Abstract
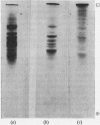
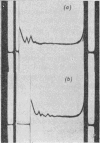
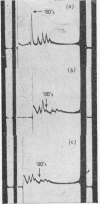
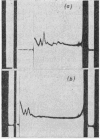
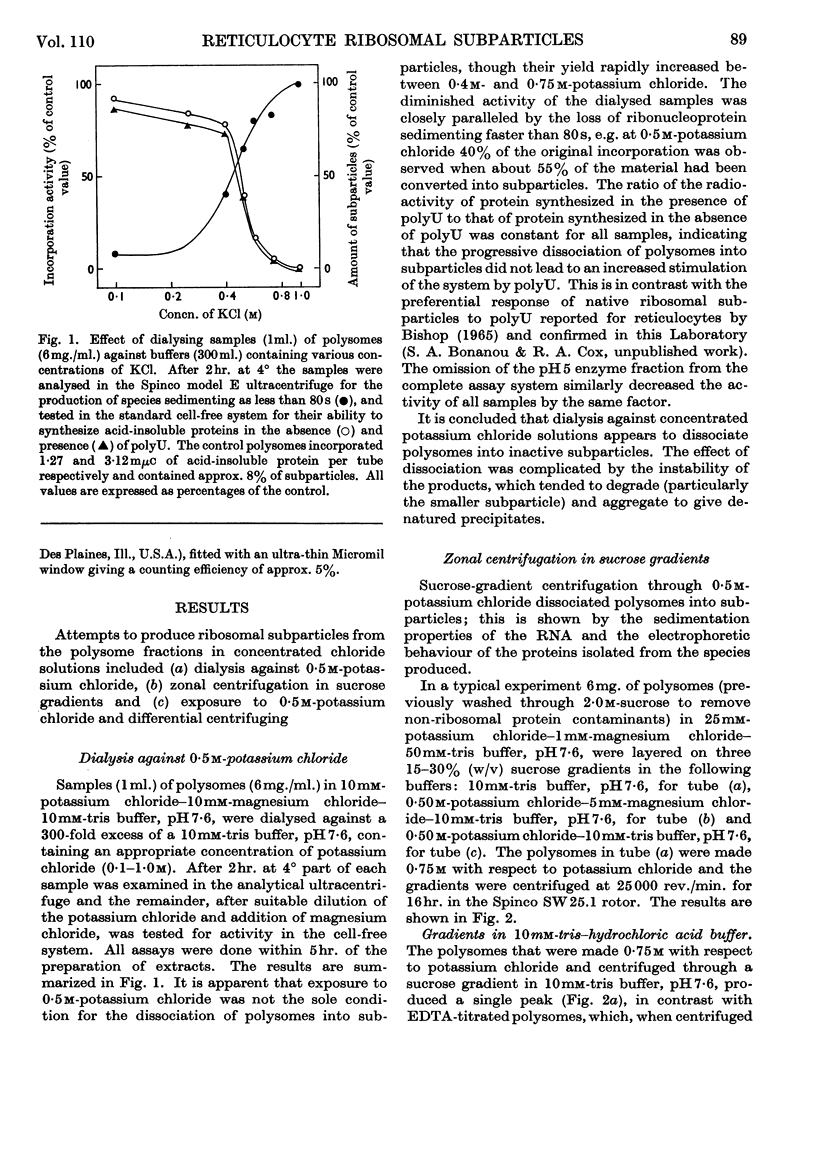
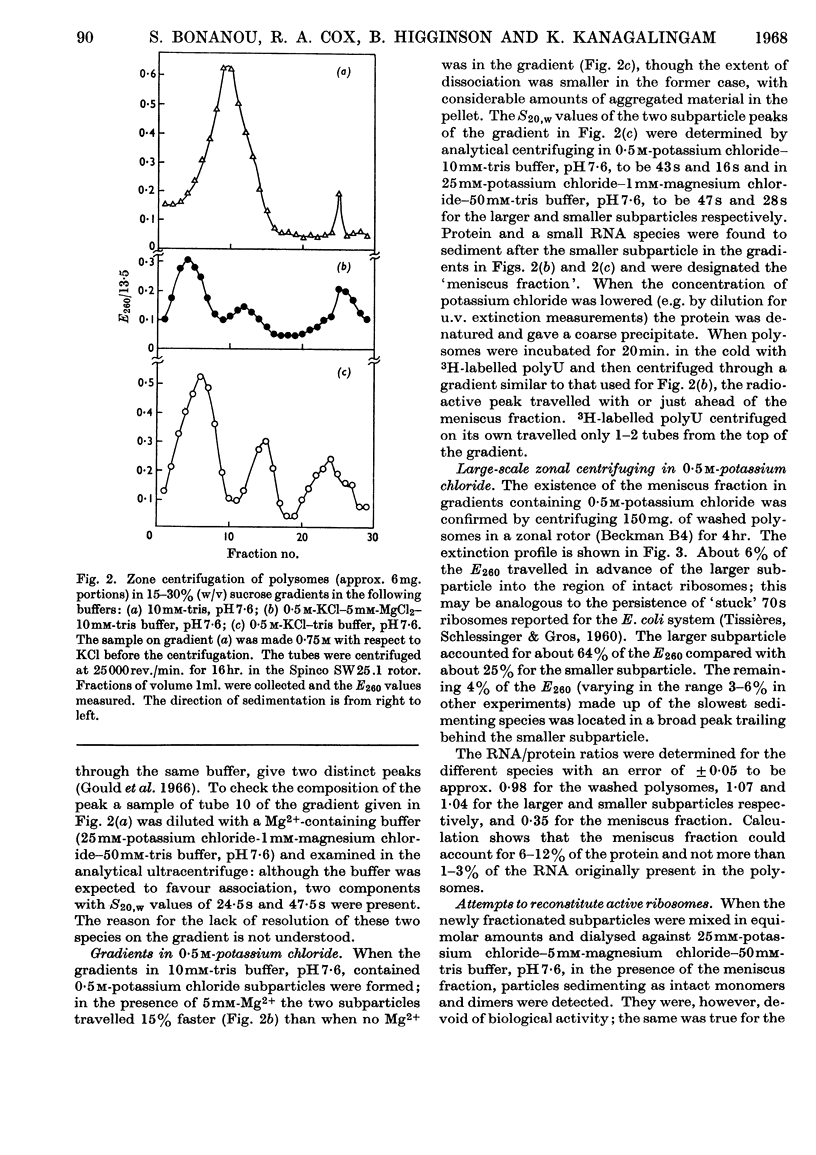
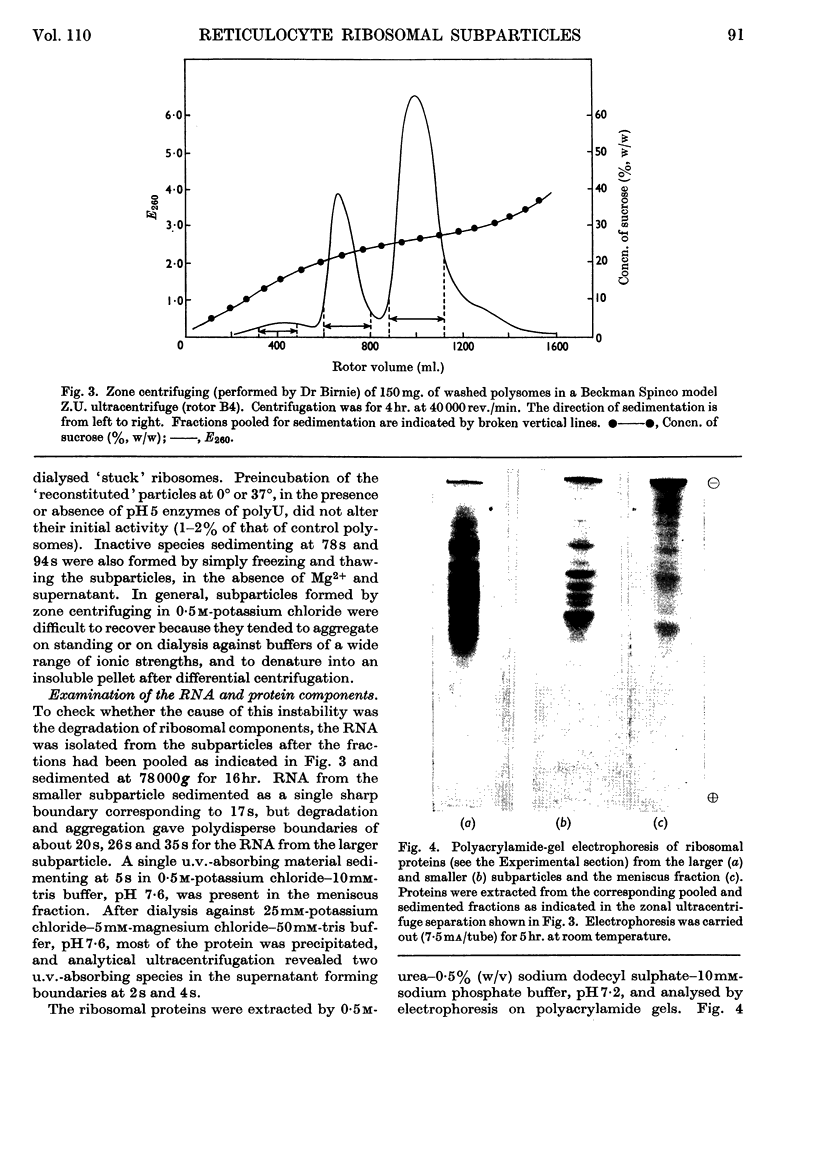
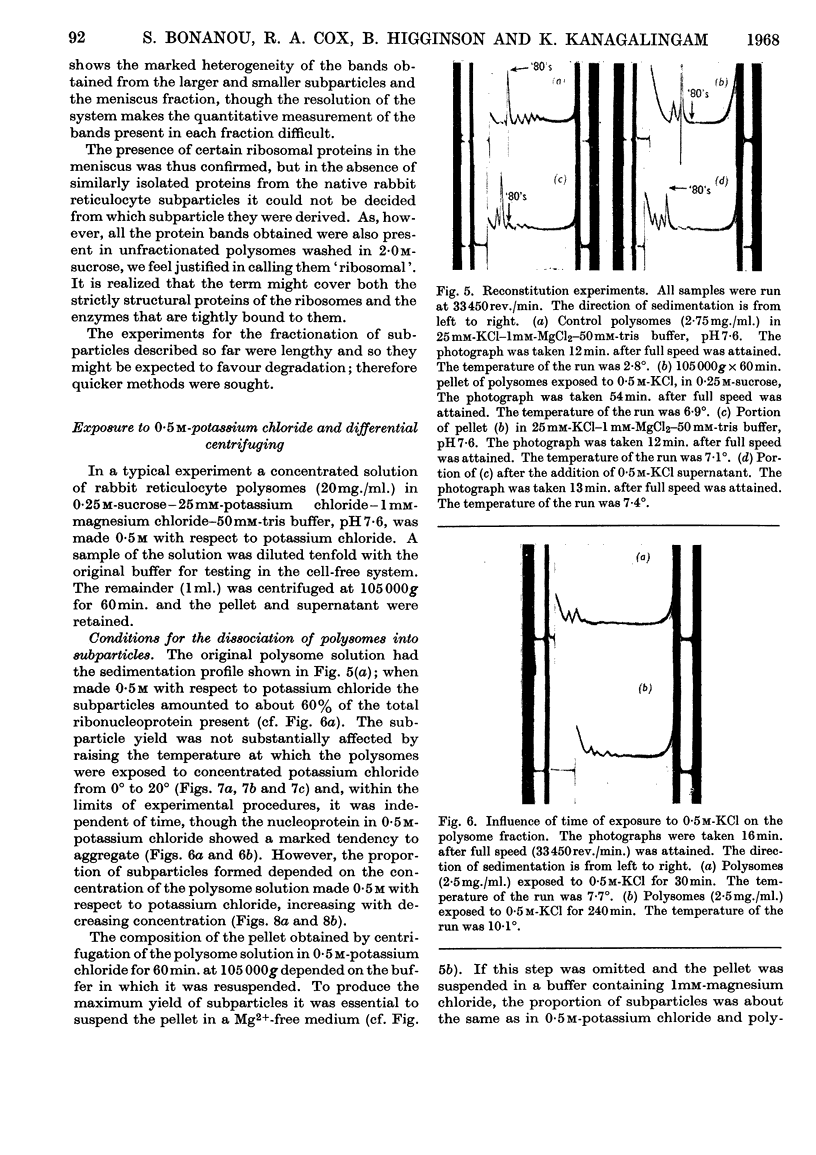
The effect of exposing rabbit reticulocyte ribosomes to concentrated solutions of potassium chloride was examined by: (a) dialysis against 0·5m-potassium chloride; (b) zone centrifugation through a sucrose gradient in 0·5m-potassium chloride; (c) differential centrifugation of a solution made 0·5m with respect to potassium chloride. The products of each treatment and their ability to support protein synthesis in a reticulocyte cell-free system, in the presence and in the absence of polyuridylic acid, were examined. The following results were found. (1) Exposing the polysomes to 0·5m-potassium chloride was not a sufficient condition for the complete dissociation of ribosomes into subparticles; the reaction showed a concentration-dependence, implying the existence of an equilibrium between the various ribosomal species. Disturbance of the equilibrium by removing certain products, as in zone centrifuging, can lead to complete dissociation. (2) The subparticles produced by dialysis or sucrose-gradient fractionation were biologically inactive and unstable. (3) The pellet obtained by differential centrifuging consisted of subparticles, if suspended in a Mg2+-free buffer; addition of Mg2+ converted about 30% of the material into heavier sedimenting species, and the preparation had 20–40% of the activity of the untreated control polysomes in the cell-free system. Addition of the 0·5m-potassium chloride supernatant fraction resulted in further apparent reconstitution of sub-particles into ribosomes and polysomes and in a 50–100% restoration of biological activity. When both polyuridylic acid and supernatant factors were present incorporations similar to or higher than those of the control were attained.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARLINGHAUS R., SHAEFER J., SCHWEET R. MECHANISM OF PEPTIDE BOND FORMATION IN POLYPEPTIDE SYNTHESIS. Proc Natl Acad Sci U S A. 1964 Jun;51:1291–1299. doi: 10.1073/pnas.51.6.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allende J. E., Seeds N. W., Conway T. W., Weissbach H. Guanosine triphosphate interaction with an amino acid polymerization factor from E. coli. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1566–1573. doi: 10.1073/pnas.58.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnstein H. R., Cox R. A., Gould H., Potter H. A comparison of methods for the isolation and fractionation of reticulocyte ribosomes. Biochem J. 1965 Aug;96(2):500–506. doi: 10.1042/bj0960500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnstein H. R., Cox R. A., Hunt J. A. The function of high-molecular-weight ribonucleic acid from rabbit reticulocytes in haemoglobin biosynthesis. Biochem J. 1964 Sep;92(3):648–661. doi: 10.1042/bj0920648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. O. Initiation of haemoglobin polypeptide chains. Biochim Biophys Acta. 1966 Apr 18;119(1):130–145. doi: 10.1016/0005-2787(66)90045-1. [DOI] [PubMed] [Google Scholar]
- Bishop J. O. Reticulocyte ribosome fraction with an exceptional capacity for polyphenylalanine synthesis. Nature. 1965 Oct 23;208(5008):361–365. doi: 10.1038/208361a0. [DOI] [PubMed] [Google Scholar]
- Brown J. C., Doty P. Protein factor requirement for binding of messenger RNA to ribosomes. Biochem Biophys Res Commun. 1968 Feb 15;30(3):284–291. doi: 10.1016/0006-291x(68)90448-8. [DOI] [PubMed] [Google Scholar]
- CHAO F. C. Dissociation of macromolecular ribonucleoprotein of yeast. Arch Biochem Biophys. 1957 Aug;70(2):426–431. doi: 10.1016/0003-9861(57)90130-3. [DOI] [PubMed] [Google Scholar]
- GILBERT W. Polypeptide synthesis in Escherichia coli. I. Ribosomes and the active complex. J Mol Biol. 1963 May;6:374–388. doi: 10.1016/s0022-2836(63)80050-9. [DOI] [PubMed] [Google Scholar]
- Gavrilova L. P., Ivanov D. A., Spirin A. S. Studies on the structure of ribosomes. 3. Stepwise unfolding of the 50 s particles without loss of ribosomal protein. J Mol Biol. 1966 Apr;16(2):473–489. doi: 10.1016/s0022-2836(66)80186-9. [DOI] [PubMed] [Google Scholar]
- Gould H. J., Arnstein H. R., Cox R. A. The dissociation of reticulocyte polysomes into subunits and the location of messenger RNA. J Mol Biol. 1966 Feb;15(2):600–618. doi: 10.1016/s0022-2836(66)80130-4. [DOI] [PubMed] [Google Scholar]
- Hamada K., Yang P., Heintz R., Schweet R. Some properties of reticulocyte ribosomal subunits. Arch Biochem Biophys. 1968 May;125(2):598–603. doi: 10.1016/0003-9861(68)90618-8. [DOI] [PubMed] [Google Scholar]
- Kaempfer R. O., Meselson M., Raskas H. J. Cyclic dissociation into stable subunits and re-formation of ribosomes during bacterial growth. J Mol Biol. 1968 Jan 28;31(2):277–289. doi: 10.1016/0022-2836(68)90444-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lerman M. I., Spirin A. S., Gavrilova L. P., Golov V. F. Studies on the structure of ribosomes. II. Stepwise dissociation of protein from ribosomes by caesium chloride and the re-assembly of ribosome-like particles. J Mol Biol. 1966 Jan;15(1):268–281. doi: 10.1016/s0022-2836(66)80226-7. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Schweet R. Isolation of a protein fraction from reticulocyte ribosomes required for de novo synthesis of hemoglobin. Arch Biochem Biophys. 1968 May;125(2):632–646. doi: 10.1016/0003-9861(68)90622-x. [DOI] [PubMed] [Google Scholar]
- Nair K. G., Arnstein H. R. Further observations on the polynucleotide-induced stimulation of protein synthesis by cell-free preparations from rabbit reticulocytes. Biochem J. 1965 Nov;97(2):595–606. doi: 10.1042/bj0970595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Lowry C. V. PHAGE f2 RNA-DIRECTED BINDING OF FORMYLMETHIONYL-TRNA TO RIBOSOMES AND THE ROLE OF 30S RIBOSOMAL SUBUNITS IN INITIATION OF PROTEIN SYNTHESIS. Proc Natl Acad Sci U S A. 1967 Sep;58(3):946–953. doi: 10.1073/pnas.58.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLESSINGER D., GROS F. STRUCTURE AND PROPERTIES OF ACTIVE RIBOSOMES OF ESCHERICHIA COLI. J Mol Biol. 1963 Oct;7:350–359. doi: 10.1016/s0022-2836(63)80029-7. [DOI] [PubMed] [Google Scholar]
- Schlessinger D., Mangiarotti G., Apirion D. The formation and stabilization of 30S and 50S ribosome couples in Escherichia coli. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1782–1789. doi: 10.1073/pnas.58.4.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley W. M., Jr, Salas M., Wahba A. J., Ochoa S. Translation of the genetic message: factors involved in the initiation of protein synthesis. Proc Natl Acad Sci U S A. 1966 Jul;56(1):290–295. doi: 10.1073/pnas.56.1.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr Evidence for virus-specific noncapsid proteins in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1965 Aug;54(2):505–513. doi: 10.1073/pnas.54.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TS'O P. O., BONNER J., VINOGRAD J. Structure and properties of microsomal nucleoprotein particles from pea seedlings. Biochim Biophys Acta. 1958 Dec;30(3):570–582. doi: 10.1016/0006-3002(58)90104-5. [DOI] [PubMed] [Google Scholar]
- Tissieres A., Schlessinger D., Gros F. AMINO ACID INCORPORATION INTO PROTEINS BY ESCHERICHIA COLI RIBOSOMES. Proc Natl Acad Sci U S A. 1960 Nov;46(11):1450–1463. doi: 10.1073/pnas.46.11.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WETTSTEIN F. O., STAEHELIN T., NOLL H. Ribosomal aggregate engaged in protein synthesis: characterization of the ergosome. Nature. 1963 Feb 2;197:430–435. doi: 10.1038/197430a0. [DOI] [PubMed] [Google Scholar]
- Yang P. C., Hamada K., Schweet R. Studies on salt-treated reticulocyte ribosomes. Arch Biochem Biophys. 1968 May;125(2):506–513. doi: 10.1016/0003-9861(68)90608-5. [DOI] [PubMed] [Google Scholar]