Abstract
Sex linked dystonia parkinsonism (XDP), also referred to as “lubag” in American literature, was described in 1975 occurring endemically in Panay, Philippines. It is an adult onset, sex linked, predominantly male, severe, progressive movement disorder with high penetrance and a high frequency of generalisation. The movement disorder is characterised by dystonic movements, usually starting in the 3rd or 4th decade, spreading to generalisation within two to five years. The dystonia coexists or is replaced by parkinsonism usually beyond the 10th year of illness. No treatment has been found to be effective. Neuroimaging shows caudate and putamenal atrophy in patients reaching the parkinsonian stage. Neuropathology reveals pronounced atrophy of the caudate and putamen, mostly in the cases with long standing illness. The sex linked pattern of inheritance has been established. Genetic studies have located the affected gene (DYT3) to Xq13.1, with one group mapping the XDP gene to a < 350 kb locus in the DXS 7117–DXS 559 region.
Keywords: dystonia, parkinsonism, sex linked recessive dystonia parkinsonism, movement disorder, Filipino dystonia parkinsonism, “lubag”
Sex linked recessive dystonia parkinsonism (XDP) is a movement disorder unique to adult Filipino men whose ancestries can be traced to Panay Island, Philippines. It is characterised by severe, progressive torsion dystonia, which dominates the first 10 to 15 years of the illness and is associated or replaced by parkinsonian features in the later years of life.
The presence of a high concentration of patients with XDP was first noted in the 1970s, when Dr GH Viterbo, of Roxas City, Capiz (a province of Panay) referred five of the six cases labelled as “dystonia musculorum deformans” to the neurology section of the Philippine General Hospital. This initiated an epidemiological survey, which resulted in the first paper published in 1976.1 Lee et al described 28 adult male patients with torsion dystonia, and 23 came from Panay Island. Six families had more than one affected male. No male to male transmission occurred; hence, the inference of sex linked recessive transmission. In addition, it was noted that some patients had parkinsonian features and relatives with parkinsonism. The phenotype of XDP was described in 42 male patients from 21 families in 1991.2 Lee et al emphasised that XDP is a movement disorder, which manifests primarily as dystonia in combination with parkinsonism. Fahn et al confirmed the coexistence of dystonia and parkinsonism in XDP.3 Fahn called the disease “lubag” based on the term used by the Ilongo speaking Filipinos to describe any movement characterised by torsion, including children with cerebral palsy.
Twenty five years after the first report, the natural history of sex linked dystonia parkinsonism has unfolded to show that, indeed, XDP is a combination of dystonia and parkinsonism presenting initially with the more disabling dystonic movements in the 3rd and 4th decade, with a subsequent diminution of movements, such that a parkinsonian-like state replaces the picture, usually beyond the 10th year of illness. Bradykinesia and postural instability, festinating gait, and blepharospasm are seen, but plastic rigidity and cogwheeling are infrequently seen.
Demographics
See table 1 ▶ for an outline of the demographics of the disease.
Table 1.
Demographics and clinical features of patients with X linked dystonia parkinsonism (XDP)
| Variable | Data |
| No. of XDP cases registered 1975–2000 | 373 |
| No. of survivors November 2000 | 268 |
| Philippine population as of 2000 | 74.7 M |
| Prevalence of surviving cases/100 000 | 0.36 |
| Prevalence of cases in Capiz/100 000 | 21.94 |
| Prevalence of cases in Aklan/100 000 | 7.72 |
| Prevalence of cases in Iloilo/100 000 | 1.43 |
| Prevalence of cases in Antique/100 000 | 0.86 |
| Prevalence of cases in Guimaras/100 000 | 0.73 |
| No. of families | 187 |
| No. of cases with positive family history | 343 |
| No. of deaths | 105 |
| No. of cases that underwent necropsy | 6 |
| Sex, male/female (%) | 370 (99)/3 (1) |
| Mean age at onset (range) | 39.5 (12–64) |
| Mean age at initial examination (range) | 40.8 (20–70) |
| Mean duration of illness from onset to present mean (range) | 13.04 (1–41) |
| Mean duration of illness from onset to generalisation of dystonia (range) | 3.8 (1–23) |
| Mean duration of illness from onset to parkinsonism (range) | 13.39 (7–25) |
| Mean age of survivors (range) | 52.73 (28–86) |
| Mean age at death (range) | 52.06 (31–81) |
| No. initially presenting with parkinsonism (%) | 24 (6) |
| No. initially presenting with dystonia (%) | 349 (94) |
| Lower extremities | 122 (33) |
| Craniofacial | 102 (27) |
| Cervical and shoulder | 92 (25) |
| Upper extremities | 52 (14) |
| Trunk | 5 (1) |
| No. of cases, spread (%) | 363 (97) |
| No. of cases, generalised (%) | 313 (84) |
| No. of cases, parkinsonian (%) | 53 (14) |
| Degree of disability of survivors | |
| No. still working (%) | 13 (5) |
| No. ambulant, not working (%) | 192 (72) |
| No. non-ambulant; able to care for self (%) | 3 (1) |
| No. wheelchair and bed bound (%) | 60 (22) |
These data are derived from the XDP Philippine Registry, 2000.
Ages and durations are in years.
Prevalence of XDP
As of year 2000, 373 cases of XDP have been registered with the Philippine XDP project based at the Philippine Children's Medical Center in Metro Manila and in Roxas City, Capiz.4 One hundred and five of these patients have died. The 268 survivors in a population of 74 million give a prevalence rate of 0.36/100 000. For the entire island of Panay, the prevalence rate is 4.77/100 000 (table 1 ▶). Among the provinces (Iloilo, Capiz, Antique, Aklan, and Guimaras), Capiz has the highest prevalence at 21.94 cases/100 000 population, which translates to 1/4000 men. Aklan has the next highest rate at 7.72/100 000. Capiz and Aklan once belonged to a single province. The figures suggest that XDP is endemic in Panay, particularly in Capiz.
Family history
Ninety four per cent of patients with XDP have a positive family history (table 1 ▶). Three hundred and seventy three cases are from 170 families. Forty two kindred have more than one affected sibling, and 39 families have more than two brothers affected. There are two sets of twin brothers affected.
Sex
There are 370 men and three women, a male to female ratio of 123 : 1 (table 1 ▶). Before 1992, all reported patients with XDP were men. The three women have affected fathers and carrier mothers by history. Two are sisters. Waters et al (1993)5 reported two sisters with XDP, one, age 61, manifesting as mild dystonia and the other, age 54, presenting with chorea. Both did not progress. Our female cases all generalised.
Age of onset
The age of onset of XDP ranges from 12 to 64 years, with a mean of 39.5 years (SD, 8.44) (table 1 ▶). The ages of onset of the female cases are above 46 years.
Initial area of involvement
Only 6% presented with parkinsonian traits (postural instability, bradykinesia, shuffling gait, and tremor); 94% presented with focal dystonia; 33% presented with symptoms (postural dystonia) in the lower extremities (for example, dorsiflexion of big toe, foot flexion, foot inversion, tremors of the feet, gait dystonia, and lower leg dystonia) and 27% presented with craniofacial symptoms (for example, blepharospasm, jaw closing, jaw opening, oromandibular, tongue protrusion or retraction, and tremors); 25% presented with symptoms in the neck and shoulder (for example, torticollis, retrocollis, anterocollis, neck stiffness, tremors, and shoulder dystonia), whereas 14% presented with symptoms involving the upper extremities (for example, tremors and cramps); only 1% have symptoms affecting the trunk (for example, flexion, tremors, torsion, and extension). Regardless of the initial area involved, the dystonia spread in 97% of cases and generalised in 84%, at a mean duration of 3.8 years (SD, 2.96) (table 1 ▶).
As the disease progressed, the predominant picture was one of nearly continuous, severe dystonic movements during the patients' waking hours (fig 1 ▶). In a few instances, sensory tricks alleviated the movements. Table 2 ▶ correlates the initial involvement and duration of illness and the final status of their dystonia, showing the spread and progression of XDP depicting the natural history. Table 2 ▶ shows that generalisation occurs as early as the first two years of illness, no matter where the initial involvement starts. In 80% of patients, generalisation has developed by the fifth year. Beyond the fifth to the seventh year, the movements become less intense, as observed on the videotapes, and gradually become less frequent. From the 10th year onwards, in addition to the gradual diminution of frequency and intensity of dystonic movements, bradykinesia was more evident and a picture of stiffness was assumed. However, examination showed that there was minimal rigidity at rest and cogwheeling was rare. The more prominent parkinsonian features are loss of postural reflexes, freezing with festinating gait, masked facies, and bradykinesia. About 20% of patients with XDP survived beyond the 15th year of illness, at which time they assumed the typical parkinsonian picture, with minimal evidence of dystonia. In most patients, however, the dystonic movements diminished and subsided, only to postural dystonia.
Figure 1.
(A) Three brothers with X linked dystonia parkinsonism (XDP) in varying stages. Patient on the left: 57 years old, after 22 years of illness, with remaining dystonia and early parkinsonism. Patient in the middle: 67 years old, after 21 years of illness, parkinsonian showing masked facies and lid retraction. Patient on the right: 53 years old , after 13 years of illness, generalised dystonia, oromandibular and leg dystonia, starting bradykinesia. (B) Generalised XDP, seventh year of illness, severe generalised dystonia with trunk extension. (C) XDP at eigth year of illness, severe generalised dystonia with trunk flexion.
Table 2.
Correlation between initial area of involvement and spread and generalisation of dystonia and progression to parkinsonism in patients with X linked dystonia parkinsonism (XDP; n = 373) as of year 2000
| Present status | ||||||
| Initial area of involvement | Duration of illness (years) | Focal n (%) | Segmental n (%) | Multifocal n (%) | Generalised n (%) | Parkinsonian n (%) |
| Lower extremities | <2 | 4 (80) | 2 (17) | 2 (28) | 37 (53) | 0 |
| n = 122 (33%) | 2–5 | 1 (20) | 5 (42) | 2 (28) | 25 (35) | 1 (4) |
| 5–10 | 0 | 3 (25) | 0 | 9 (12) | 4 (15) | |
| 10–15 | 0 | 1 (8) | 3 (44) | 0 | 2 (7) | |
| >15 | 0 | 1 (8) | 0 | 0 | 20 (74) | |
| Total | 5 | 12 | 7 | 71 | 27 | |
| Upper extremities | <2 | 0 | 1 (25) | 0 | 13 (42) | 0 |
| n = 52 (14%) | 2–5 | 0 | 1 (25) | 2 (40) | 14 (45) | 0 |
| 5–10 | 0 | 1 (25) | 1 (20) | 3 (10) | 1 (8) | |
| 10–15 | 0 | 1 (25) | 2 (40) | 1 (3) | 2 (17) | |
| > 15 | 0 | 0 | 0 | 0 | 9 (75) | |
| Total | 0 | 4 | 5 | 31 | 12 | |
| Cervix and shoulder | <2 | 0 | 3 (25) | 0 | 29 (47) | 0 |
| n = 92 (25%) | 2–5 | 2 (67) | 4 (33) | 2 (50) | 27 (43) | 2 (18) |
| 5–10 | 0 | 4 (33) | 0 | 5 (8) | 2 (18) | |
| 10–15 | 1 (33) | 0 | 2 (50) | 1 (2) | 4 (36) | |
| >15 | 0 | 1 (9) | 0 | 0 | 3 (28) | |
| Total | 3 | 12 | 4 | 62 | 11 | |
| Trunk | <2 | 0 | 1 (50) | 0 | 1 (33) | 0 |
| n = 5 (1%) | 2–5 | 0 | 1 (50) | 0 | 1 (33) | 0 |
| 5–10 | 0 | 0 | 0 | 1 (33) | 0 | |
| 10–15 | 0 | 0 | 0 | 0 | 0 | |
| >15 | 0 | 0 | 0 | 0 | 0 | |
| Total | 0 | 2 | 0 | 3 | 0 | |
| Craniofacial | <2 | 0 | 1 (6) | 1 (20) | 24 (38) | 0 |
| n = 102 (27%) | 2–5 | 1 (50) | 4 (25) | 2 (40) | 33 (52) | 2 (12) |
| 5–10 | 0 | 5 (31) | 1 (20) | 5 (8) | 1 (6) | |
| 10–15 | 1 (50) | 3 (19) | 0 | 1 (2) | 6 (38) | |
| >15 | 0 | 3 (19) | 1 (20) | 0 | 7 (44) | |
| Total | 2 | 16 | 5 | 63 | 16 | |
One hundred and five patients died; the status of dystonia at time of death was considered.
Monitoring of the severity of movements and disability was done using the Fahn-Marsden scale6 and the unified Parkinson's disease rating scale (UPDRS).7 Electrophysiological studies could not be done in Panay until 1999, so that only recently have we obtained electromyography (EMG) documentation of the movements. Observations are mostly neurological evaluations and videotape recordings. Wilhelmsen et al noted the presence of both parkinsonism and dystonia in the family they described from Iloilo in 1991.8
To date (table 1 ▶), only 13% of the 268 survivors are still working, 72% are ambulant but not working, 1% non-ambulant but able to care for themselves, and 22% are wheelchair or bed bound.
Analysis of province of origin and age of onset, in addition to the severity of the disease, and the dystonic involvement, did not reveal a significant difference, suggesting that XDP from Panay Island does not vary among Filipinos.
However, there was some intrafamilial variation in manifestations of the movements: within the same kindred there tends to be some commonality—for example, symptoms were predominantly oromandibular in families 96, 38, and 7 whereas leg dystonia predominated in families 48, 16, and 14 (XDP registry).
Response to treatment and surgery
Oral treatment in the form of anticonvulsants, antihistamines, anticholinergics, antiparkinsonian drugs, and antipsychotic drugs has shown no consistent beneficial effects.2 The use of botolinum toxin has been beneficial only for focal dystonia and provided temporary relief of focally impaired areas such as lingual or oromandibular dystonia in generalised cases.
Surgical treatment (thalamotomy in four, cerebellar implantation in one) provided partial relief, but one patient had residual hemiparesis. The patient with implantation became infected and developed an abscess, which caused his death.1
Two patients with XDP had undergone pallidotomy in the USA with unsuccessful results.9 One of these patients died 48 hours after surgery.
Diagnostic studies
Laboratory, metabolic, and biochemical studies of patients with XDP have revealed no particular abnormalities to date.2 Electrophysiological electroencephalograph (EEG) and EMG studies in some patients showed no specific abnormalities.
Among the 373 patients followed up in the Philippines, 47 (13%) underwent brain imaging studies, 32 had computed tomography (CT) scans, and 16 had magnetic resonance imaging (MRI) studies. Twelve CT scans and 16 MRIs were abnormal.
MRI findings in XDP
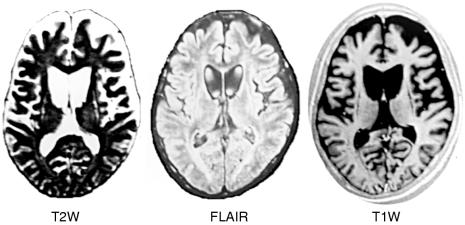
In a prospective series, 16 male patients with XDP, ranging in age from 27 to 68 years, were evaluated by MRI and the results were correlated with clinical findings, such as the age of the patient, the length of disease, the number of years of generalisation of dystonia, and the extent of dystonic and parkinsonian features. Two different kinds of MRI findings were identified (fig 2 ▶).
Figure 2.
T2 weighted (T2W), T1W, and infrared (IR) images demonstrate mild caudate atrophy and mild putaminal atrophy with peripheral high signal rim on TW2 images. Clinically, the patient has dystonia but no parkinsonism.
In 10 of 16 patients, there is no atrophy or only mild atrophy of the caudate nuclei and the putamen. However, there are bilateral curvilinear increases in signal intensity of the rims of the putamen on T2 weighting. Clinically, all 10 of these patients are in the earlier stages of disease when dystonic features predominate and parkinsonism is absent. The pattern of an outer rim of high signal in the putamen has been described previously in other diseases such as in Wilson's disease (presented by K Tan at the 10th Asian and Oceanian Congress of Neurology, Pasay City, Philippines, January 2000) (fig 3 ▶).
Figure 3.
T2 weighted (T2W), FLAIR (fluid attenuated inversion recovery), and T1W images demonstrate severe head atrophy with ex vacuo dilatation of the frontal horns and severe atrophy of the putamina with “slit-like” high signal intensity on T2W and low signal intensity on T1W. Clinically, this patient has both dystonia and parkinsonism.
In six of the 16 patients, there is bilateral and symmetric moderate to severe atrophy of the putamen and heads of the caudate nuclei, associated with ex vacuo dilatation of the frontal horns. The residual putamen demonstrates a slit-like appearance, with greatly increased signal intensity. Clinically, these patients are in the later stages of disease, when parkinsonism dominates or when parkinsonism coexists with dystonia. A similar MRI pattern has previously been described for juvenile Huntington's disease (K Tan, 2000, unpublished data).
Positron emission tomography
Two positron emission tomography (PET) studies on XDP have been published. Eidelberg et al reported PET studies on three patients with XDP.10 In all three patients, a selective reduction in normalised striatal glucose metabolism (rCMRGIc/GMR) was observed compared with 15 normal volunteer subjects. Presynaptic nigrostriatal function was assessed in these patients using fluorodopa and PET. Striatal rate constants for fluorodopa uptake were in the normal range in all three patients with XDP. These findings suggest that the extrapyramidal manifestations of XDP are metabolically localised to the striatum and that clinical parkinsonism in these patients may be secondary to extranigral factors. Waters et al reported PET studies on two patients with XDP.5 Fluorodopa PET in both the man and his affected cousin revealed reduced striatal uptake rate constants consistent with nigrostriatal involvement.
Pathology of XDP
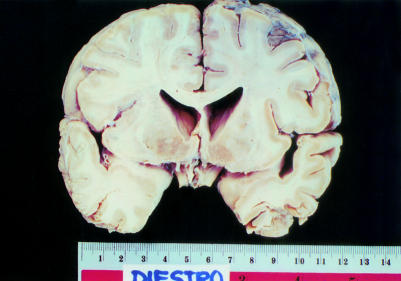
Postmortem examination of the brain was carried out on six patients. Their ages ranged from 42 to 59 years. The duration of illness ranged from three to 23 years. All six brains consistently showed varying degrees of atrophy of the caudate nucleus and putamen (fig 4 ▶). The patient with the shortest duration of illness had almost grossly normal appearing basal ganglia. The patient with the longest duration of illness showed pronounced atrophy of the putamen and caudate, with concave depression of the caudate in relation to the lateral ventricle. Neuronal loss and astrogliosis involved the putamen and caudate nucleus including the head, body, and tail (fig 5 ▶). In general, the region of the nucleus accumbens was the least affected. The degree of neuronal loss and the astrogliosis in each case parallelled the degree of gross atrophy.
Figure 4.
Caudate atrophy. Note the linear, rather than the normal convex outline of the caudate in relation to the lateral ventricle.
Figure 5.
Neuronal loss and astroliosis of the putamen in X linked dystonia parkinsonism. (A) Haematoxylin and eosin stain. (B) Glial fibrillary acidic protein stain.
In general, the cerebral cortices, thalamus, subthalamic nuclei, substantia nigra, and pons were unremarkable. Altrocchi and Forno (1983)11 and Waters et al (1993)9 each published one neuropathological case report of Filipinos with XDP showing caudate and putamenal atrophy.
Molecular genetics of XDP
The high rate of dystonia in Panay (5/100 000 population) is indicative of a genetic founder effect. A single mutation occurring in a common ancestor may have been carried on in this geographical isolate owing to the genetically non-lethal nature of the disorder. This theory is supported by the knowledge of several individuals with the disease living elsewhere in the Philippines and in the USA whose mothers or maternal ancestors originated from Panay. The identification of the XDP gene has been pursued using the positional cloning approach, which facilitates the isolation of the gene on the basis of its map position alone, even without the knowledge of its defective gene product. It is largely dependent on the availability of polymorphic markers that are linked to the disease—loci markers, which lie close to the disease gene in the same chromosome. The alleles in these loci will tend to pass together in each gamete. By inference, if the marker locus is known then the disease gene can be mapped to that region. Physical mapping can start with construction of a yeast artificial chromosome (YAC) contig map, followed by gene isolation, expression, and characterisation of the gene product.
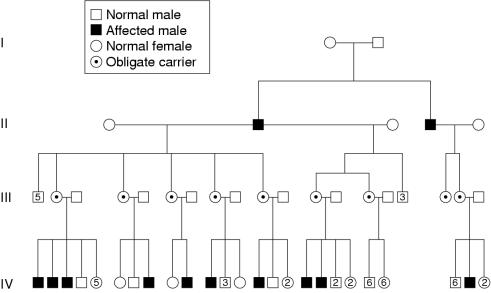
Figure 6 ▶ (family 38) is an example of an updated pedigree previously reported by Lee et al (1976)1 with 28 patients, all male, belonging to six families, which indicated that the disease under study is X linked.2 Previous reports described the disease as autosomal recessive or dominant.12 The X linked inheritance pattern was firmly established in 1990 by Kupke et al in a study of 21 families, with 36 affected males, where 17 of the kindreds had a family history of dystonia. There was no male to male transmission.13
Figure 6.
Pedigree of family 38 (as of year 2000).
Family linkage analysis with polymorphic DNA markers that span the X chromosome made it possible to assign the XDP gene to subchromosomal locations. In 1990, Kupke et al studied seven families with living patients with torsion dystonia and obligate carriers by means of linkage analysis with X chromosome (Xq21).14 In 1991, Wilhelmsen et al,8 in their study of a large Filipino family with eight affected men in three generations (total of 51 individuals), used DNA markers—34 restriction fragment length polymorphism (RFLP) markers and one CA repeat polymorphism, which span Xp 11.22 to Xq21.31—and reported that the XDP gene is located in the pericentromeric region of the X chromosome.8
In addition, linkage disequilibrium studies,15 where frequencies of alleles for tightly linked markers in affected individuals differed from the rest of the normal population, contributed to the narrowing of the suballocation in the X chromosome to Xq13. The authors studied 47 unrelated families with affected and unaffected members of a three generation XDP pedigree and 105 unrelated normal Filipino male controls. They used polymorphic dinucleotide tandem repeats (DNTRs) from Xq13 derived YACs (that encompass either PGK1 or the proximally adjacent locus DXS56). A DNTR locus 4548–7 (locus DXS56) recombination event was observed, indicating that DNTR locus 4548–7 is a distal flanking marker. In 1994, Muller and colleagues16 narrowed the approximate location to 3.0 Mb, which is flanked by DXS106 and DXS559, using short tandem repeat polymorphism at six loci through linkage disequilibrium and haplotype analysis.
Table 3 ▶ summarises the methods and markers used in the chromosomal sublocalisation of the XDP gene.
Table 3.
Linkage analysis of the X linked dystonia parkinsonism (XDP) gene using different markers
| Year | First author | Analysis | Marker | Location |
| 1990 | Kupke13 | Linkage | RFLPs | Xq21 |
| 1991 | Wilhelmsen8 | Linkage | RFLPs CA repeats | Xp11.22–q21.3 |
| 1992 | Kupke14 | Linkage Disequilibrium | Xq12–q21.1 | |
| 1992 | Graeber15 | Linkage | DNTRs | Xq13 |
| 1994 | Muller16 | Disequilibrium Haplotype | STRs | DXS106–DXS559 |
DNTRs, dinucleotide tandem repeats; RFLPs, restriction fragment length polymorphisms; STRs, sequence tagged sites.
Table 4 ▶ summarises the studies in the physical mapping of the XDP gene. In 1995, Haberhausen and colleagues17 were able to assign DYT3 to a small region within a 1.8 Mb YAC contig constructed of Xq13.1 (1.8 Mb), which included loci DXS453, DXs348, IL2R gamma, GJB1, CCG1, and DX559, using nine sequence tagged sites (STSs) and four short tandem repeat polymorphic markers, and were able to pinpoint the gene location to within 1 Mb (DXS7117–DXS1124). The latest study is that of Nemeth's18 group (1999), who mapped the XDP gene to a < 350 kb locus in the DXS7117–DXS559 region. They have constructed a sequence ready contig of 700 kb spanning the region where nine genes and novel expressed sequence tags have been mapped. They have already excluded two of these genes as the cause of XDP.
Table 4.
Physical mapping of the XDP gene (DYT3 locus)
| Year | Investigator | Analysis | Markers | X region |
| 1995 | Haberhausen17 | Disequilibrium | STSs, STRs | YAC contig Xq13.1 |
| 1998 | Wilhelmsen | Disequilibrium | New polym | DXS7117–DXS1124 |
| 1999 | Nemeth18 | Haplotype | New polym | DXS7117–DXS559 |
STSs, sequence tagged sites; YAC, yeast artificial chromosome.
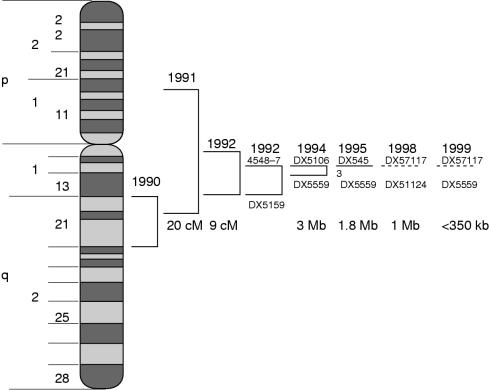
Figure 7 ▶ is a schematic diagram showing the time wise development in the physical mapping of the XDP gene in the X chromosome up to the approximation of the locus to a candidate interval of < 50 kb segment.
Figure 7.
Tracking down the X linked dystonia parkinsonism (XPD) gene.
In 1997, Peters and colleagues19 considered AFX1 and p54nrb as candidate genes for XDP because both genes mapped to a YAC contig of Xq13.1 and both genes are expressed in the brain among other tissues. However, AFX1 and p54rb were both excluded as candidates when no differences were seen on sequencing of the exons and the flanking intronic sequences in a patient with XDP and a control and by northern blot analysis.
Summary
Dystonia is the main feature of familial dopa responsive dystonia, dystonic juvenile parkinsonism, and other diseases caused by basal ganglia lesions that develop in childhood.20 In contrast, basal ganglia lesions that occur in adulthood manifest in parkinsonism.
However, XDP defies these generalities. Its onset is in adulthood, by the end of the 3rd decade or early 4th decade, but it manifests predominantly as dystonia. This spreads and generalisation occurs after two to five years; this plateaus until the 10th year, then becomes less severe or is replaced by a Parkinsonian picture by the 15th year of illness.
The PET scan findings and the MRI results have suggested involvement of the striatum. This has been verified by the neuropathological findings of caudate and putamenal atrophy found mostly in those with parkinsonian manifestations.
So far, no neurohistochemical analyses have been made, but it is tempting to speculate that at the time when dystonia manifests in patients with XDP, there is pronounced activity of tyrosine hydroxylase at the terminals of the nigrostriatal dopamine (NS-DA) neurones, although they are already in the 3rd and 4th decades of life. It is possible that as the decrease in NS-DA activities occurs, as expected with age variation (usually in the first 3 decades), parkinsonism replaces the dystonia.
The location of the degenerative process may also modify the motor manifestations; depending on whether the direct or indirect pathway is affected. The direct pathway, located in the ventral area of the striatum, matures earlier, whereas the indirect pathway, in the dorsal area, matures later.
Functional MRI and PET studies, in addition to neurohistochemical investigations, should help to clarify these issues so that the mystery of XDP might be elucidated while awaiting the genetic answers and the definition of the defect.
Acknowledgments
The authors express their appreciation to the following PCMC staff: Ms O Peralta and Ms J Jara for technical assistance, Ms M Lagula for clerical assistance, and Dr C Rivera. We also sincerely thank Ms R Borres, Dr J Arancillo, Mrs M Viterbo, Ms C del Rosario Andrade, and Mayor A Del Rosario from Roxas City. Dr E Maranon and Dr C Javellosa-Demaisip from Iloilo City are acknowledged for their continuous support in this study.
References
- 1.Lee LV, Pascasio FM, Fuentes FD, et al. Torsion dystonia in Panay, Philippines. Adv Neurol 1976;14:137–51. [PubMed] [Google Scholar]
- 2.Lee LV, Kupke KG, Gonzaga-Caballar F, et al. The phenotype of the X-linked dystonia-parkinsonism syndrome: an assessment of the 42 cases in the Philippines. Medicine (Baltimore) 1991;70:179–87. [DOI] [PubMed] [Google Scholar]
- 3.Fahn S, Moskowitz CB. X-linked recessive dystonia and parkinsonism in Filipino males. Ann Neurol 1988;24:179. [Google Scholar]
- 4.Lee LV. Phenotype of X-linked dystonia of Panay, epidemiology and spectrum of movement disorder [abstract]. 10th Asian and Oceanian Congress of Neurology in the third millennium, January 2000, Manila, Philippines: Philippine Neurological Society:26–7.
- 5.Waters CH, Takahashi H, Wilhelmsen KC, et al. Phenotype expression of X-linked dystonia-parkinsonism (lubag) in 2 women. Neurology 1993;43:1555–8. [DOI] [PubMed] [Google Scholar]
- 6.Fahn S, Marsden CD, Calne DB. Classification and investigation of dystonia. Mov Disord 1987;2:332–58. [Google Scholar]
- 7.Lang AE, Fahn S. Assessment of Parkinson's disease. In: Munsat TL, ed. Quantification of neurologic deficit. Boston: Butterworths, 1989:285–309.
- 8.Wilhelmsen KC, Weeks DE, Nygaard TC, et al. Genetic mapping of “lubag” (X-linked dystonia-parkinsonism) in a Filipino kindred to the pericentromeric region of the X-chromosome. Ann Neurol 1991;29:124–31. [DOI] [PubMed] [Google Scholar]
- 9.Waters CH, Faust PL, Powers J, et al. Neuropathology of lubag (X-linked dystonia parkinsonism). Mov Disord 1993;8:387–90. [DOI] [PubMed] [Google Scholar]
- 10.Eidelberg D, Takikawa S, Wilhelmsen K, et al. Positron emission tomographic findings in Filipino X-linked dystonia parkinsonism. Ann Neurol 1993;34:185–91. [DOI] [PubMed] [Google Scholar]
- 11.Altrocchi PH, Forno LS. Spontaneous oral-facial dyskinesia: neuropathology of a case. Neurology 1983;33:802–5. [DOI] [PubMed] [Google Scholar]
- 12.Hayes MW, Ouvrier RA, Evans W, et al. X-linked dystonia-deafness syndrome. Mov Disord 1998;13:303–8. [DOI] [PubMed] [Google Scholar]
- 13.Kupke KG, Lee LV, Viterbo GH, et al. X-linked recessive torsion dystonia in the Philippines. Am J Med Genet 1990;36:237–42. [DOI] [PubMed] [Google Scholar]
- 14.Kupke KG, Lee LV, Muller U. Assignment of the X-linked torsion dystonia gene to Xq21 by linkage analysis. Neurology 1990;40:1438–42. [DOI] [PubMed] [Google Scholar]
- 15.Graeber MB, Kupke KG, Muller U. Delineation of the dystonia-parkinsonism syndrome locus in Xq13. Proc Natl Acad Sci U S A 1992;89:8245–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller U, Haberhausen G, Wagner T, et al. DXS106 and DXS559 flank the X-linked dystonia-parkinsonism syndrome locus (DYT3). Genomics 1994;23:114–17. [DOI] [PubMed] [Google Scholar]
- 17.Haberhausen G, Schmitt I, Kohler A, et al. Assignment of the dystonia-parkinsonism syndrome locus, DYT3, to a small region within a 1.8 Mb YAC contig of Xq13.1. Am J Hum Genet 1995;57:644–50. [PMC free article] [PubMed] [Google Scholar]
- 18.Nemeth AH, Nolte D, Dunne E, et al. Refined linkage disequilibrium and physical mapping of the gene locus for X-linked dystonia-parkinsonism (DYT3). Genomics 1999;60:320–9. [DOI] [PubMed] [Google Scholar]
- 19.Peters U, Haberhausen G, Kostrzewa M, et al. AFX1 and p54 nrb: fine mapping, genomic structure, and exclusion as candidate genes of X-linked dystonia parkinsonism. Hum Genet 1997;100:569–72. [DOI] [PubMed] [Google Scholar]
- 20.Segawa M. Development of the nigrostriatal dopamine neuron and the pathways in the basal ganglia. Brain Dev 2000;S1:1–4. [DOI] [PubMed] [Google Scholar]