Abstract
Guillain Barré syndrome is one of the best examples of a post infectious immune disease and offers insights into the mechanism of tissue damage in other more common autoimmune diseases. Controlled epidemiological studies have linked it to infection with Campylobacter jejuni in addition to other viruses including cytomegalovirus and Epstein Barr virus. The syndrome includes several pathological subtypes, of which the most common is a multifocal demyelinating disorder of the peripheral nerves in close association with macrophages. Evidence from histological examination of peripheral nerve biopsy and postmortem samples suggests that both cell mediated and humoral mechanisms are involved in the pathogenesis. Immunological studies suggest that at least one third of patients have antibodies against nerve gangliosides, which in some cases also react with constituents of the liposaccharide of C jejuni. In the Miller Fisher variant of the disease, these antiganglioside antibodies have been shown to produce neuromuscular block, and may in part explain the clinical signs of that disorder. Treatment with both intravenous immunoglobulin and plasma exchange reduces the time taken for recovery to occur, although mortality remains around 8%, with about 20% of patients remaining disabled.
Keywords: Guillain Barré syndrome, Campylobacter jejuni, antiganglioside antibodies, intravenous immunoglobulin treatment, plasma exchange
Guillain Barré syndrome remains one of the most fascinating yet challenging conditions despite considerable advances in its understanding and treatment over the past 10 years.
Described originally by French physicians working in the Sixth Army camp during the First World War,1 it has remained relatively rare, but so striking in its presentation that few doctors will not remember the clinical features. Current epidemiological studies suggest an incidence of between 1 and 2/100 000 with slightly, more male individuals affected than females.2 The incidence rises with age, although there is a minor peak among young adults.3 Its relation to infection and its place as an autoimmune disease have stimulated much research over the years, which has been rewarded by the discovery of antiganglioside antibodies in at least one third of patients.4 These antibodies appear to crossreact with antigens in the lipopolysaccharide of some antecedent infective agents, providing a possible mechanism for the disease.5 Although the clinical syndrome presents with a rapidly progressive neuropathy, it is now recognised that several pathologically and probably aetiologically distinct subtypes exist (table 1 ▶).
Table 1.
Subtypes of Guillain Barré syndrome
| Acute inflammatory demyelinating polyneuropathy |
| Acute motor axonal neuropathy |
| Acute motor and sensory axonal neuropathy |
| Miller Fisher syndrome |
It should also be remembered that Guillain Barré syndrome is but one of a spectrum of diseases, identified by its rapid presentation, but closely allied to more chronic diseases, such as subacute inflammatory demyelinating polyneuropathy and chronic inflammatory demyelinating neuropathy (table 2 ▶). The diagnostic criteria and treatment of these disorders differ, although there are many pathological similarities.
Table 2.
Spectrum of demyelinating neuropathies
| Guillain Barré syndrome |
| Subacute demyelinating polyneuropathy |
| Chronic inflammatory demyelinating polyneuropathy |
Clinical features
Criteria for the diagnosis of Guillain Barré syndrome were initially devised to investigate the possible association of this disease with swine flu vaccination in the 1970s.6 These criteria have been redefined in the light of advances in the electrophysiology of Guillain Barré syndrome.7 Required criteria for the diagnosis include progressive weakness of more than two limbs, areflexia, and progression for no more than four weeks. Other causes of an acute neuropathy such as lead poisoning, vasculitis, botulism, and porphyria require exclusion. Supportive criteria include relatively mild sensory signs, raised protein in the cerebrospinal fluid (CSF), with a relatively normal cell count, and neurophysiological evidence of conduction block. Weakness is frequently proximal and distal, unlike dying back axonopathies, and respiratory involvement occurs in about a quarter of cases. The CSF protein may be normal in the first week of the illness8 but may then rise to several g/dl. The CSF cell count usually remains below 500 cells/litre. Oligoclonal bands are sometimes found in the CSF. Routine blood tests sometimes reveal a raised sedimentation rate with hyponatraemia from inappropriate antidiuretic hormone release, and mild impairment of liver function tests is not uncommon.
Antecedent events
Although Guillain, Barré, and Strohl did not comment on the association of this illness with infection, extensive clinical observations supported by epidemiological studies suggest that about 75% of patients have a history of preceding symptoms of infection.9 Serological studies reveal evidence of antecedent infection in about 30%9–11 to 50% of cases.12 These data are supported by accounts of outbreaks of Guillain Barré syndrome and an association between clinical cases of food poisoning and Guillain Barré syndrome within communities. Case controlled studies confirm a significant association with C jejuni,13 cytomegalovirus,14 and probably Epstein-Barr virus.14 Of these, the association with C jejuni remains the most highly studied. Numerous anecdotal reports of associations with other infections exist in the literature. Some immunisations also appear to be recognised triggers of the disease, including swine flu15 and rabies.16 Serological evidence of C jejuni infection occurs in about 30% of patients with Guillain Barré syndrome and appears to be associated with slightly more severe disease and with acute motor axonal neuropathy (AMAN) variants.13,17 Many examples of persistent excretion of this organism in the stools of clinical cases of Guillain Barré syndrome are described, strengthening the association.18
Pathology
The studies of Asbury and colleagues19 suggested that the earliest hall mark of Guillain Barré syndrome was the presence of perifascicular lymphocytic cuffs of small vessels in the endoneurium and perineurium. This appears to be associated with demyelination, which is typically macrophage associated.20 In this regard, the pathology has many similarities with the animal model, experimental allergic neuritis (EAN).21 More recent pathological studies have shown that several pathological subtypes of Guillain Barré syndrome exist, although the demyelinating form of the disease is the most common, and probably represents at least 75% of cases.22 Some cases of Guillain Barré syndrome are associated with a primarily axonal process, in which macrophages may be found in close proximity to the axon, with sparing of myelin.23 This histological finding has been interpreted as indicating an immunological attack on antigens of axonal origin, rather than a myelin antigen in demyelinating forms of the disease.
Still other cases of the disease appear to involve both sensory and motor axons and such cases are termed acute motor and sensory axonal neuropathy (AMSAN). This variant of the disease appears to be the most uncommon and perhaps accounts for only 5% of the clinical syndrome.
Electrophysiology
Early neurophysiological studies revealed that, despite the demyelinating pathology, many patients retained normal conduction velocities until the disease was well established. The earliest changes appear to be a delay in F waves (implying root demyelination)24 and reduction in nerve motor action potentials. This last abnormality may be difficult to determine precisely for technical reasons until the abnormality is severe. Patients with early Guillain Barré syndrome frequently have conduction block or dispersion of the responses at sights of natural nerve compression, such as carpal tunnel. The extent of reduction in the motor nerve action potentials appears to correlate with prognosis. It is exceptional for extensive neurophysiological tests to be normal in Guillain Barré syndrome, but this does sometimes occur, presumably because demyelinating lesions have occurred in anatomical sites that are exclusively proximal and not amenable to easy neurophysiological study.
Immunology
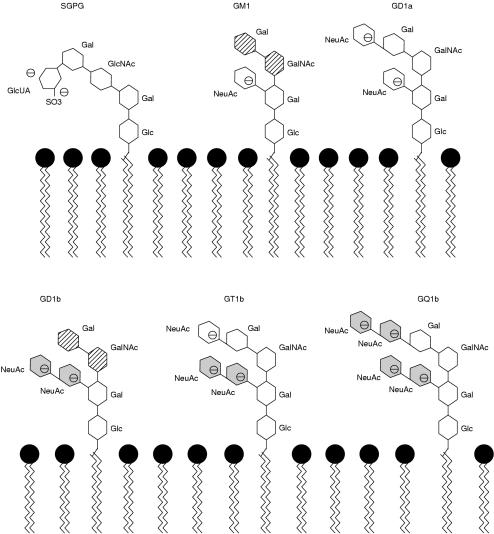
The earliest immunological studies of Guillain Barré syndrome were limited to crude complement fixation tests to nerve antigens. Such studies suggested minor abnormalities in only a small proportion of cases.25 Nevertheless, the dramatic response of demyelinating cases of Guillain Barré syndrome to treatment with plasma exchange strengthened the view that a plasma derived factor must have a role in the aetiology of the syndrome. In the mid-1980s Koski et al described a C1 esterase technique that appeared to detect subtle complement fixation in most patients with Guillain Barré syndrome26 and, furthermore, the concentrations fell during the convalescent stage of the disease. Unfortunately, this test proved difficult to reproduce and few other laboratories could demonstrate such striking abnormalities. The discovery of antiganglioside antibodies in the serum of patients with Guillain Barré syndrome has sparked of an enormous proliferation of publications. The frequency of such antibodies varies from as low as 29%27 up to nearly 70%,28 although the average figure is probably around 30%. Patients with Miller Fisher syndrome have detectable anti-GQ1b antibodies at a much higher frequency, probably around 95%.29,30 Gangliosides are widely distributed in the nervous system and may have a variety of functional roles. The structure of gangliosides (fig 1 ▶) involves several repeating subunits, which can be antigenic. Thus, antiganglioside antibodies have different specificities and these may overlap. Antibodies that recognise the NeuACNeuAC epitope will crossreact with several different gangliosides, making the importance of antiganglioside antibodies more difficult to interpret. The pattern of reactivity of a particular patientapos;s serum with several different gangliosides helps to define the exact specificity of the antibody. This can be seen most clearly when monoclonal antibodies against gangliosides are produced.32 The presence of antiganglioside antibody in a proportion of patients with Guillain Barré syndrome does not imply that the antibodies are pathogenetic. However, there appears to be a growing body of evidence in favour of the hypothesis that the fine specificity of the antiganglioside antibodies in at least a proportion of patients with Guillain Barré syndrome determines the pattern of clinical and pathological involvement. In support of this hypothesis is the observation that patients with axonal forms of Guillain Barré syndrome are more likely to have antiganglioside antibodies that recognise the ganglioside GD1a.23
Figure 1.
The NeuAc motif is shared between gangliosides GD1b, GT1b, and GQ1b (shaded, not hatched). Other similarities include the Gal (β1-3) GAL/NAc epitope shared between GM1 and GD1b. SGPG, sulphate-3-glucuronyl paragloboside; Glc, glucose; Gal, galactose; GalNAc, N-acetylgalactosamine; GlcNAc, N-actylglucosamine; GlcUA, glucuronic acid; NeuAc, N-acetylneuramic acid (sialic acid). From Dalakas and Quarles.31
In Miller Fisher syndrome, the clinical symptoms relate to dysfunction of the third, fourth, and sixth cranial nerves, similar to ocular myasthenia. Biochemical studies suggest that these cranial nerves contain a considerable amount of GQ1b,33 and Miller Fisher serum as well as monoclonal antibodies will immunostain these nerves in section.34 Serum from patients with Miller Fisher syndrome contains a blocking factor, which will initially depolarise the neuromuscular junction in a mouse hemidiaphragm preparation and then completely block it in a mechanism that resembles that of the toxin Lathratoxin.34 The responsible factor in the serum appears to be in the IgG fraction. Furthermore, monoclonal anti-GQ1b antibody will immunostain the neuromuscular junction of the mouse, and the pattern of staining exactly mimics that found with fluorescent labelled bungarotoxin, which is known to combine with the α subunit of the acetyl choline receptor.34 This evidence strongly suggests that at least in Miller Fisher syndrome the ophthalmoparesis results from a direct action of anti-GQ1b antibodies on the neuromuscular junction between the cranial nerves and ocular muscles.
Complete proof of this hypothesis requires production of ocular dysfunction by passive transfer of antibody, and this has not yet been achieved, largely because of the lack of an appropriate animal model. In Guillain Barré syndrome, the lack of antiganglioside antibodies in a large proportion of patients argues against this mechanism in all cases, but it could explain a proportion of cases.
Another possible antibody that might be relevant is some of these patients is antibody directed towards the myelin protein PMP 22. Genetic abnormalities of PMP 22 expression underlie the demyelinating form of Charcot Marie tooth disease and point mutations of PMP 22 can produce the same phenotype. The serum of patients with Guillain Barré syndrome contains antibodies against PMP 22,35 and the protein can induce EAN in Lewis rats in an experimental model. It is possible that Guillain Barré syndrome may turn out to be the result of a variety of different antibodies, of which antiganglioside antibodies are simply the most common.
The classic histological studies of Guillain Barré syndrome noted the presence of infiltrates of lymphocytes around small vessels,19 and the similarities of this finding with EAN suggested a role for T cells in the pathogenesis of Guillain Barré syndrome. Initial studies of T cells in Guillain Barré syndrome were conflicting, but only a very small proportion of cases had evidence of T cell proliferation to putative protein antigens.36 The peripheral blood of patients with inflammatory neuropathy contains activated T cells37 and peripheral nerve can be shown to express class II human major histocompatibility (HLA) antigens in appropriate circumstances.38 Adhesion molecules such as intercellular cell adhesion molecule (ICAM)39 and vascular cell adhesion molecule (VCAM) (S Hughes, 2000, personal communication) are expressed by endothelium in sections of nerve biopsy material, pointing to a role for T cells. Furthermore, the antiganglioside antibodies in serum from patients with Guillain Barré syndrome are usually of the IgG1 or IgG3 isotype, which is characteristic of a T helper type 2 (Th2) dependent antibody response.40
Non-classic T cell responses from γδ T cells may play a role because such cells have been isolated from peripheral nerve in culture41 and can be detected in nerve section in Guillain Barré syndrome by immunohistochemistry (J Cooper et al, 2000, personal communication). Such T cells are restricted by CD1, which has also been detected in Guillain Barré syndrome biopsy samples.42 The role of T cell help in antibody production remains to be defined and is the subject of continued research interest.
Treatment
The mainstay of treatment of Guillain Barré syndrome remains good intensive care, with respiratory support where required and early recognition of respiratory failure. The routine use of prophylaxis for deep venous thrombosis is generally accepted, although it has never been subjected to controlled trial. Positive pressure ventilation with frequent turning to avoid atelectasis and frequent physiotherapy are also useful. Passive limb movement helps to prevent contractures that hinder rehabilitation.
Several specific attempts at treatment have been tried. Unlike the more chronic disorder, chronic inflammatory demyelinating polyneuropathy, Guillain Barré syndrome has not been shown to respond to treatment with oral or intravenous steroids.43 Several controlled clinical trials have shown that both plasma exchange and intravenous immunoglobulin shorten the time to recovery when used in the early stages of the neuropathy.44–47 In the largest study of plasma exchange carried out in North America, this procedure improved the time to achieve walking unaided by 32 days.45 Whereas plasma exchange clearly removes a blood borne substance mediating the neuropathy, possibly an antibody, the mechanism of action of intravenous immunoglobulin administration is more complicated. This probably includes blockage of Fc receptors, increased catabolism of autoimmune immunoglobulin, and possible roles in providing anti-idiotypic antibodies and in promoting remyelination.
Prognosis
Studies of outcome in Guillain Barré syndrome suggest that at the end of one year from onset of the neuropathy 65% of patients achieve an almost complete cure so that they regain the ability to perform manual work.48 Of the 35% who do not, about 8% will die in the acute stage,48 usually from cardiac arrhythmias or pulmonary emboli.
Unfortunately, these figures of persistent deficit have not been significantly altered by the advent of plasma exchange and intravenous immunoglobulin, which mainly reduce the time taken to recover and not the percentage of patients making a good recovery. It seems likely that the proportion of patients with extensive axonal damage following the acute phase of the disease is not altered by present forms of treatment. Clearly, further and better treatments are needed for this group. There is interest in the use of nerve growth factors. Trials are under way to examine a combined role for steroids and intravenous immunoglobulin, β-interferon treatment, and repeated courses of intravenous immunoglobulin. There are also those who favour immunoabsorption rather than simple plasma exchange.49 At the moment all these forms of treatment are experimental.
Conclusion
Much has been learned about the mechanism of neuropathy in Guillain Barré syndrome but treatment remains disappointing after the major advances that occurred in the 1980s. Better and more specific treatments are clearly needed. It is hoped that recent advances in our understanding of pathogenesis may lead to better treatments in the next few years.
References
- 1.Guillain G, Barré J, Strohl A. Sur un syndrome de radiculo-nevrite avec hyperalbuminose du liquide cephalorachidien sans reaction cellulaire. Remarques sur les characteres clinique et graphique des reflexes tendinaux. Bulletins et Memories de la Societe Medicale des Hopitaux de Paris 1916;40:1462–70. [Google Scholar]
- 2.Alter M. The epidemiology of Guillain-Barré syndrome. Ann Neurol 1990:27(suppl):S7–12. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan JE, Katona P, Hurwitz ES, et al. Guillain-Barré syndrome in the United States, 1979–1980 and 1980–1981. Lack of an association with influenza vaccination. JAMA 1982;248:698–700. [PubMed] [Google Scholar]
- 4.Carpo M, Pedotti R, Allaria S, et al. Clinical presentation and outcome of Guillain-Barré and related syndromes in relation to anti-ganglioside antibodies. J Neurol Sci 1999;168:78–84. [DOI] [PubMed] [Google Scholar]
- 5.Yuki N. Molecular mimicry between gangliosides and lipopolysaccharides of Campylobacter jejuni isolated from patients with Guillain-Barré syndrome and Miller Fisher syndrome. J Infect Dis 1997;176(suppl 2):S150–3. [DOI] [PubMed] [Google Scholar]
- 6.Asbury AK, Arnason BG, Karp HR, et al. Criteria for the diagnosis of Guillain-Barré syndrome. Ann Neurol 1978;3:565–6. [DOI] [PubMed] [Google Scholar]
- 7.Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barré syndrome [see comments]. Ann Neurol 1990;27(suppl):S21–4. [DOI] [PubMed] [Google Scholar]
- 8.Hughes R. Guillain-Barré syndrome. London: Springer-Verlag, 1990.
- 9.Winer JB, Hughes RA, Anderson MJ, et al. A prospective study of acute idiopathic neuropathy. II. Antecedent events. J Neurol Neurosurg Psychiatry 1988;51:613–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vriesendorp FJ, Mishu B, Blaser MJ, et al. Serum antibodies to GM1, GD1b, peripheral nerve myelin, and Campylobacter jejuni in patients with Guillain-Barré syndrome and controls: correlation and prognosis [see comments]. Ann Neurol 1993;34:130–5. [DOI] [PubMed] [Google Scholar]
- 11.Mishu B, Ilyas AA, Koski CL, et al. Serologic evidence of previous Campylobacter jejuni infection in patients with the Guillain-Barré syndrome. Ann Intern Med 1993;118:947–53. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs BC, Rothbarth PH, van der Meche FG, et al. The spectrum of antecedent infections in Guillain-Barré syndrome: a case–control study. Neurology 1998;51:1110–15. [DOI] [PubMed] [Google Scholar]
- 13.Rees JH, Gregson NA, Hughes RA. Anti-ganglioside GM1 antibodies in Guillain-Barré syndrome and their relationship to Campylobacter jejuni infection. Ann Neurol 1995;38:809–16. [DOI] [PubMed] [Google Scholar]
- 14.Dowling PC, Cook SD. Role of infection in Guillain-Barré syndrome: laboratory confirmation of herpesviruses in 41 cases. Ann Neurol 1981;9(suppl):44–55. [DOI] [PubMed] [Google Scholar]
- 15.Langmuir AD, Bregman DJ, Kurland LT, et al. An epidemiologic and clinical evaluation of Guillain-Barré syndrome reported in association with the administration of swine influenza vaccines. Am J Epidemiol 1984;119:841–79. [DOI] [PubMed] [Google Scholar]
- 16.Toro G, Vergara I, Roman G. Neuroparalytic accidents of antirabies vaccination with suckling mouse brain vaccine. Clinical and pathologic study of 21 cases. Arch Neurol 1977;34:694–700. [DOI] [PubMed] [Google Scholar]
- 17.Ho TW, Hsieh ST, Nachamkin I, et al. Motor nerve terminal degeneration provides a potential mechanism for rapid recovery in acute motor axonal neuropathy after Campylobacter infection [see comments]. Neurology 1997;48:717–24. [DOI] [PubMed] [Google Scholar]
- 18.Hariharan H, Naseema K, Kumaran C, et al. Detection of Campylobacter jejuni/C.coli infection in patients with Guillain-Barré syndrome by serology and culture. New Microbiol 1996;19:267–71. [PubMed] [Google Scholar]
- 19.Asbury AK, Arnason BG, Adams RD. The inflammatory lesion in idiopathic polyneuritis. Its role in pathogenesis. Medicine 1969;48:173–215. [DOI] [PubMed] [Google Scholar]
- 20.Prineas JW. Pathology of the Guillain-Barré syndrome. Ann Neurol 1981;9(suppl):6–19. [DOI] [PubMed] [Google Scholar]
- 21.Lampert P. Mechanism of demyelination in experimental allergic neuritis. Electron microscopic studies. Lab Invest 1969;20:127–38. [PubMed] [Google Scholar]
- 22.Hadden RD, Comblath DR, Hughes RA, et al. Electrophysiological classification of Guillain-Barré syndrome: clinical associations and outcome. Plasma exchange/Sandoglobulin Guillain-Barré syndrome trial group. Ann Neurol 1998;44:780–8. [DOI] [PubMed] [Google Scholar]
- 23.Ho TW, Willison HJ, Nachamkin I, et al. Anti-GD1a antibody is associated with axonal but not demyelinating forms of Guillain-Barré syndrome. Ann Neurol 1999;45:168–73. [DOI] [PubMed] [Google Scholar]
- 24.Kimura J. Proximal versus distal slowing of motor nerve conduction velocity in the Guillain-Barré syndrome. Ann Neurol 1978;3:344–50. [DOI] [PubMed] [Google Scholar]
- 25.Melnick S. 38 cases of the Guillain-Barré syndrome; an immunological study. BMJ 1963;1:368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koski CL, Humphrey R, Shin ML. Anti-peripheral myelin antibody in patients with demyelinating neuropathy: quantitative and kinetic determination of serum antibody by complement component 1 fixation. Proc Natl Acad Sci U S A 1985;82:905–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gregson NA, Koblar S, Hughes RS. Antibodies to gangliosides in Guillain-Barré syndrome: specificity and relationship to clinical features [see comments]. Q J Med 1993;86:111–17. [PubMed] [Google Scholar]
- 28.Kusunoki S. Antiganglioside antibodies in the pathogenesis of autoimmune neuropathies. Rinsho Shinkeigaku 1999;39:93–5. [PubMed] [Google Scholar]
- 29.Chiba A, Kusunoki S, Obata H, et al. Serum anti-GQ1b IgG antibody is associated with ophthalmoplegia in Miller Fisher syndrome and Guillain-Barré syndrome: clinical and immunohistochemical studies. Neurology 1993;43:1911–17. [DOI] [PubMed] [Google Scholar]
- 30.Willison HJ, Veitch J, Paterson G, et al. Miller Fisher syndrome is associated with serum antibodies to GQ1b ganglioside. J Neurol Neurosurg Psychiatry 1993;56:204–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalakas MC, Quarles RH. Autoimmune ataxic neuropathies (sensory ganglionopathies): are glycolipids the responsible autoantigens. Ann Neurol 1996;39:419–22. [DOI] [PubMed] [Google Scholar]
- 32.Goodyear CS, Oapos;Hanlon GM, Plomp JJ, et al. Monoclonal antibodies raised against Guillain-Barré syndrome-associated Campylobacter jejuni lipopolysaccharides react with neuronal gangliosides and paralyze muscle-nerve preparations. J Clin Invest 1999;104:697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiba A, Kusunoki S, Obata H, et al. Ganglioside composition of the human cranial nerves, with special reference to pathophysiology of Miller Fisher syndrome. Brain Res 1997;745:32–6. [DOI] [PubMed] [Google Scholar]
- 34.Plomp JJ, Molenaar PC, Oapos;Hanlon GM, et al. Miller Fisher anti-GQ1b antibodies: alpha-latrotoxin-like effects on motor end plates [published erratum appears in Ann Neurol1999;45:823]. Ann Neurol 1999;45:189–99. [DOI] [PubMed] [Google Scholar]
- 35.Gabriel C, Gregson N, Hughes R. Anti-PMP22 antibodies in patients with inflammatory neuropathy. J Neuroimmunol 2000;104:139–46. [DOI] [PubMed] [Google Scholar]
- 36.Hughes RA, Gray IA, Gregson NA, et al. Immune responses to myelin antigens in Guillain-Barré syndrome. J Neuroimmunol 1984;6:303–12. [DOI] [PubMed] [Google Scholar]
- 37.Hartung HP, Hughes RA, Taylor WA, et al. T cell activation in Guillain-Barré syndrome and in MS: elevated serum levels of soluble IL-2 receptors. Neurology 1990;40:215–18. [DOI] [PubMed] [Google Scholar]
- 38.Pollard JD, Baverstock J, McLeod JG. Class II antigen expression and inflammatory cells in the Guillain-Barré syndrome. Ann Neurol 1987;21:337–41. [DOI] [PubMed] [Google Scholar]
- 39.Putzu G, Figarella-Branger D, Bouvier-Labit C, et al. Immunohistochemical localization of cytokines, C5b-9 and ICAM-1 in peripheral nerve of Guillain-Barré syndrome. J Neurol Sci 2000;174:16–21. [DOI] [PubMed] [Google Scholar]
- 40.Ogino M, Orazio N, Latov N. IgG anti-GM1 antibodies from patients with acute motor neuropathy are predominantly of the IgG1 and IgG3 subclasses. J Neuroimmunol 1995;58:77–80. [DOI] [PubMed] [Google Scholar]
- 41.Ben-Smith A, Gaston JS, Barber PC, et al. Isolation and characterisation of T lymphocytes from sural nerve biopsies in patients with Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry 1996;61:362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khalili-Shirazi A, Gregson N, Hughes R. CD1 expression in human peripheral nerve of GBS patients. Biochem Soc Trans 1997;5:172S. [DOI] [PubMed] [Google Scholar]
- 43.Hughes R, van der Meche FG. Corticosteroids for treating Guillain-Barré syndrome. Cochrane Database Syst Rev 2000;2:CD001446. [DOI] [PubMed] [Google Scholar]
- 44.The Guillain-Barré Syndrome Study Group. Plasmapheresis and acute Guillain-Barré syndrome. Neurology 1985;35:1096–104. [PubMed] [Google Scholar]
- 45.French Cooperative Group on Plasma Exchange in Guillain-Barré Syndrome. Efficiency of plasma exchange in Guillain-Barré syndrome: role of replacement fluids. Ann Neurol 1987;22:753–61. [DOI] [PubMed] [Google Scholar]
- 46.van der Meche FG, Schmitz PI. A randomized trial comparing intravenous immune globulin and plasma exchange in Guillain-Barré syndrome. Dutch Guillain-Barré study group [see comments]. N Engl J Med 1992;326:1123–9. [DOI] [PubMed] [Google Scholar]
- 47.Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group. Randomised trial of plasma exchange, intravenous immunoglobulin, and combined treatments in Guillain-Barré syndrome [see comments]. Lancet 1997;349:225–30. [PubMed] [Google Scholar]
- 48.Rees JH, Thompson RD, Smeeton NC, et al. Epidemiological study of Guillain-Barré syndrome in south east England [see comments]. J Neurol Neurosurg Psychiatry 1998;64:74–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watanabe T, Matsuura O, Natsume J, et al. Dramatic improvement with immunoabsorption therapy in a 7-year-old girl with severe Guillain-Barré syndrome after unsuccessful gammaglobulin therapy. No To Hattatsu 1998;30:255–60. [PubMed] [Google Scholar]