Abstract
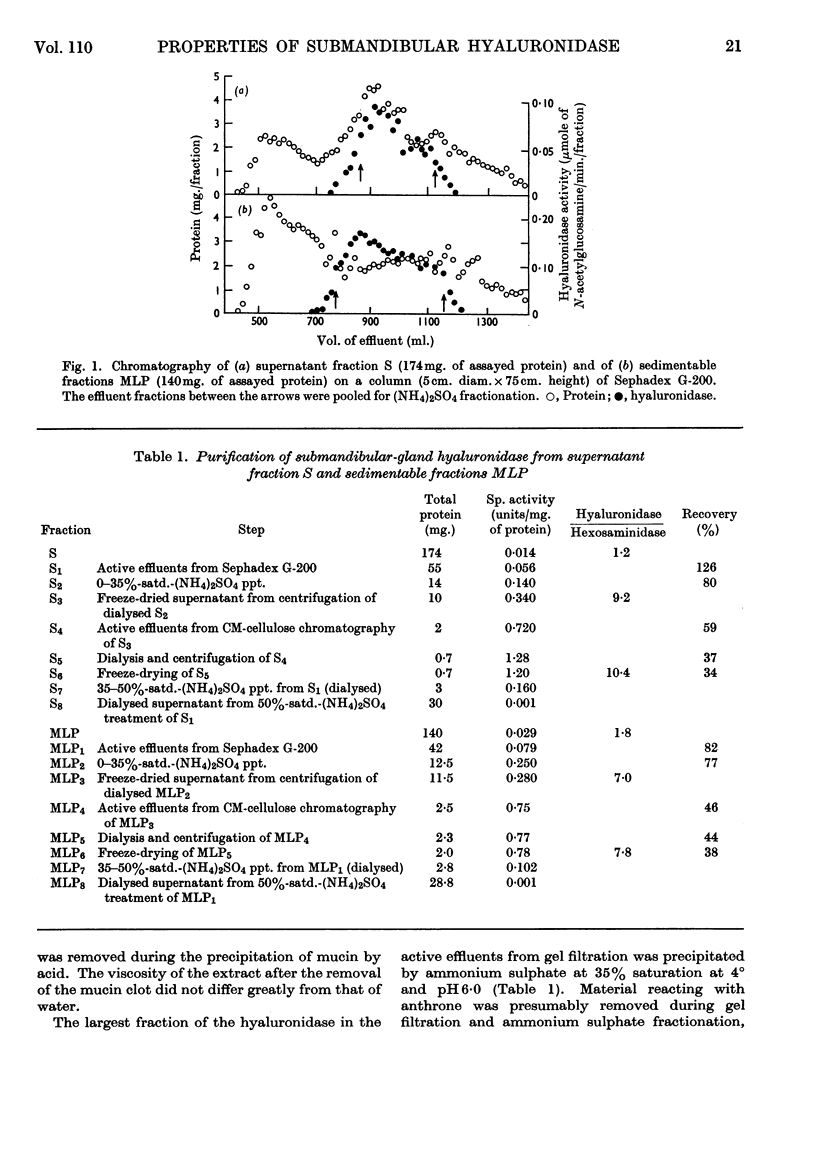
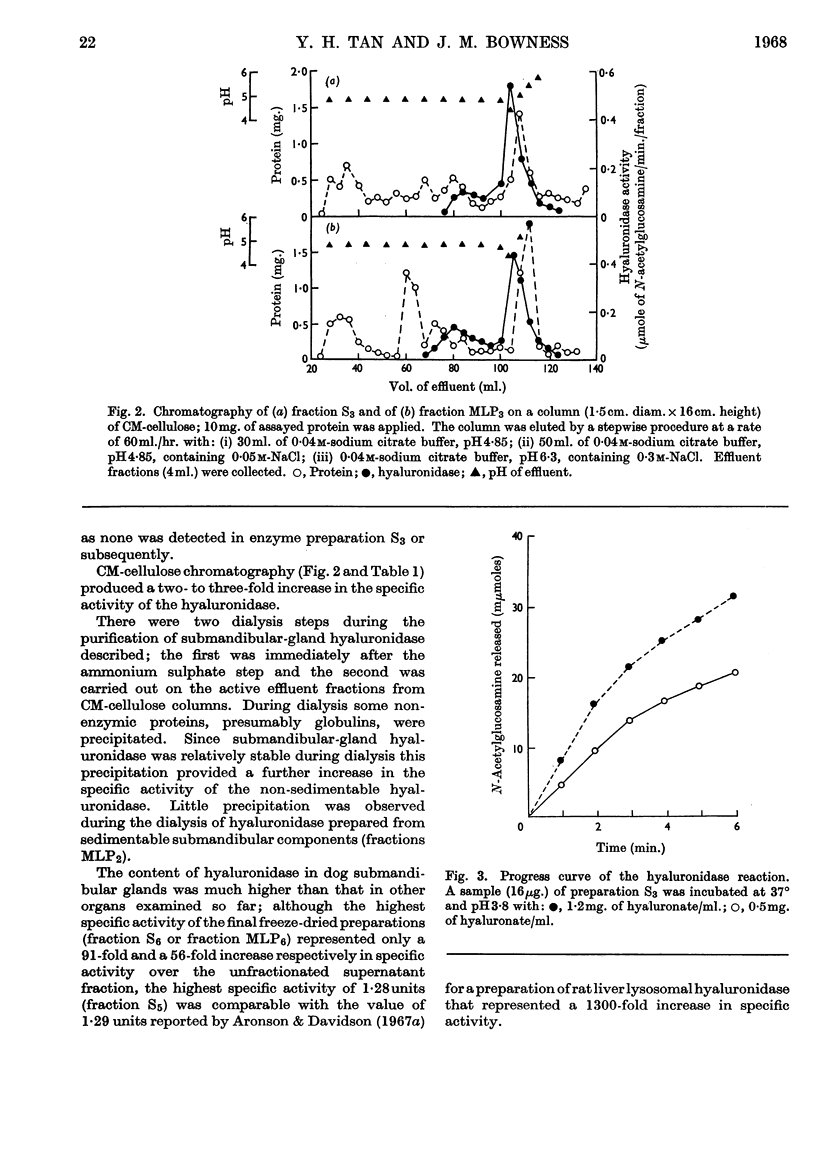
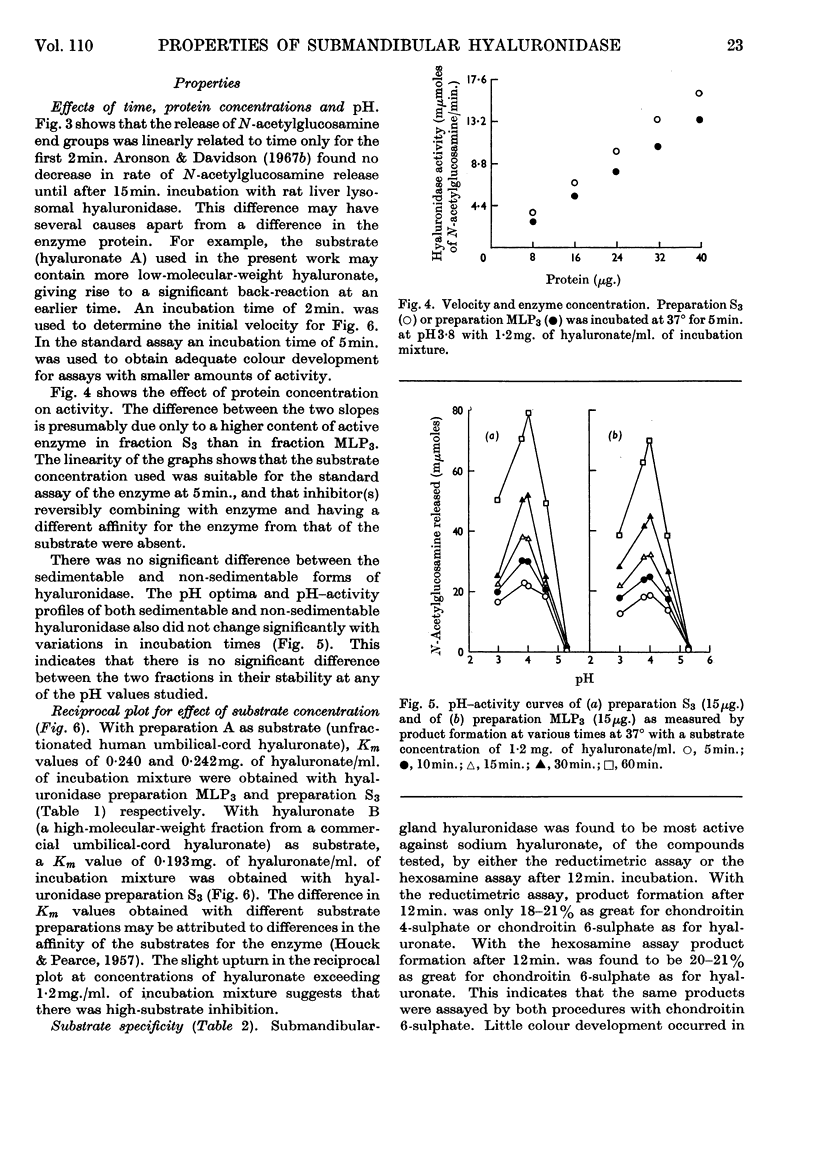
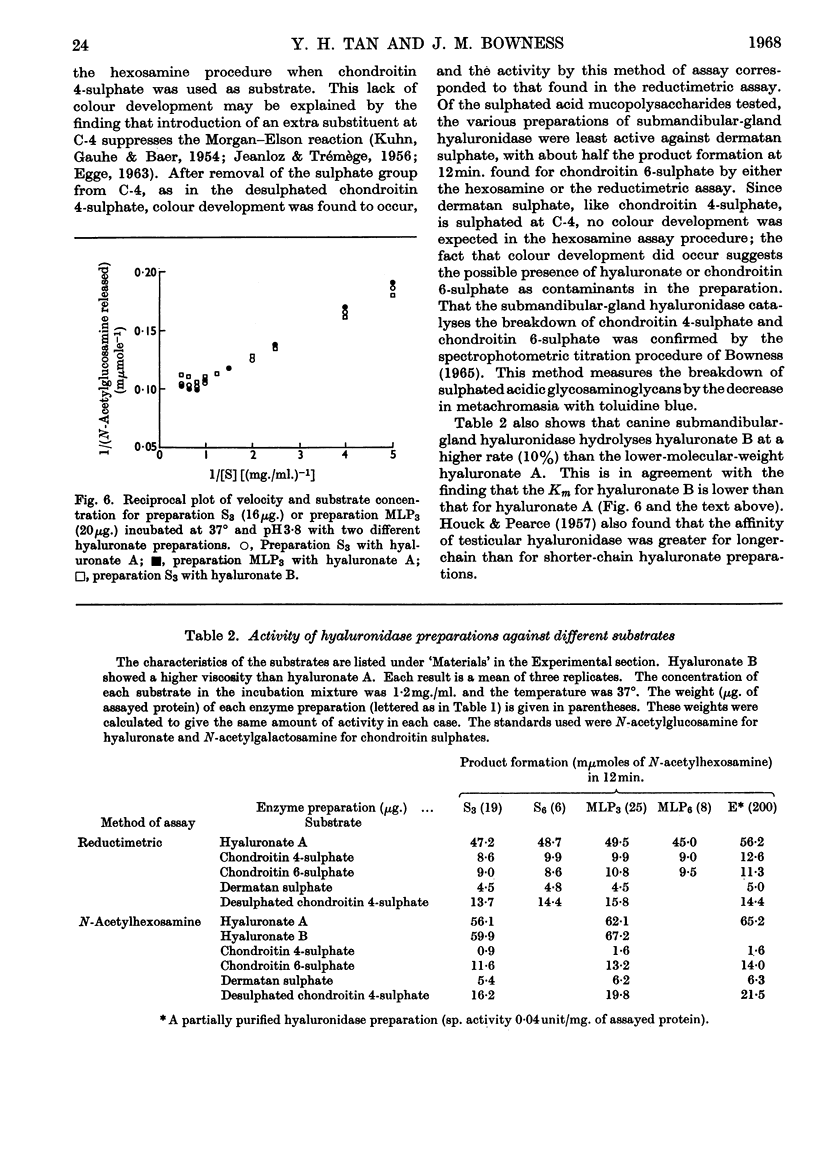
1. Methods for the purification of dog submandibular-gland hyaluronidase from sedimentable and non-sedimentable portions of a homogenate and from the whole homogenate are presented. The method consists of three main steps: removal of mucin by acid precipitation or gel filtration on Sephadex G-200, ammonium sulphate precipitation and CM-cellulose chromatography. By this method specific activities of up to 1·28 and 0·78μmoles of N-acetylglucosamine/min./mg. of protein were obtained for the purified freeze-dried non-sedimentable hyaluronidase and for the sedimentable hyaluronidase respectively. 2. A comparison of some of the properties of the non-sedimentable and the sedimentable hyaluronidase preparation indicated that there was little difference between the two and that they both resembled lysosomal hyaluronidase from rat liver.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson N. N., Jr, Davidson E. A. Lysosomal hyaluronidase from rat liver. I. Preparation. J Biol Chem. 1967 Feb 10;242(3):437–440. [PubMed] [Google Scholar]
- Aronson N. N., Jr, Davidson E. A. Lysosomal hyaluronidase from rat liver. II. Properties. J Biol Chem. 1967 Feb 10;242(3):441–444. [PubMed] [Google Scholar]
- BOAS N. F. Method for the determination of hexosamines in tissues. J Biol Chem. 1953 Oct;204(2):553–563. [PubMed] [Google Scholar]
- DODGSON K. S., PRICE R. G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem J. 1962 Jul;84:106–110. doi: 10.1042/bj0840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goggins J. F., Fullmer H. M., Steffek A. J. Hyaluronidase activity of human gingiva. Arch Pathol. 1968 Mar;85(3):272–274. [PubMed] [Google Scholar]
- HOUCK J. C., PEARCE R. H. The effect of substrate size upon hyaluronidase action. Biochim Biophys Acta. 1957 Sep;25(3):607–613. doi: 10.1016/0006-3002(57)90534-6. [DOI] [PubMed] [Google Scholar]
- JEANLOZ R. W., FORCHIELLI E. Studies on hyaluronic acid and related substances. I. Preparation of hyaluronic acid and derivatives from human umbilical cord. J Biol Chem. 1950 Oct;186(2):495–511. [PubMed] [Google Scholar]
- NISIZAWA K., PIGMAN W. The composition and properties of the mucin clot from cattle submaxillary glands. Arch Oral Biol. 1959 Oct;1:161–170. doi: 10.1016/0003-9969(59)90008-1. [DOI] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Tan Y. H., Bowness J. M. Canine submandibular-gland hyaluronidase. Identification and subcellular distribution. Biochem J. 1968 Nov;110(1):9–17. doi: 10.1042/bj1100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaes G. Hyaluronidase activity in lysosomes of bone tissue. Biochem J. 1967 Jun;103(3):802–804. doi: 10.1042/bj1030802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaes G., Jacques P. Studies on bone enzymes. The assay of acid hydrolases and other enzymes in bone tissue. Biochem J. 1965 Nov;97(2):380–388. doi: 10.1042/bj0970380. [DOI] [PMC free article] [PubMed] [Google Scholar]


