Abstract
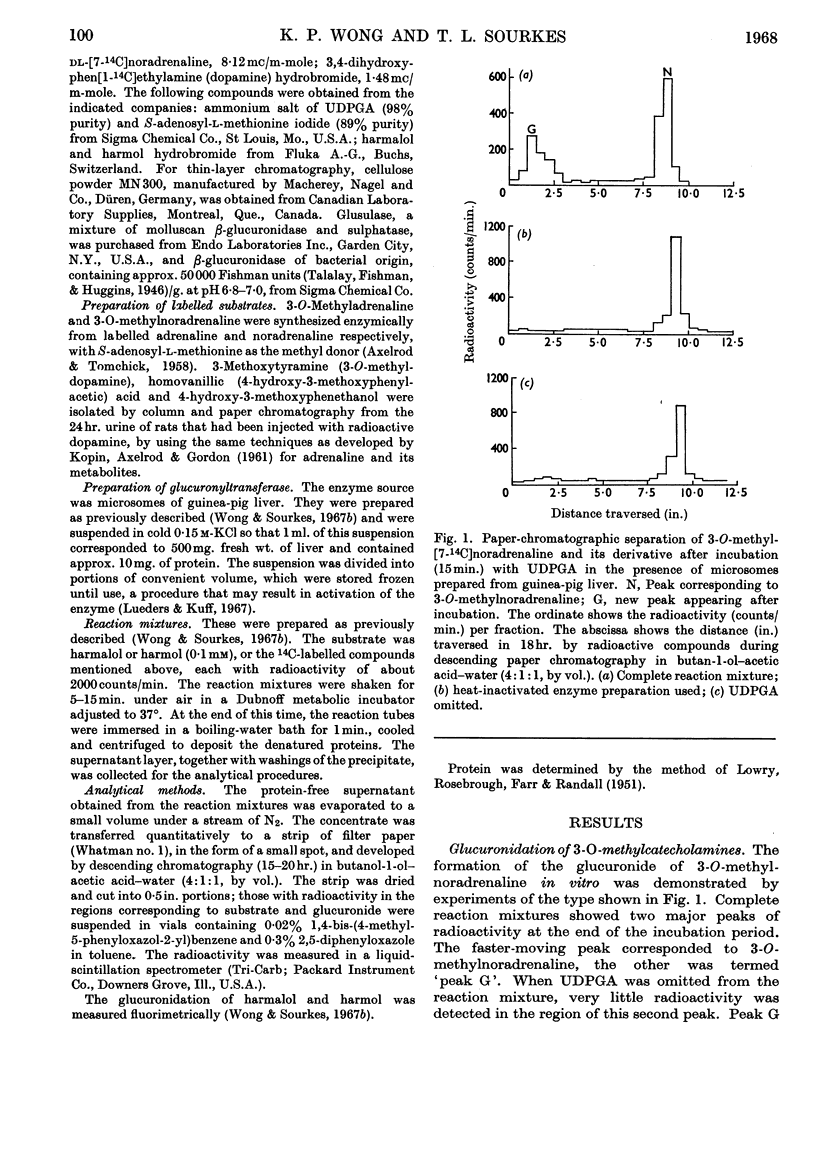
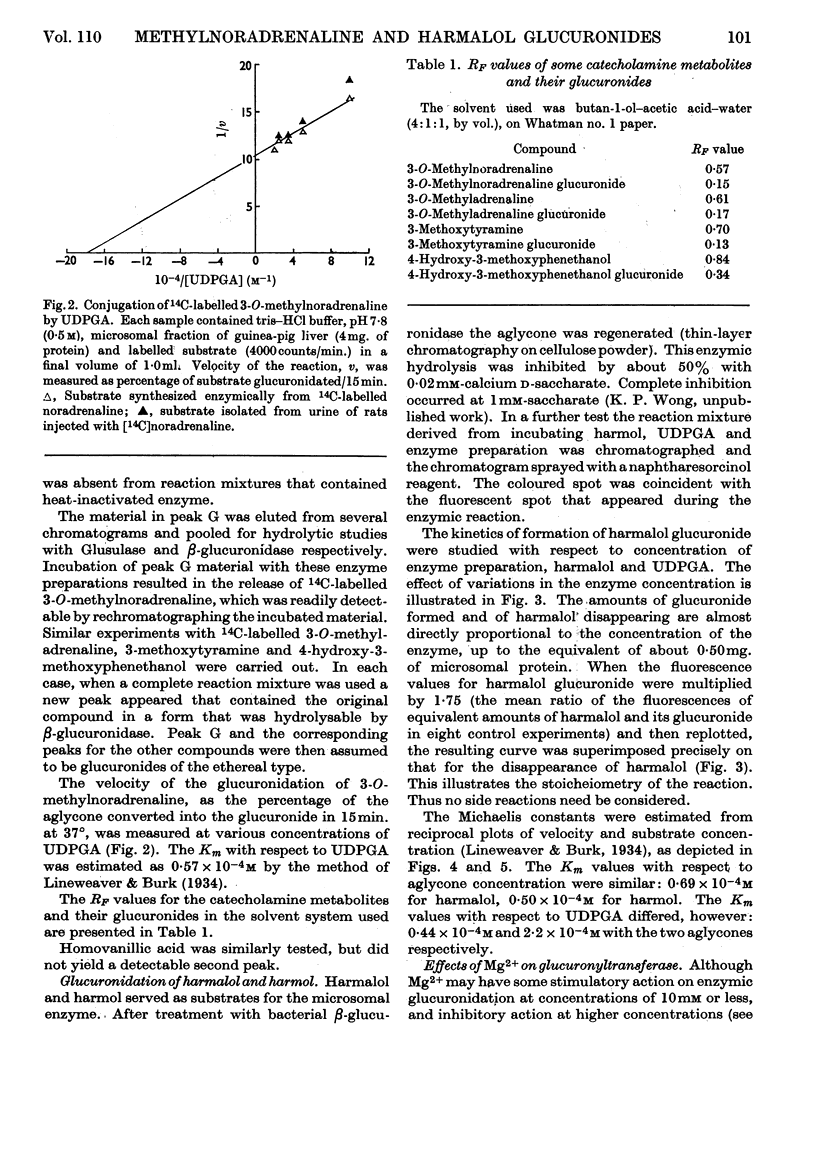
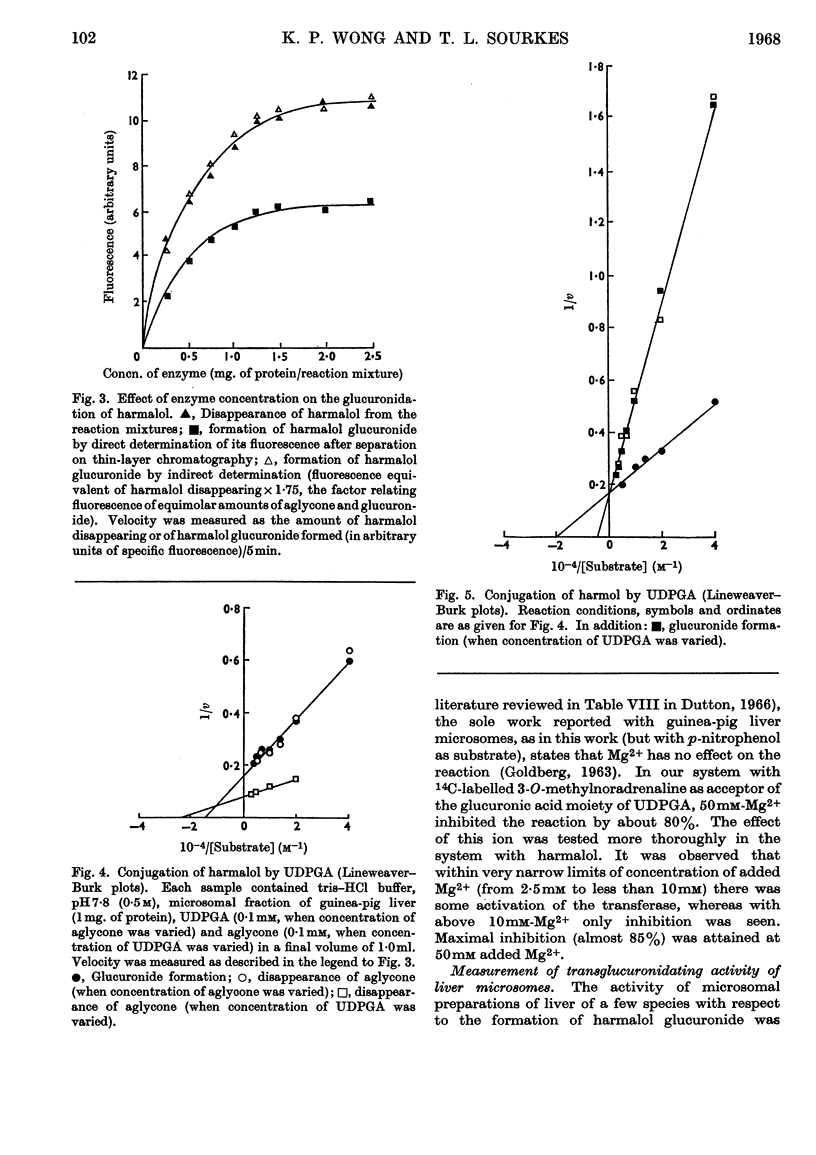
1. The following compounds were glucuronidated in the presence of UDP-glucuronic acid and a microsomal preparation made from guinea-pig liver: 14C-labelled 3-O-methyladrenaline, 3-O-methylnoradrenaline, 3-methoxytyramine and 4-hydroxy-3-methoxyphenethanol, as well as unlabelled harmalol and harmol. 2. [14C]Homovanillic (4-hydroxy-3-methoxyphenylacetic) acid was not a substrate for the microsomal glucuronyltransferase. 3. The Km values for harmalol and harmol were 0·69×10−4m and 0·50×10−4m respectively. 4. The Km values for UDP-glucuronic acid, in the presence of 14C-labelled 3-O-methylnoradrenaline, harmalol and harmol as aglycones, were 0·57×10−4m, 0·44×10−4m and 2·20×10−4m respectively. 5. Mg2+ added at 2·5–10mm activated glucuronyltransferase, with harmalol as substrate. Concentrations above 10mm inhibited the enzymic activity. 6. The overall, or net, transglucuronidating activity of microsomal preparations of the liver, with harmalol as substrate, was greatest for guinea pig, and very much lower for rabbit, mouse and rat.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG M. D., McMILLAN A. Studies on the formation of 3-methoxy-4-hydroxy-D-mandelic acid, a urinary metabolite of norepinephrine and epinephrine. Pharmacol Rev. 1959 Jun;11(2 Pt 2):394–401. [PubMed] [Google Scholar]
- AXELROD J., INSCOE J. K., SENOH S., WITKOP B. O-methylation, the principal pathway for the metabolism of epinephrine and norepinephrine in the rat. Biochim Biophys Acta. 1958 Jan;27(1):210–211. doi: 10.1016/0006-3002(58)90316-0. [DOI] [PubMed] [Google Scholar]
- AXELROD J., SENOH S., WITKOP B. O-Methylation of catechol amines in vivo. J Biol Chem. 1958 Sep;233(3):697–701. [PubMed] [Google Scholar]
- AXELROD J., TOMCHICK R. Enzymatic O-methylation of epinephrine and other catechols. J Biol Chem. 1958 Sep;233(3):702–705. [PubMed] [Google Scholar]
- AXELROD J. The metabolism of catecholamines in vivo and in vitro. Pharmacol Rev. 1959 Jun;11(2 Pt 2):402–408. [PubMed] [Google Scholar]
- AXELROD J., WEIL-MALHERBE H., TOMCHICK R. The physiological disposition of H3-epinephrine and its metabolite metanephrine. J Pharmacol Exp Ther. 1959 Dec;127:251–256. [PubMed] [Google Scholar]
- HERTTING G. THE FATE OF 3H-ISO-PROTERENOL IN THE RAT. Biochem Pharmacol. 1964 Aug;13:1119–1128. doi: 10.1016/0006-2952(64)90112-1. [DOI] [PubMed] [Google Scholar]
- HSIA D. Y., RIABOV S., DOWBEN R. M. INHIBITION OF GLUCURONOSYL TRANSFERASE BY STEROID HORMONES. Arch Biochem Biophys. 1963 Nov;103:181–185. doi: 10.1016/0003-9861(63)90392-8. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev. 1966 Jun;18(2):925–964. [PubMed] [Google Scholar]
- KIRSHNER N., GOODALL M., ROSEN L. Metabolism of dl-adrenaline-2-C14 in the human. Proc Soc Exp Biol Med. 1958 Jul;98(3):627–630. doi: 10.3181/00379727-98-24129. [DOI] [PubMed] [Google Scholar]
- KIRSHNER N., TERRY L., POLLARD D. D. The metabolism of dl-adrenaline-2-C14 in the cat. I. Urinary catabolites. Arch Int Pharmacodyn Ther. 1961 May 1;131:421–432. [PubMed] [Google Scholar]
- KOPIN I. J., AXELROD J., GORDON E. The metabolic fate of H3-epinephrine and C14-metanephrine in the rat. J Biol Chem. 1961 Jul;236:2109–2113. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Spontaneous and detergent activation of a glucuronyltransferase in vitro. Arch Biochem Biophys. 1967 Apr;120(1):198–203. doi: 10.1016/0003-9861(67)90614-5. [DOI] [PubMed] [Google Scholar]
- STOREY I. D. SOME DIFFERENCES IN THE CONJUGATION OF O-AMINOPHENOL AND P-NITROPHENOL BY THE URIDINE DIPHOSPHATE TRANSGLUCURONYLASE OF MOUSE-LIVER HOMOGENATES. Biochem J. 1965 Apr;95:209–214. doi: 10.1042/bj0950209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourkes T. L., Poirier L. J. Neurochemical bases of tremor and other disorders of movement. Can Med Assoc J. 1966 Jan 8;94(2):53–60. [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS C. M., BABUSCIO A. A., WATSON R. In vivo alteration of the pathways of dopamine metabolism. Am J Physiol. 1960 Oct;199:722–726. doi: 10.1152/ajplegacy.1960.199.4.722. [DOI] [PubMed] [Google Scholar]
- Wong K. P., Sourkes T. L. Determination of UDPG and UDPGA in tissues. Anal Biochem. 1967 Dec;21(3):444–453. doi: 10.1016/0003-2697(67)90319-3. [DOI] [PubMed] [Google Scholar]
- Wong K. P., Sourkes T. L. Uridine nucleotides in brain. J Neurochem. 1968 Mar;15(3):201–203. doi: 10.1111/j.1471-4159.1968.tb06196.x. [DOI] [PubMed] [Google Scholar]


