Abstract
Fetal magnetocardiography (fMCG) is a non-invasive technique that measures the magnetic fields associated with fetal heart electrical activity outside of the maternal abdomen. fMCG has high temporal precision for measuring fetal heart rate and its variability which reflects fetal neurodevelopment. Free of cryogenics and low-cost sensors called microfabricated optically pumped magnetometers (OPMs) have emerged as an alternate to cryogenic SQUID (Superconducting Quantum Interference Device) systems to record fMCG. Previous research has demonstrated the ability of the OPMs to measure the fMCG at different maternal positions by taking the advantage of the conformal and geometric flexibility of the sensors. In this work, we designed and configured a bed-based stand-alone array of OPMs to obtain serial recordings of fMCG. 72 combined OPM-SQUID recordings were conducted at different gestational ages in 22 pregnant women. We were able to obtain fMCG with similar detectability as the gold standard SQUID from OPM sensors mounted on a novel belly-shape patient interface design with movable sensor holders. While additional translational research is needed, the outcome of this study can further facilitate the development of a non-cryogenic low-cost smaller footprint device to increase the use of OPMs for fetal research and clinical applications.
Keywords: Biomagnetism, Fetus, Magnetocardiography, Optically pumped magnetometers, Pregnancy
Subject terms: Cardiology, Medical research, Preclinical research, Developmental biology, Intrauterine growth, Physiology, Reproductive biology
Introduction
Fetal Magnetocardiography (fMCG) is a non-invasive technique that records the magnetic fields associated with fetal heart activity outside the maternal abdomen. Compared to fetal electrocardiography (fECG), fMCG has a high signal-to-noise ratio (SNR) because magnetic fields propagate relatively undisturbed through the maternal tissues. fMCG is capable in measuring fetal heart rate and fetal heart rate variability (FHRV)1 with good precision due to its high temporal resolution. FHRV is one of the most useful parameters for assessing fetal neurodevelopment, reflecting the maturation of the autonomic nervous system. Currently, fMCG signals are measured with SQUID (Superconducting Quantum Interference Device) systems2. Although advances have been made with the SQUID systems in the study of fetal heart electrophysiology, several drawbacks exist, such as high maintenance costs and lack of flexibility in the positioning of the sensors. Cryogenic-free biomagnetometer systems based on microfabricated optically pumped magnetometers (OPM) have been used as a low-cost alternative for fetal cardiac assessment3–7. OPMs offer similar biomagnetic data comparable to SQUIDs without relying on cryogenic cooling8,9. Previous studies have shown the equivalence of OPMs to the gold standard SQUIDs by simultaneously recording the fMCG from both systems in a three-layered shielded room5. Additionally, previous research has demonstrated the ability of OPM sensors to measure the fMCG signals at different maternal positions to take advantage of the conformal and geometric flexibility of the OPM sensors6,7. Recently, our research study introduced a prototype bed-based system to assess both the performance of OPMs and patient ergonomics in a large three-layered shielded room10. Based on this prototype, we designed and configured a novel bed-based array of OPMs that conforms to the shape of the maternal abdomen to record fMCG signals in a stand-alone small footprint cylindrical shielded room. In this study, we report results of serial fMCG recordings that were conducted at different gestational ages of pregnant women. To validate the bed-based array of OPMs, we performed back-to-back recordings with the SQUID Array for Reproductive Assessment (SARA) system. We compared the detectability of fMCG signals between the two systems, and quantified FHRV and standard cardiac time intervals to assess the fMCG waveforms. This OPM system is the first step towards the development of a novel non-cryogenic, low-cost, smaller-footprint device to increase the use of OPM sensors for fetal translational research and clinical applications.
Methods
Stand-alone system
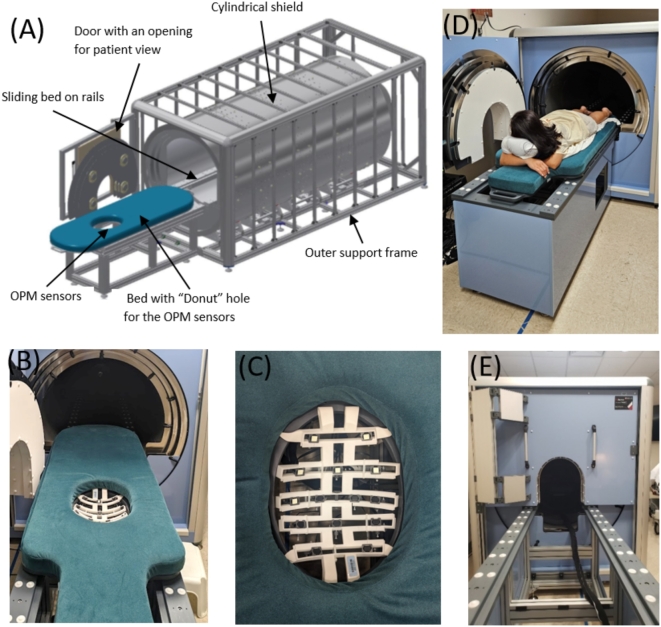
We have developed a 14 sensor (28 channel) stand-alone OPM system with a custom-designed bed and patient interface housed in a cylindrical 3-layer shield built by Magnetic Shields Ltd., UK. Figure 1 shows our stand-alone OPM system. The bed, patient-sensor interface and ergonomics were developed based on previous studies6,7. The mother was lying flat, face-down on her abdomen in a “donut hole” mattress with the sensors placed from below. The 14 sensors (QuSpin Inc., combination of Gen 2 and Gen 3 dual axis sensors) were operated in dual-axis mode to measure biomagnetic fields in the y–z directions. The z-axis of the sensors was pointing towards the maternal abdomen and the y-axis was tangential to her body. The shielded space has a cylindrical shape (10 ft long and 6 ft diameter), and the bed slides on railing into the shielded enclosure. The shield consisted of three layers of Mu metal 1.5 mm each with an outer covering Aluminum 5 mm and an inner PVC internal liner. The weight of the shield is around 1300 kg. One end of the cylinder was conical shaped with a small opening for cables. In order to minimize external fields, on the other end of the cylinder a door was added with an opening for patient view. The main shield is passive with a degaussing system to further lower the residual field, and there is an active coil around the door of the shield to lower the field further/improve homogeneity inside the shield. The goal was to achieve lower noise floor performance with residual fields below 15 nano Tesla (nT). The pregnant woman lies abdomen-down with her head positioned towards the door of the shielded enclosure with a view outside of the shielded space. The “donut” hole in the bed houses the OPM sensor array with the maternal abdomen nestled against the sensors. We used a sensor holder to support the sensors from below with adequate spacing between them for air circulation and to avoid sensor overheating during use. The sensor holder can accommodate up to 21 sensors. Each sensor has an attachment with a lockable hinge, allowing the sensors to be adjusted to the shape of the maternal abdomen. The OPM system is installed on the hospital’s fifth floor adjacent to labor and delivery, and next to our SQUID system.
Fig. 1.
Stand-alone 14-sensor OPM system (QuSpin Inc., USA) with a customized bed. (A) Small footprint shielded enclosure (Magnetic Sheilds Ltd, UK). (B) Bed with sensor setup. (C) Sensor holder (designed by UAMS and production by Cerca Magnetics Ltd, UK) . The z-axis of each sensor was pointing towards the maternal abdomen and the y-axis was tangential to the mother’s body. (D) Subject lying flat with the abdomen over the sensors. (E) Recording performed inside the shielded enclosure.
Measurements
Empty room measurement
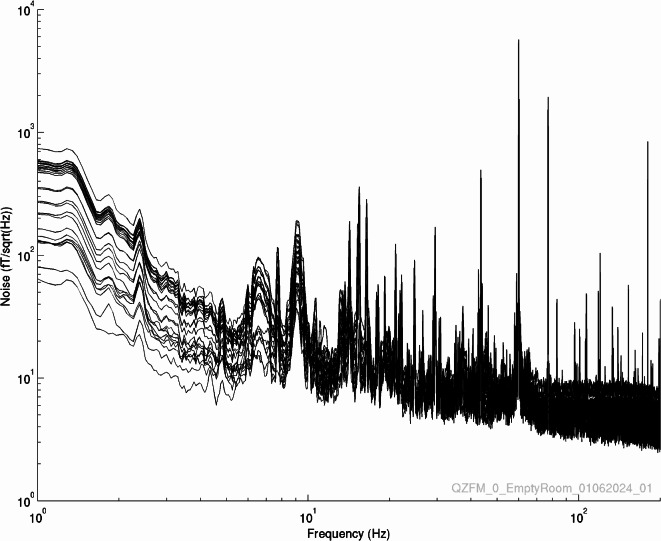
The stand-alone OPM system is installed inside the shielded enclosure as described before and the DC fields were reduced with the global field coils to below 15 nT over the region of the sensor array. The signals were recorded of the empty room for 360 s. The noise spectra were recorded at 1 kHz sampling rate and are shown in Fig. 2. Stand-alone OPM system provides an average of noise floor across OPM sensors of 11 fT/Hz1/2 in the frequency band of 10 – 200 Hz, and 62 fT/Hz1/2 in the band of 1 – 10 Hz.
Fig. 2.
Noise floor in the empty magnetically shielded enclosure.
Participant data
The stand-alone 14-sensor (28 channel) OPM array was used to record six minutes of biomagnetic data from 22 low-risk pregnant women between 28 and 38 weeks of gestational age (GA) in the y–z directions. The signals were recorded at 1 kHz sampling rate with repeated measurements every two weeks. An ultrasound was performed before SQUID and OPM recordings to localize the fetal heart. Next, right before the OPM recording, a gold standard study was conducted with the SQUID system to validate the fMCG signals from the OPM system. The SQUID system (CTF Systems, Port Coquitlam, BC, Canada) called SARA (SQUID Array for Reproductive Assessment) was used for SQUID recordings11–14. The device consists of 151 SQUID sensors (first order gradiometers with a baseline of 8 cm) that are uniformly distributed in a shell shaped structure matching the form of the maternal abdomen. Sensors are spaced approximately 3 cm apart over an area of 850 cm2. The pregnant woman sits on the device leaning forward with the maternal abdomen resting on the sensor array. The University of Arkansas for Medical Sciences Institutional Review Board approved this study, and the pregnant women provided written informed consent to participate. Research was performed in accordance with the Declaration of Helsinki and in accordance with relevant guidelines/regulations.
Signal processing and fMCG data extraction
OPM data was notch-filtered for power line attenuation and band-pass filtered between 0.5–50 Hz. A 4th order Butterworth bandpass filter was applied using functions in the MATLAB software (Mathworks Inc) specifically Butter() and filtfilt(). In the filtfilt() function. The data is filtered twice, once, forward in time and then once in reverse time order. The phase shifts at each frequency sum to zero. For the notch filter, we selected the zero phase IIR filter. Our notch filter was designed with a 1.5 Hz bandwidth; therefore, it has little effect on the output of the Butterworth passband. The independent component analysis (ICA) was used to remove background noise such as maternal breathing. We used Infomax ICA which is part of the Brainstorm software suite (https://neuroimage.usc.edu/brainstorm/). ICA has been used before in fetal MCG OPM applications6,7,10,15,16. Similar to previous work6,7,10, a projection operator algorithm based on the minimum norm (POMN)17 was used to remove the maternal cardiac signals. For OPM data, we visually inspected the fMCG data using Brainstorm software to remove bad channels, marked bad segments, and manually selected and inspected the selected ICA components to attenuate background noise18,19. To extract fMCG from SQUID data, we used same filters, and we applied our standard Orthogonal projection (OP) algorithm based on Signal-Space Projection technique20,21. For SQUID data we did not apply ICA algorithm because signals have better SNR due to high-order gradiometer noise cancellation configuration13. Similar to OPM data, the SQUID fMCG data was visually inspected with Brainstorm software to remove bad channels and marked bad segments.
fMCG R peak detection and cardiac complex data extraction
For both OPM and SQUID, extraction of the R peaks of the continuous fMCG traces were performed using a peak detection algorithm22. R peak detection algorithm automatically discards outliers via thresholding techniques (see more details in Ulusar et al22). Once R peaks of the fMCG data are extracted, we computed FHRV metrics: fetal heart rate (FHR) in beats per minute and R-R duration in milliseconds. R-R was calculated as the grand average of R-R values from the whole fMCG recording. To obtain the fMCG PQRST complex, we performed standard averaging of fMCG time series23–26 .
FHRV analysis
We extracted descriptive comparisons between FHRV metrics obtained from OPM and SQUID recordings. To accomplish this and as described before, traditional FHRV metrics were calculated: mean fetal heart rate (mFHR, bpm); mean R-R intervals (mRR millisecond, ms). Descriptive statistics including means and standard deviations (SD) were extracted to characterize and quantify FHRV metrics for both OPM and SQUID systems. We present descriptive comparisons of FHRV for the following GA interval groups1: 28–30 weeks of GA, 31–33 weeks, 34–35 weeks, and ≥ 36 weeks.
Fetal cardiac time interval analysis
As described before and to further improve the SNR of the fMCG signal, we performed standard signal averaging by using the R peaks of the fMCG traces from multiple 60-s windows. After being averaged, the resulting traces were analyzed to verify the existence of P wave, QRS complex, and T wave. Thus, tracings from all OPM and SQUID sensors were superimposed to define better cardiac time intervals27. To measure the fetal cardiac intervals, our pediatric cardiologist (author EB) reviewed and scored the superimposed tracings to select the averaged fMCG in which the PQRST waveforms were most sharply defined, especially the T wave. The cardiac time intervals (P, PR, QRS and QT) were measured using previously published standards28, and the cardiologist was blinded to group assignment. The fetal PR, QRS, and QT durations were extracted to obtain descriptive comparisons between OPM and SQUID data. We present descriptive comparisons of cardiac time intervals for the following GA interval groups1: 28–30 weeks of GA, 31–33 weeks, 34–35 weeks, and ≥ 36 weeks.
Results
Detection of fMCG
We successfully collected serial OPM and SQUID data from 22 pregnant women within 28 to 38 weeks of gestational age. Participants had a mean age of 28.8 ± 5.3 years and pre-pregnancy BMI of 29.8 ± 7.0 kg/m2. Within the overall group, 41% were white, 59% were African-American, and 91% of all participants had an anterior placenta position. Among the fetuses, 36% were females. We recorded 72 combined OPM-SQUID sessions from 28 to 38 weeks of GA. One session included a visit from a consenting pregnant woman at a given GA to record fMCG with OPM and SQUID systems. Six of the 72 recorded sessions were discarded due to technical issues obtaining a detection rate of 91.67%.
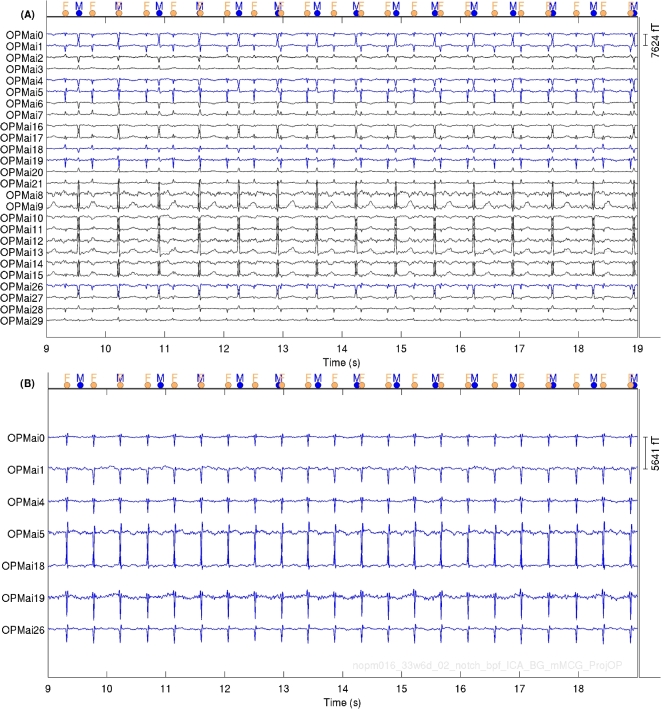
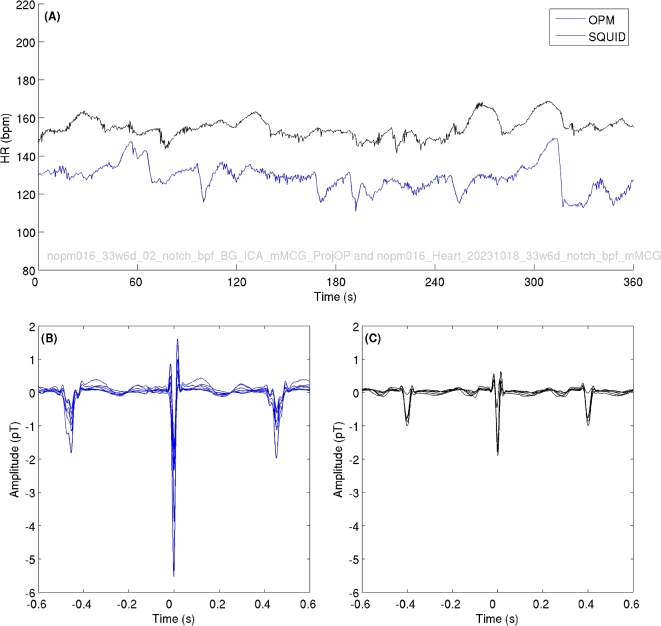
Figure 3 shows 10-s OPM fMCG time series from a pregnant woman at 33 weeks of GA. The fMCG signal amplitudes ranged from 2–6 pT (pico-Tesla). The dots on the top of Fig. 3 displays the position of R peaks of the maternal (blue dots, labeled as ”M”) and fetal (orange dots, labeled as ”F”) R peaks from the corresponding cardiac activities. Figure 4 shows fetal heart rate and averaged fMCG from same pregnant woman at 33 weeks of GA for OPM (blue traces) and SQUID (black traces) data. It can be observed that fetal heart rates are slightly different which can be attributed to the back-to-back measurements performed with OPM and SQUID rather than a simultaneous measure.
Fig. 3.
Ten-second OPM fMCG data from a pregnant woman at 33 weeks of gestational age: (A) Biomagnetic data before removing maternal cardiac activities (B) Extracted fMCG after signal processing. Blue traces in Fig. 3A correspond to the fetal cardiac activities displayed in Fig. 3B.
Fig. 4.
Heart rate extracted from back-to-back OPM and SQUID measurements. (A) Shows the FHR for OPM (blue traces) and SQUID (black traces). (B) Averaged OPM signals. (C) Averaged SQUID signals. Note that measurements were performed back-to-back, we expected to observed FHR difference between recordings.
Comparative FHRV analysis
To perform a descriptive FHRV comparison between OPM and SQUID data, seven out of the 66 combined OPM-SQUID sessions were discarded due to either low SNR or fMCG that had only a small portion with continuous R peaks (3 sessions), or were due to incomplete SQUID recordings (4 sessions). Thus, we obtained 59 OPM/SQUID pairs of sessions with 6-min (360 s) of continuous R-peaks for FHRV analysis. We obtained 11 pairs of sessions for 28–30 weeks of GA, 17 for 31–33 weeks, 13 for 34–35 weeks, and 18 for ≥ 36 weeks. Figure 5 provides a comparison of FHRV analysis (see Supplementary Table 1 for descriptive statistics). As shown in Fig. 5, the range of FHRV metrics obtained from OPM overlapped with those obtained from SQUID data.
Fig. 5.
FHRV analysis. Pairs of OPM (blue) and SQUID (black) data within GA groups: (A) Heart rate (HR) in bpm (beats per minute). (B) R-R intervals in milliseconds.
Comparative cardiac time intervals
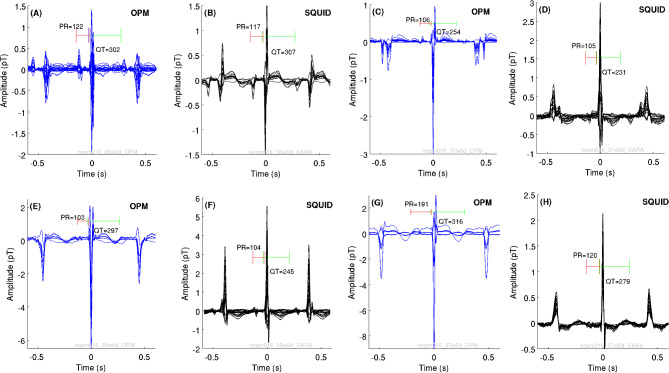
From the 59 pairs of sessions available, we detected complete PQRST complex in 43 (72.88%) sessions. We obtained six pairs from 11 sessions at 28–30 weeks’ GA, 14 out of 17 for 31–33 weeks, 10 out of 13 for 34–35 weeks, and 13 out of 18 for ≥ 36 weeks. Figure 6 presents examples of averaged fMCG of OPM (blue traces) and SQUID (black traces) waveforms with the corresponding scores. The waveforms had similar amplitude between the OPM and SQUID recordings, however among all results, OPM amplitudes were higher that SARA amplitudes. Figure 7 summarizes the results of the fetal cardiac time intervals of OPM and SQUID data per GA group (see Supplementary Table 2 for descriptive statistics). Similarly, the range of fetal cardiac time interval durations obtained from OPM overlapped with those obtained from SQUID data.
Fig. 6.
Examples of averaged fMCG waveforms for OPM (blue signals) and SQUID (black signals) recordings within a GA group: (A-B) 30 weeks of GA. (C-D) 31 weeks of GA. (E–F) 35 weeks of (G-H) 37 weeks of GA. Green and red brackets indicate the QT interval and PR interval, respectively.
Fig. 7.
Durations of the fetal cardiac time intervals in milliseconds. Pairs of OPM (blue) and SQUID (black) data within a GA groups: (A) P wave. (B) PR interval. (C) QRS complex. (D) QT interval.
Discussion
This study goes beyond the earlier feasibility studies in affirming that the less expensive non-cryogenic OPM alternative could provide similar electrophysiological and quantitative characteristics compared to the SQUID system that has used a larger shielded room5,7. In previous work, we designed the bed and the belly-shaped patient interface and tested the ergonomics in a large-shielded room. In the current work, we have refined our design by customizing the shape of the sensor interface with moveable sensor holders based on our previous prototype, and we have incorporated the bed and sensor interface to fit in a cylindrical enclosure. This study tests for the first time a completed integrated system that focused on sensor interface and ergonomics in a small footprint shield enclosure.
In this study, we successfully performed serial recordings that could be used to understand the dynamics of the fetal autonomic nervous system to track fetal neurodevelopment using OPM-fMCG. This new OPM configuration shows a similar fMCG time series in comparison with the SQUID system. Even though in the current study we used a semi-rigid array and fewer sensors than the SQUID, we were able to sometimes obtain higher amplitudes of the OPM data than SQUID system; thus demonstrating the potential of the OPM sensors in capturing high SNR fMCG signals. It is possible that fetus was in closer position to the OPM array in comparison with SQUID system at some of the back-to-back recordings. Previous works when using OPMs to obtain adult brain data have demonstrated that OPM signals could be higher in the magnitude than SQUID signals8,9. This new OPM patient interface has the advantage of moveable sensor holders that allow sensor for repositioning if required to ensure closer proximity between the maternal abdomen and the sensor.
Our new OPM configuration was able to capture OPM-fMCG to quantify FHR and its variability. Although we were limited by the fact that SQUID recordings were not simultaneously measured with OPM and different positions of the mother on each of the systems (prone for OPM versus recline for SQUID), our overall results showed an overlap in the ranges of the FHRV and cardiac time interval metrics between systems. Even though fMCG-OPM data was collected with an array of 14 sensors (28 channels) as compared to 151 channels of the SQUID system, we were able to obtain high SNR OPM-fMCG signals. As noted above, when comparing SNR, we are comparing gradiometer SQUID data to magnetometer data (OPMs), with the latter being more sensitive to interference. However, with our signal processing methods, we were able to extract high-quality OPM-fMCG data (see our examples of time series at Figs. 3, 4, 6). Our study shows the potential of a stand-alone OPM system showing similar performance to the established SQUID-based sensors.
Further, the results show that the morphology of the averaged fMCG obtained by OPM is similar to the SQUID system. Despite the cardiac time intervals having more variability in OPM than SQUID (as expected due to the difference in resolution between the two techniques), with only 20% of sensors compared to SQUID, OPM is still able to provide signals with higher amplitudes (see traces of Fig. 3B, and averaged fMCG waves on Fig. 4B-4C). In future, adding more OPM sensors in the system could improve the quality of the PQRST complex, and be able to capture fetal cardiac signals during movement, especially in early third trimester thus reducing the potential for missed beats. This will represent an important step forward covering more regions in the maternal abdomen for capturing other biomagnetic signals beyond fMCG. In the future, we plan to develop procedures to obtain OPM sensor location and orientation29–32, and co-registration with maternal abdominal anatomy, thus enabling us to extract spatial information to understand the dynamics of the biomagnetic signals via source modeling.
From a clinical perspective, fMCG is a valuable tool since several aspects of fetal cardiac function can be captured including the waveform morphology, FHRV and fetal cardiac time intervals. The wider adoption of fMCG has been hindered by high cost and the need of a cryogenic-based SQUID system. As we have shown with a low-cost OPM-based system, we can identify the beat-to-beat R-R intervals and characterize R-R intervals using the standard FHRV statistics. To complement this, the parameters extracted using the components of the cardiogram (cardiac time intervals) help to unravel the finer details of heart development in the fetus. With regards to the waveform morphology fMCG can aid in complementing standard fetal echocardiography in identifying abnormal cardiac conditions. For example, it is important to distinguish premature atrial contractions (PAC) from premature ventricular contractions (PVC) which can be associated with structural heart disease, myocardial disease, tumors and even extracardiac disease such as drug exposure. While a PAC can be observed on fMCG as an early systole preceded by a P-wave, the PVC is recorded as a wider beat and has a different axis or morphology from the normal beats. Additionally, it is possible to identify a PAC conducted with aberrancy on the fMCG. Also, PACs are common in fetuses and rarely progress to SVT. They are typically detected as an irregular heartbeat on routine screening by the obstetrician and can often be confirmed by fetal echocardiography. However, many times the arrhythmia is transient and not present during the brief rhythm analysis portion of a fetal echocardiogram or the echocardiographic windows are inadequate for accurate diagnosis. Although PACs most commonly resolve before birth, they can precede events of SVT. Long periods of SVT are associated with cardiac failure, nonimmune hydrops fetalis and fetal death. Based on longer period of rhythm analysis and the mechanism of the rhythm obtained from fMCG tracings the diagnosed condition can be further evaluated. In the case of congenital complete heart blockage, it is sometimes difficult to distinguish from atrial bigeminy with blocked PACs on standard imaging methods. However, fMCG will be very useful in distinguishing the two rhythms. Clearly identifiable P-waves with unequal P to P intervals and slightly different morphologies would indicate the more benign rhythm of atrial bigeminy as opposed to a complete AV Block with atrioventricular dissociation. However, fMCG is very useful in distinguishing the two rhythms.
Limitations in the current study exist with our custom stand-alone OPM system such as the number of sensors– here we contrast 14-OPM sensors (28 channels) to 151-SQUID channels. The number of sensors is important for signal acquisition, the more channels to average for fMCG extraction, the better the SNR will be, which could better characterize the fMCG waveforms, especially T-waves. Our study is a moderate sample size and we achieved parity between systems, and obtained OPM fMCG signals that were superior to signals from the SQUID system. The noise level of our stand-alone OPM system remains higher than that of the SQUID system, due to the floor vibration but with our developed signal processing techniques, we were able to obtain quality fMCG data. Further research needs to be conducted to increase the SNR of OPMs closer to that of SQUIDs, particularly at low frequencies. Given the moderate sample size, we subsequently collapsed the fMCG data into GA groups allowing an overall examination. However, this is the first study measuring serial recordings on pregnant women with a custom stand-alone OPM array design. In the future, we will increase our sample size of serial recordings for continuous fetal monitoring and neurodevelopmental assessments.
In summary, findings indicate that our stand-alone OPM system can obtain serial fMCG signals with enough signal-to-noise ratio, and to extract FHRV metrics and PQRST complexes. The advantage of the semi-rigid stand-alone OPM system allows flexibility to ensure close proximity of the OPM sensors to the pregnant abdomen for better characterization of the fMCG signals.
Conclusion
Using a unique belly-shape patient interface design with movable sensor holders, we performed serial fMCG recordings using OPM sensors in a small footprint cylindrical enclosure while optimizing patient comfort. FHR was extracted from low-risk fetuses across different gestational ages for continuum neurodevelopmental assessments. Fetal heart rate and averaged fMCG waveforms visually showed similar values to the gold standard SQUID measurement. We believe that with lower cost and maintenance requirements of a stand-alone OPM array system, that fetal biomagnetometry could be translated from research to widespread clinical applications.
Supplementary Information
Acknowledgements
The authors would like to thank Karina Leal and Heather Moody for their assistance in recruiting and data acquisition.
Author contributions
D.E.V: Investigation, Methodology, Formal analysis, Writing – original draft, Writing – review & editing. A.R: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. E. R.S: Formal analysis, Writing – original draft, Writing – review & editing. E. H. B: Formal analysis, Methodology, Supervision. H. E: Conceptualization, Formal analysis, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing.
Funding
This work was supported by the U.S. National Institute of Health (NIH) under Grant R01HL164303-01A1.
Data availability
The data that support the findings of this study are available upon reasonable request to the corresponding author following the institutional guidelines and policies.
Code availability
We used publicly available open-source Brainstorm software and custom scripts written in Matlab to analyze our data.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-90846-y.
References
- 1.Lowery, C. L. et al. Noninvasive antepartum recording of fetal ST segment with a newly developed 151-channel magnetic sensor system. Am. J. Obstet. Gynecol.188(6), 1491–1497 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Vrba, J. Multichannel SQUID biomagnetic systems 61–138 (Springer, 2000). [Google Scholar]
- 3.Alem, O. et al. Fetal magnetocardiography measurements with an array of microfabricated optically pumped magnetometers. Phys. Med. Biol.60(12), 4797 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Wyllie, R., Kauer, M., Wakai, R. T. & Walker, T. G. Optical magnetometer array for fetal magnetocardiography. Opt. Lett.37(12), 2247–2249 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eswaran, H., Escalona-Vargas, D., Bolin, E. H., Wilson, J. D. & Lowery, C. L. Fetal magnetocardiography using optically pumped magnetometers: a more adaptable and less expensive alternative?. Prenat. Diagn.37(2), 193–196 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Escalona-Vargas D, Eswaran H, editors. Adaptable Sensor Arrays for Fetal Magnetocardiographic Measurements Using Optically-Pumped Magnetometers: A Pilot Study. 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC) 2020 IEEE. [DOI] [PMC free article] [PubMed]
- 7.Escalona-Vargas, D., Bolin, E. H., Lowery, C. L., Siegel, E. R. & Eswaran, H. Recording and quantifying fetal magnetocardiography signals using a flexible array of optically-pumped magnetometers. Physiol. Meas.10.1088/1361-6579/abc353 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boto, E. et al. On the potential of a new generation of magnetometers for MEG: a beamformer simulation study. PLoS ONE11(8), e0157655 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boto, E. et al. A new generation of magnetoencephalography: Room temperature measurements using optically-pumped magnetometers. Neuroimage149, 404–414 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escalona-Vargas, D., Siegel, E. R., Bolin, E. H. & Eswaran, H. Fetal magnetocardiographic recordings with a prototype bed-based array system of optically-pumped magnetometers. Med. Eng. & Phys.10.1016/j.medengphy.2024.104175 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eswaran, H. et al. Magnetoencephalographic recordings of visual evoked brain activity in the human fetus. Lancet360(9335), 779–780 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Preissl, H., Lowery, C. L. & Eswaran, H. Fetal magnetoencephalography: current progress and trends. Exp. Neurol.190, 28–36. 10.1016/j.expneurol.2004.06.016 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Eswaran, H., Lowery, C. L., Wilson, J. D., Murphy, P. & Preissl, H. Fetal magnetoencephalography–a multimodal approach. Brain Res. Dev. Brain Res.154(1), 57–62. 10.1016/j.devbrainres.2004.10.003 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Preissl, H., Lowery, C. L. & Eswaran, H. Fetal magnetoencephalography: viewing the developing brain in utero. Int. Rev. Neurobiol.68, 1–23. 10.1016/S0074-7742(05)68001-4 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Wurm, D. et al. A Small Scale Optically Pumped Fetal Magnetocardiography System. J. Clin. Med.12(10), 3380 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wakai R. Fetal Magnetocardiography with OPMs. In Flexible High Performance Magnetic Field Sensors: On-Scalp Magnetoencephalography and Other Applications Etienne Labyt, Tilmann Sander, Ronald Wakai (eds) Springer 2022 283–97.
- 17.Wilson, J. D. & Haueisen, J. Separation of physiological signals using minimum norm projection operators. IEEE Trans. Biomed. Eng.64(4), 904–916 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tadel, F., Baillet, S., Mosher, J. C., Pantazis, D. & Leahy, R. M. Brainstorm: A user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci.2011, 1–13 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niso, G. et al. Brainstorm Pipeline Analysis of Resting-State Data From the Open MEG Archive. Front. neurosci.10.3389/fnins.2019.00284 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vrba, J. et al. Fetal MEG redistribution by projection operators. IEEE Trans. Biomed. Eng.51(7), 1207–1218. 10.1109/TBME.2004.827265 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Escalona-Vargas, D., Wu, H.-t, Frasch, M. G. & Eswaran, H. A comparison of five algorithms for fetal magnetocardiography signal extraction. Cardiovas. Eng. & Technol.10.1007/s13239-018-0351-4 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Ulusar UD, Govindan RB, Wilson JD, Lowery CL, Preissl H, Eswaran H. Adaptive rule based fetal QRS complex detection using Hilbert transform Conf Proc IEEE Eng Med Biol Soc. 2009 2009:4666–9 Epub 2009/12/08 10.1109/IEMBS.2009.5334180 PubMed PMID: 19964648 PMCID: 2954062. [DOI] [PMC free article] [PubMed]
- 23.Van Leeuwen, P. et al. Reproducibility and reliability of fetal cardiac time intervals using magnetocardiography. Physiol. Meas.25(2), 539 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Bolin, E. H. et al. Magnetocardiographic identification of prolonged fetal corrected QT interval in women receiving treatment for opioid use disorder. J. Obstet. & Gynaecol. Res.45(10), 1989–1996. 10.1111/jog.14055 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Bolin, E. H., Whittington, J. R., Mehl, S. T., Escalona-Vargas, D. & Eswaran, H. Fetal magnetocardiography for the diagnosis of fetal dysrhythmias: single-center experience over 8 Years. Clin. Electrophysiol.8(9), 1161–1163 (2022). [DOI] [PubMed] [Google Scholar]
- 26.Bolin, E. et al. Cardiac time intervals derived by magnetocardiography in fetuses exposed to pregnancy hypertension syndromes. J. Perinatol.36(8), 643–648 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Cuneo, B. F. et al. In utero diagnosis of long QT syndrome by magnetocardiography. Circulation128(20), 2183–2191 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grimm, B. et al. Recommended standards for fetal magnetocardiography. Pacing & clin. electrophysiol.26(11), 2121–2126 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Hill, R. M. et al. Multi-channel whole-head OPM-MEG: Helmet design and a comparison with a conventional system. NeuroImage.219, 116995 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang, Y. et al. Co-registration of OPM-MCG signals with CT using optical scanning. Iscience.26(11), 108235 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao, Z., Cao, F., An, N. & Ning, X. Automatic co-registration of OPM-MEG and MRI using a 3D laser scanner. Measurement.223, 113729 (2023). [Google Scholar]
- 32.Gu, W. et al. Real-Time Localization of OPMs in a Flexible Array during MEG Recordings. IEEE Trans. Instrumen. & Meas.10.1109/TIM.2023.3346516 (2023). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon reasonable request to the corresponding author following the institutional guidelines and policies.
We used publicly available open-source Brainstorm software and custom scripts written in Matlab to analyze our data.