains elusive.
JCV is a human neurotropic polyomavirus infecting more than 80% of the human population early in life, and associated with the subacute demyelinating disease, progressive mulitifocal leucoencephalopathy, in immunosuppressed individuals.28 It has been associated with chromosomal damage in infected individuals,29 and has also been shown to produce PNETs in several animal systems, both through direct inoculation of whole virus or expression of only the early genes, which include T antigen.6,9,10,30 At present, the JCV and T antigen serological status of patients with paediatric brain tumours is not well characterised.
To test the hypothesis that JCV might also be associated with PNETs in humans, we recently analysed a series of medulloblastomas for the presence of JCV DNA sequences and T antigen expression.12 The PCR analysis of nearly 50% of those tumours demonstrated sequences of N-terminal T antigen, C-terminal T antigen, and VP-1 capsid protein corresponding to regions of both the early and late JCV genome. In addition, 25% of tumours analysed immunohistochemically for T antigen expression showed nuclear positivity. These results raise the possibility that activation of the JCV early promoter may be an important event in some medulloblastomas.
The data presented in our current study support that possibility by demonstrating a significant relation between T antigen expression and overexpression of p53 in medulloblastomas. We also present evidence that both T antigen and p53 may be expressed in the same cells of these tumours. This suggests a T antigen–p53 association in medulloblastomas. Previous studies have shown that p53 mutations are not common in medulloblastomas. Although loss of heterozygosity in the region of chromosome 17 that contains the p53 gene has been reported in 30–40% of medulloblastomas,21,31,32 only 5–10% of these tumours contain p53 mutations.19,33 However, between 40% and 60% of medulloblastomas show raised p53 content when studied immunohistochemically.18,34 In light of the low frequency of p53 mutations, most tumours with immunocytochemical positivity for p53 must overexpress the wild-type protein. It is also of interest that high p53 expression in medulloblastomas (as measured by intensity of immunoreactivity) has been correlated with poor patient prognosis.18 Thus, it is possible that JCV induction of medulloblastomas (as demonstrated by the findings of T antigen positivity and p53 overexpression) might serve as a prognostic indicator for patient survival following diagnosis.
Although immunohistochemistry detected nuclear labelling for pRb in five of the eight medulloblastomas in our study, the percentage of staining was significantly greatest in those tumours that showed T antigen and p53 expression. The observed immunoreactivity of antibodies to the Rb related proteins p107 and Rb2/p130 in most of the T antigen positive cases was also significant. These findings are not unexpected because pRb staining has been described previously in medulloblastomas.35 The interpretation of these data is less obvious than that for p53. Although the Rb gene does not appear to be mutated in medulloblastomas,36 pRb is integral to polyomavirus tumour induction. Binding of a product of the polyoma early region—small T antigen—to pRb is an important factor in the anti-apoptotic activities integral to polyoma tumour formation.37 Transgenic mice bearing SV40 T antigen genes deficient in pRb binding regions fail to develop the choroid plexus tumours found in other SV40 T antigen transgenics.38 In addition, T antigen–pRb complex formation has been reported to occur in human medulloblastomas.39 However, one should be wary of extending observations based on pRb immunoreactivity in tissue sections. Although antibodies to pRb stain some normal human tissues, such as respiratory epithelium, lung tumours derived from this epithelium often show little or no immunoreactivity.27 In addition, reports of pRb immunoreactivity in human glial tumours show a variable percentage of positive cells, perhaps depending on the number of wild-type Rb alleles retained by the neoplasm.39,40 Nonetheless, the finding of immunoreactivity for pRb and the Rb family of proteins within these tumours suggests that associations with T antigen and/or regulation of protein expression independent of T antigen might play a role in the evolution of medulloblastomas.
In summary, our study has demonstrated that medulloblastomas that express polyomavirus T antigen are also likely to demonstrate high expression of both p53 and the Rb family of proteins. p53 and pRb may also be expressed in the same cells as T antigen. The possibility that these findings represent an association of T antigen with these proteins and the role that such an interaction might play in the development of these tumours should receive further consideration.
Acknowledgments
We would like to thank Ms C Shriver for editorial assistance, Dr LB Rorke (Department of Pathology, Children's Hospital of Philadelphia) for intellectual input, and E Gracely (Department of Biostatistics, MCP/ HU) for biostatistical advice. This work was supported by PHS grant NS36466 to KK.
References
- 1.Davis FG, Preston-Martin S. Epidemiology: incidence and survival. In: Bigner DD, McLendon RE, Bruner JM, eds. Russell and Rubenstein's pathology of tumors of the nervous system, 6th ed. New York: Oxford University Press, 1998:5–45.
- 2.Molenaar WM, Trojanowski JQ. Primitive neuroectodermal tumors of the central nervous system in childhood: tumor biological aspects. Crit Rev Oncol Hematol 1994;17:1–25. [DOI] [PubMed] [Google Scholar]
- 3.Hart MN, Earl KM. Primitive neuroectodermal tumors of the brain in children. Cancer 1973;32:890–7. [DOI] [PubMed] [Google Scholar]
- 4.Rorke LB. The cerebellar medulloblastoma and its relationship to primitive neuroectodermal tumors. J Neuropathol Exp Neurol 1983:42:1–15. [PubMed] [Google Scholar]
- 5.Provias JP, Becker LE. Cellular and molecular pathology of medulloblastoma. J Neurooncol 1996;29:35–43. [DOI] [PubMed] [Google Scholar]
- 6.Walker DL, Padgett BL, ZuRhein GM, et al. Human papovavirus (JC): induction of brain tumors in hamsters. Science 1973;181:674–6. [DOI] [PubMed] [Google Scholar]
- 7.Zhen HN, Zhang X, Bu XY, et al. Expression of the simian virus 40 large T antigen (Tag) and formation of tag–p53 and tag–pRb complexes in human brain tumors. Cancer 1999;68:2124–32. [PubMed] [Google Scholar]
- 8.Frisque RJ, White FA, III. The molecular biology of JC virus, causative agent of progressive multifocal leukoencephalopathy. In: Roos, R, ed. Molecular neurovirology. Totowa, New Jersey, USA: Humana Press, 1992:26–160.
- 9.Franks RR, Rencic A, Gordon J, et al. Formation of undifferentiated mesenteric tumors in transgenic mice expressing human neurotropic polyomavirus early protein. Oncogene 1996;12:2573–8. [PubMed] [Google Scholar]
- 10.Krynska B, Otte J, Franks R, et al. Human ubiquitous JCV (CY) T-antigen gene induces brain tumors in experimental animals. Oncogene 1999;18:39–46. [DOI] [PubMed] [Google Scholar]
- 11.Khalili K, Krynska B, Del Valle L, et al. Medulloblastomas and the human neurotropic polyoma virus JC virus. Lancet 1999;353:1152–3. [DOI] [PubMed] [Google Scholar]
- 12.Krynska B, Del Valle L, Croul S, et al. Detection of human neurotropic JC virus DNA: sequence and expression of the viral oncogenic protein in pediatric medulloblastomas. Proc Natl Acad Sci U S A 1999;96:11519–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bollag B, Chuke WF, Frisque RJ. Hybrid genomes of the polyomaviruses JC virus, BK virus, and simian virus 40: identification of sequences important for efficient transformation. J Virol 1989;63:863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krynska B, Gordon J, Otte J, et al. Role of cell cycle regulators in tumor formation in transgenic mice expressing the human neurotropic virus, JCV, early protein. J Cell Biochem 1997;67:223–30. [DOI] [PubMed] [Google Scholar]
- 15.Major EO, Traub RG. JC virus T protein during productive infection in human fetal brain and kidney cells. Virology 1986;148:221–5. [DOI] [PubMed] [Google Scholar]
- 16.Rencic A, Gordon J, Otte J, et al. Detection of JC virus DNA sequence and expression of the viral oncoprotein, tumor antigen, in brain of immunocompetent patient with oligoastrocytoma. Proc Natl Acad Sci U S A 1996;93:7352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ressetar HG, Prakash O, Frisque RJ, et al. Expression of viral T-antigen in pathological tissues from transgenic mice carrying JC-SV40 chimeric DNAs. Mol Chem Neuropathol 1993;20:59–79. [DOI] [PubMed] [Google Scholar]
- 18.Jaros E, Lunec J, Perry RH, et al. p53 overexpression defines neuroectodermal tumors with poor prognosis. Cancer Res 1993;51:639–43. [Google Scholar]
- 19.Adesina AM, Nalbantoglu J, Cavanee WK. p53 gene mutation and mdm2 gene amplification are uncommon in medulloblastoma. Cancer Res 1994;54:5649–51. [PubMed] [Google Scholar]
- 20.Burnett ME, White EC, Sih S, et al. Chromosome arm 17p deletion analysis reveals molecular genetic heterogeneity in supratentorial and infratentorial primitive neuroectodermal tumors of the central nervous system. Cancer Genet Cytogenet 1997;97:25–31. [DOI] [PubMed] [Google Scholar]
- 21.Cogen PH, McDonald JD. Tumor suppressor genes and medulloblastoma. J Neurooncol 1996;29:103–12. [DOI] [PubMed] [Google Scholar]
- 22.Nozaki M, Tada M, Matsumoto R, et al. Rare occurrence of inactivating p53 gene mutations in primary non-astrocytic tumors of the central nervous system: reappraisal by yeast functional assay. Acta Neuropathol (Berl) 1998;95:291–6. [DOI] [PubMed] [Google Scholar]
- 23.Dyson N, Bernards R, Friend S, et al. Large T-antigens of many polyoma viruses are able to form complexes with the retinoblastoma protein. J Virol 1990;64:1353–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiebert SW, Chellappan SP, Horowitz JM, et al. The interaction of RB with E2F coincides with an inhibition of the transcriptional activity of E2F. Genes Dev 1992;6:177–85. [DOI] [PubMed] [Google Scholar]
- 25.Johnson DG, Schwarz JK, Cress WD, et al. Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature 1993;365:349–52. [DOI] [PubMed] [Google Scholar]
- 26.Ludlow JW. Interactions between SV40 large-tumor antigen and the growth suppressor proteins pRb and p53. FASEB J 1993;7:866–71. [DOI] [PubMed] [Google Scholar]
- 27.Baldi A, Esposito V, DeLuca A, et al. Differential expression of the retinoblastoma gene family members pRb/p105, p107, and pRb2/p130 in lung cancer. Clin Cancer Res 1996;2:1239–45. [PubMed] [Google Scholar]
- 28.Padgett BL, Walker DL. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive mulitifocal leukoencephalopathy. J Infect Dis 1973;127:467–70. [DOI] [PubMed] [Google Scholar]
- 29.Neel JV, Major EO, Awa AA, et al. Hypothesis: “rouge cell”-type chromosomal damage in lymphocytes is associated with infection with the JC human polyoma virus and has implications for oncopoiesis. Proc Natl Acad Sci U S A 1996;93:2690–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ZuRhein GM. Studies of JC virus-induced nervous system tumors in the Syrian hamsters: a review. In: Sever JL, Madden DL, eds. Polyomavirus and human neurological diseases. 1983:205–21. [PubMed]
- 31.Bigner SH, McLenBigdon RE, Fuchs H, et al. Chromosomal characteristics of childhood brain tumors. Cancer Genet Cytogenet 1997;97:125–34. [DOI] [PubMed] [Google Scholar]
- 32.Pietsch T, Koch A, Wiestler OD. Molecular genetic studies in medulloblastomas: evidence for tumor suppressor genes at the chromosomal regions 1q31–32 and 17p13. Klin Padiatr 1997;209:150–5. [DOI] [PubMed] [Google Scholar]
- 33.Ohgaki H, Eibl RH, Wiestler OD, et al. p53 mutations in nonastrocytic human brain tumors. Cancer Res 1993;51:6202–5. [PubMed] [Google Scholar]
- 34.Karamitopoulou E, Perentes E, Diamantis I. p53 protein expression in central nervous system tumors: an immunohistochemical study with CM1 polyvalent and DO-7 monoclonal antibodies. Acta Neuropathol (Berl) 1993;85:611–16. [DOI] [PubMed] [Google Scholar]
- 35.Korshunov AG, Sycheva RV, Golanov AV. Immunohistochemical study of neoplasm-associated proteins in cerebellar medulloblastomas. Arkh Patol 1998;60:8–14. [PubMed] [Google Scholar]
- 36.Lee WH, Bookstein R, Hong F, et al. Human retinoblastoma susceptibility gene: cloning, identification and sequence. Science 1987;235:1394–9. [DOI] [PubMed] [Google Scholar]
- 37.Kolzau T, Hansen RS, Zahra D, et al. Inhibition of SV40 large T antigen induced apoptosis by small T antigen. Oncogene 1999;18:5598–603. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Tobin GJ, Pipas JM, et al. T-antigen mutant activities in vivo: roles of p53 and pRb binding in tumorigenesis of the choroid plexus. Oncogene 1992;7:1167–75. [PubMed] [Google Scholar]
- 39.Burns KL, Ueki K, Jhung SL, et al. Molecular genetic correlates of p16, cdk4, and pRb immunohistochemistry in glioblastomas. J Neuropathol Exp Neurol 1998;57:122–30. [DOI] [PubMed] [Google Scholar]
- 40.Rathore A, Kamarajan P, Mathur M, et al. Simultaneous alterations of retinoblastoma and p53 protein expression in astrocytic tumors. Pathol Oncol Res 1999;5:21–7. [DOI] [PubMed] [Google Scholar]