Abstract
Aims: To establish that cells from the murine mammary carcinoma cell line, EMT6, express type I insulin-like growth factor receptor (IGF-IR), tissue-type plasminogen activator (tPA), and urokinase-type plasminogen activator (uPA). To investigate the role of IGF-IR in growth, transformation, and tumorigenesis in addition to its relation to tPA and uPA in EMT6 cells. To assess the suitability of the EMT6/syngeneic mouse model for studying the role of IGF-IR in tumorigenesis.
Methods: The presence of transcripts for IGF-IR, tPA, and uPA was determined by northern blot analysis using poly (A+) RNA derived from EMT6 cells transfected with an antisense IGF-IR construct or a construct lacking the antisense IGF-IR insert. Flow cytometry was used to measure IGF-IR protein. Assays were performed to determine cell proliferation, transformation, and the tumorigenicity of antisense IGF-IR transfected EMT6 cells and control transfected EMT6 cells.
Results: There was strong expression of IGF-IR, tPA, and uPA in EMT6 cells. EMT6 cells from clones carrying antisense IGF-IR displayed a significant decrease in cell proliferation and lost the ability to form colonies in soft agar. A decrease in tumour size occurred when cells carrying the antisense IGF-IR were injected into syngeneic mice. Reduced expression of tPA and uPA was seen in EMT6 cells carrying the antisense IGF-IR construct.
Conclusions: The IGF-IR plays a role in the progression, transformation, and tumorigenesis of EMT6 murine mammary carcinoma cells. The suppression of IGF-IR mRNA in EMT6 cells decreases tPA and uPA expression. EMT6 cells and the syngeneic mouse provide a suitable model for studying the role of IGF-IR in breast tumour progression.
Keywords: cell transfection, EMT6 cells, animal models, tissue-type plasminogen activator, urokinase-type plasminogen activator
The insulin-like growth factor (IGF) family of ligands, receptors, and binding proteins is intimately involved in the normal growth and development of the human fetus and adult.1 The biological effects of IGF-I and IGF-II are primarily mediated by the IGF-I receptor (IGF-IR), a transmembrane heterotetramer with tyrosine kinase activity. The actions of this receptor require the use of intermediate docking proteins for the propagation of its numerous signal transduction cascades. Consistent with its well documented role in cell cycle progression2 and cell survival,3,4 inappropriate IGF-IR signalling is thought to play an important role in cancer, including the ability of tumour cells to invade and metastasise. Indeed, it has been suggested that when expressed above a certain threshold, the IGF-IR behaves like an oncogenic protein and is capable of promoting neoplastic growth in vivo.5
Breast cancer kills around 50 000 women each year in North America with world figures about 10 times higher.6 Overexpression of the IGF-IR often occurs in human breast tumours and is recognised as a factor that contributes to poor prognosis in a subgroup of patients with oestrogen receptor negative neoplasms.7 Indeed, the crucial role that the IGF-IR plays in breast cancer growth has been confirmed by several different strategies that interfered with receptor function.8–10 A recent study from our laboratory showed that the treatment of oestrogen receptor negative MDA-MB-435s human breast cancer cells with an antisense IGF-IR expression construct resulted in inhibition of cell growth, in addition to the ability of the cells to form colonies in soft agar.11 Moreover, there was suppression of tumorigenesis, a reduction in the metastatic potential, and prolonged survival after the injection of MDA-MB-435s cancer cells expressing antisense IGF-IR into the mammary fat pads of immune compromised mice. Alternatively, it has been shown that overexpression of the IGF-IR promotes aggregation, growth, and cell survival in MCF-7 human breast cancer cells in vitro.12
“There was suppression of tumorigenesis, a reduction in the metastatic potential, and prolonged survival after the injection of MDA-MB-435s cancer cells expressing antisense IGF-IR into the mammary fat pads”
Tumour recurrence and the development of metastases are common problems in the treatment of breast cancer. Many transformed or malignant tumour cells including cells from breast cancers are known to produce plasminogen activator (PA) which is a serine protease existing in two forms known as tissue-type (tPA) and urokinase-type (uPA).13 Plasminogen activators contribute to the lysis of tumour associated fibrin, which is a crucial step in the generation of metastases.14 Indeed, both tPA and uPA play crucial roles in tumour growth and metastasis for several types of cancer including breast cancer.15–19 A previous study from our laboratory showed that uPA and tPA were decreased in rat prostate cancer cells transfected with an antisense IGF-IR construct.20 Moreover, a recent report has demonstrated that the disruption of IGF-IR in human breast cancer cell lines suppressed invasion through Matrigel and decreased the expression of uPA mRNA.21
To investigate the role of IGF-IR in the pathogenesis of breast cancer, we have characterised the murine mammary carcinoma cell line, EMT6, for the expression of IGF-IR gene transcripts and for protein expression. In addition, we determined whether the EMT6 cells expressed tPA and uPA at the mRNA level. We then applied an antisense strategy that targeted the IGF-IR to EMT6 cells to test the hypothesis that IGF-IR plays a role in cell growth and tumorigenesis in this cell line. In our study, we provide data to show that targeting the IGF-IR in EMT6 murine breast cancer cells reversed the malignant phenotype in vitro and reduced tumour growth in vivo. Moreover, the data revealed that the treatment of EMT6 cells with an antisense IGF-IR construct resulted in decreased expression of tPA and uPA. These studies provide a basis for future experiments that will allow us to elucidate the function of the IGF-IR in breast cancer in an immune competent syngeneic animal model system.
METHODS
Cell line and cell culture
The EMT6 murine mammary carcinoma cells that were used in our study were kindly provided by Dr Emmanuel Akporiaye (University of Arizona, Tucson, Arizona, USA). The EMT6 cell line is a subline of an early passage of the KHJJ line that was derived from a mammary carcinoma arising in a BALB/c mouse.22 EMT6 cells form progressive tumours in syngeneic mice and immunisation of mice with irradiated EMT6 cells does not generate significant protective immunity against this tumour.23 The cells were maintained in minimum essential medium α (αMEM) (Life Technologies, Gaithersburg, Maryland, USA) supplemented with 10% fetal bovine serum (FBS; HyClone Laboratories, Logan, Utah, USA). Culture medium for transfected EMT6 cells was supplemented with geneticin at 500 μg/ml (Life Technologies) to maintain selection pressure. All of the experiments were carried out using cells from early passages (P2 or P3). Before injection into 4–6 week old female syngeneic BALB/c mice the cells (1 × 105) were washed once in phosphate buffered saline (PBS), treated with versene (1/5000 dilution; Life Technologies) for three to five minutes and resuspended in PBS.
Construction of vectors
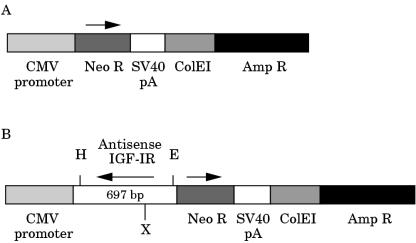
To assemble the pRcII/cytomegalovirus (CMV) vector (fig 1A ▶) used in these experiments a pRc/CMV vector (Invitrogen, Carlsbad, California, USA) was modified by removing DNA sequences from nucleotide position 995 (ApaI site in the multiple cloning site) to nucleotide position 2100 (SmaI site upstream of the neomycin resistance gene). Resulting DNA fragments were separated by agarose gel electrophoresis. A 4.3 kb vector fragment was isolated with a US Bioclean kit (United States Biochemical Corp, Cleveland, Ohio, USA) and re-ligated with T4 DNA ligase (Boehringer Mannheim, Indianapolis, Indiana, USA). After transformation the modified vector pRcII/CMV was grown in Escherichia coli and then purified by using a plasmid isolation kit (Qiagen, Chatsworth, California, USA). The human IGF-IR cDNA 697 bp fragment (nucleotide position 42 in exon 1 to nucleotide position 738 in exon 3; Genbank accession number M24599) that was obtained from total RNA isolated from T47D human breast cancer cells by reverse transcription polymerase chain reaction was cloned into the HindIII/EcoRI sites of the pRcII/CMV vector in the antisense orientation (fig 1B ▶). The directional cloning of the antisense IGF-IR cDNA insert was confirmed by restriction mapping.
Figure 1.
Schematic representation of antisense insulin-like growth factor I receptor (IGF-IR) construct (IGF-IRAS) and the control construct. (A) The construct used for control transfections lacked the antisense IGF-IR insert. (B) The insert for the antisense IGF-IR construct was assembled using the 697 bp human IGF-IR fragment in the antisense orientation. The final insert following recloning was 748 bp. Both constructs also contain sense neomycin. The transcription of IGF-IR antisense and neomycin sense are under the control of the constitutive cytomegalovirus (CMV) promoter. Amp R, ampicillin resistance gene; Neo R, neomycin resistance gene; SV40pA, Simian virus 40 polyadenylation signal; ColE1, ColE1 origin of replication; H, HindIII; E, EcoRI; X, XhoI.
Transfection
EMT6 murine mammary cancer cells were transfected with the antisense IGF-IR vector or with the pRcII/CMV control vector using Lipofectin (Life Technologies), according to the supplier's instructions. Geneticin (G-418 sulfate; Life Technologies) at a concentration of 1 mg/ml was used to select for cell clones that were neomycin resistant, indicating that the vector was present in the cells. Several (n = 9) individual cell clones were isolated from the population of cells carrying the antisense IGF-IR vector. All of the transfected cell clones were maintained in αMEM with 10% FBS and G418 (0.5 mg/ml).
Northern blot analysis
Total RNA was isolated from cells with Trizol reagent (Life Technologies). Poly A+ RNA was then selected using the Messagemaker reagent assembly (Life Technologies), according to the manufacturer's instructions. Samples (8 μg poly A+ RNA) were electrophoresed on a 1% denaturing agarose gel followed by transfer to a Hybond-N nylon membrane (Amersham Lifesciences, Arlington Heights, Illinois, USA). The cDNA probes—a 0.7 kb fragment from the human IGF-IR sequence, a 1.7 kb fragment from the mouse tPA sequence, a 1.3 kb fragment from the human uPA sequence, and a 2.2 kb fragment of chicken β actin—were labelled with α [32P]dCTP (DuPont NEN Research Products, Boston Massachusetts, USA) using the random hexanucleotide primer method.24 Northern blot hybridisation was carried out in 5× saline sodium citrate (SSC), 5× Denhardt's solution, 10% (wt/vol) dextran sulfate, 0.1% (wt/vol) sodium dodecyl sulfate (SDS), and 100 μg/ml denatured salmon sperm DNA at 65°C for 18 hours. After hybridisation, the membrane was washed for 15 minutes at room temperature with 2× SSC, followed by a final 15 minute wash in a solution consisting of 0.5× SSC and 0.5% SDS (wt/vol) at 65°C. The membranes were exposed to x ray film for five minutes to 12 hours at room temperature or at −80°C. x Ray films were analysed with a SciScan 5000 laser densitometer (United States Biochemical Corp, Cleveland, Ohio, USA) and normalised relative to β actin mRNA.
Flow cytometry
The EMT6 cells (1 × 106) were plated into 100 mm Petri dishes and grown in medium containing 10% FBS for 24 hours. The cells were rinsed with PBS and detached with versene, (1/5000 dilution; Life Technologies) at 37°C for 10 minutes, followed by washing in PBS containing 2% FBS. Cells (2 × 105) were transferred to Eppendorf tubes, spun at 300 ×g for three minutes at 4°C and washed twice in PBS containing 2% FBS. The primary antibody, mouse monoclonal antibody α-IR-3 (Oncogene Research Products, Cambridge, Massachusetts, USA) was used at a 1/30 dilution for 30 minutes at 4°C, followed by two rinses in PBS containing 2% FBS. The secondary antibody, B-phycoerythrin (PE) conjugated goat antimouse IgG Fab (Jackson ImmunoResearch Laboratories, West Grove, Pennsylvania, USA) was applied at a 1/60 dilution for 30 minutes at 4°C in the dark. Cells were rinsed as above and suspended in freshly prepared 2% paraformaldehyde at 4°C in the dark. Controls consisted of incubation with no antibodies or incubation with the secondary antibody only. Data were acquired using an EPICS XL-MCL (Beckman Coulter, Miami, Florida, USA). System II (Version 3) acquisition software was used to acquire and analyse the data. A 488 nm air cooled argon ion laser, operating at 15 mW, was used to excite the PE conjugated antibody, and a 575 nm band pass optical filter was used to collect the emitted light. Fluorescence data were visualised as a histogram. The fluorescent signals for the cells from clones carrying the antisense IGF-IR construct were compared with those of the cells carrying the CMV construct. The experiment was repeated four times.
Proliferation assays
The tetrazolium salt (MTT) method involving conversion of MTT to coloured formazan by cells serves an as indirect measurement of cell growth and is an accurate and reproducible means of measuring cell viability.25 Therefore, a modified MTT assay was used to determine cell proliferation rates in EMT6 murine mammary cancer cells from clones H, J, and O carrying antisense IGF-IR or from the clone carrying the control vector. Cells were seeded into each well (1 × 102 cells/well) of 96 well plates. MTT (20 μl, 5 mg/ml in PBS; Sigma Chemical Co, St Louis, Missouri, USA) was added on day 0, day 1, day 3, or day 5 of culture. The reaction was stopped after one hour of incubation at 37°C by the addition of 100 μl of solubilisation buffer (20% SDS (wt/vol) in 50% dimethylformamide; pH 5). Plates were then incubated at 37°C for four to 16 hours. An enzyme linked immunosorbent assay reader (Dynatech MR 5000; Dynatech Laboratories, Chantilly, Virginia, USA) was used to measure the absorbance at a wavelength of 570 nm and a reference wavelength of 630 nm. The reported cell numbers were extrapolated from a standard curve generated for each cell line and experiment relating absorbance to a known cell number. Data points are represented as the mean (SE) of six wells. All assays were repeated several times.
Soft agar colony forming assay
For soft agar assays, a bottom layer of 1 ml of the corresponding culture medium containing 0.6% granulated agar (Difco Laboratories, Detroit, Michegan, USA) and 10% FBS was prepared, placed in 35 mm culture dishes (Corning Glass Works, Corning, New York, USA) and allowed to solidify. Cells (1 × 103) from the H, J, and O clones carrying antisense IGF-IR or cells transfected with the vector lacking the insert were suspended in 50 μl of αMEM containing 10% FBS and 0.5 mg/ml G418. Culture medium (1 ml) containing 0.3% agar (BiTekTM; Difco Laboratories) was added to the cell suspension before seeding on the dishes. Triplicates were performed for each type of cell. Cells were incubated at 37°C in 5% CO2 atmosphere. Dishes were examined twice a week and colonies were counted manually after two weeks. Results are expressed as mean (SE).
Assay of tumour growth in animal models
Female syngeneic Balb/c mice 4–6 weeks of age were used. Mice were purchased from the Jackson Laboratory (Bar Harbor, Maine, USA) and maintained in the animal resource facility at Case Western Reserve University. The EMT6 murine mammary cancer cells from clones H, J, and O carrying the antisense IGF-IR construct or the control cells carrying the construct lacking the antisense IGF-IR insert were injected through a 22 gauge needle over the right scapula of the mice. All cells were injected in a volume of 100 μl at a concentration of 1 × 105 viable cells, as determined by trypan blue exclusion. The tumour take rate when the control cells were injected in the scapular region was 100%. Animals were sacrificed on day 14 and the tumours excised and weighed.
RESULTS
The goal of these studies was to characterise the EMT6 murine mammary cancer cell line for IGF-IR expression, in addition to the expression of the plasminogen activators, tPA and uPA. We also wanted to establish whether the EMT6 cell line would provide a suitable syngeneic animal model for examining the role of IGF-IR in tumorigenesis using an antisense gene targeting approach.
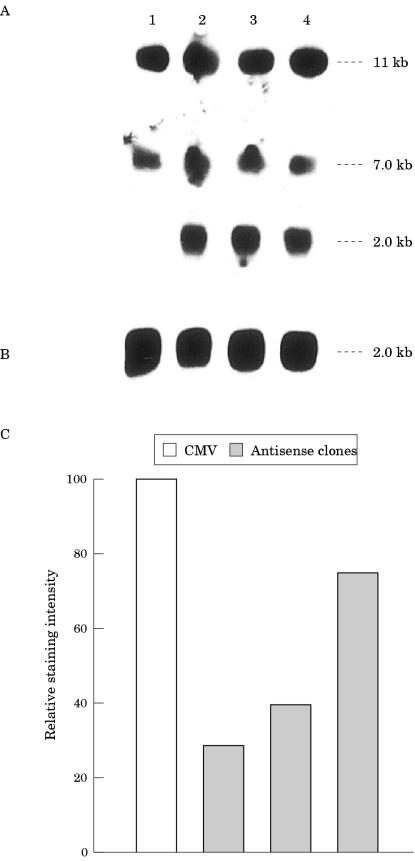
The first objective of the series of experiments carried out in our study was to determine whether the EMT6 murine mammary carcinoma cells express IGF-IR. The second aim was to establish that EMT6 cells could be transfected with our antisense IGF-IR expression construct. After transfection of the EMT6 cells with the control vector (construct lacking the IGF-IR inserts) and clonal selection, northern blot analysis was carried out using poly (A+) RNA derived from cells. The EMT6 cells carrying the control construct were found to express both the 7 kb and 11 kb transcripts for IGF-IR (fig 2A ▶). After transfection of the EMT6 murine mammary cancer cells with a vector containing the IGF-IR cDNA in an antisense orientation, several clones (n = 9) were identified and maintained in culture. All of these cell clones were assessed for the presence of the antisense IGF-IR expression vector (data not shown). The three clones, H, J, and O (fig 2A ▶) that displayed the strongest signal for antisense IGF-IR expression by northern blot analysis were used to assess further the role of IGF-IR in cell growth and tumour formation. The 7 kb and 11 kb transcripts for IGF-IR were apparent in cells from the J, H, and O clones carrying the antisense IGF-IR expression construct (fig 2A ▶). The expression of endogenous IGF-IR in the antisense IGF-IR cell clones was not decreased compared with that of the cells from the clone carrying the control vector when northern blots were analysed using laser densitometry (data not shown). The filter was rehybridised with a cDNA probe for chicken β actin to verify the integrity and amount of poly (A+) RNA in the samples (fig 2B ▶). Flow cytometric analysis using an IGF-IR antibody showed that IGF-IR protein was reduced in the cells from the clones carrying the antisense IGF-IR construct (fig 2C ▶) compared with cells from the clone carrying the construct minus the antisense IGF-IR insert (fig 2C ▶).
Figure 2.
Effects of antisense insulin-like growth factor I receptor (IGF-IR) treatment on mRNA and protein expression in EMT6 murine mammary cancer cells. (A) Expression of antisense IGF-IR transcripts and endogenous IGF-IR transcripts in cultured EMT6 murine mammary carcinoma cells from the clone carrying the control vector (lane 1), and in cells from the H (lane 2), J (lane 3), and O (lane 4) clones carrying the antisense IGF-IR vector. An 8 μg aliquot of poly A+ RNA was used in each lane, hybridised with the 32P labelled 0.7 kb IGF-IR cDNA fragment, and the membrane was exposed to an x ray film for 12 hours at −80°C. The molecular sizes of IGF-IR antisense (2 kb) and the endogenous IGF-IR transcripts (11 kb and 7 kb) are shown on the right. (B) Rehybridisation of the same filter with a cDNA probe for chicken β actin. (C) Flow cytometric analysis of IGF-IR protein in cultured EMT6 cells carrying the control vector (cytomegalovirus; CMV; lane 1), and in cells from clones H (lane 2), J (lane 3), and O (lane 4). Cells were stained with a mouse monoclonal IgG antibody α-IR-3.
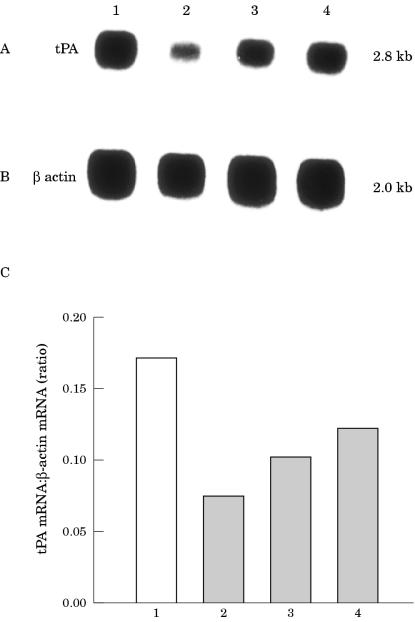
To determine whether the treatment of EMT6 cells with antisense IGF-IR RNA could affect the expression of the plasminogen activator, tPA, we analysed poly (A+) RNA derived from EMT6 cells from clones, H, J, and O, and compared it with poly (A+) RNA derived from EMT6 cells from the clone with the CMV vector by northern blot hybridisation. We found that tPA was expressed by all of the cell samples (fig 3A ▶). The expression of tPA mRNA in the IGF-IR antisense transfected clones was reduced by 60% in clone H, by 40% in clone J , and by 30% in clone O relative to that in the cells from the control transfected clone (fig 3C ▶). The filter was rehybridised with a cDNA probe for chicken β actin to verify the integrity and amount of poly (A+) RNA in the samples (fig 3B ▶).
Figure 3.
Expression of tissue type plasminognen (tPA) activator in EMT6 murine mammary carcinoma cells by northern blot analysis of mRNA derived from transfected EMT6 cells. (A) Poly (A+) RNA (8 μg/lane) derived from cells from the cytomegalovirus control transfected cell clone (lane 1) and from cells from clones H (lane2), J (lane 3), and O (lane 4) carrying the antisense insulin-like growth factor I receptor (IGF-IR) construct was analysed using a tPA cDNA hybridisation probe. (B) Rehybridisation of the same filter was performed with a β actin probe. The mRNA signals were scanned using the laser densitometer SciScan 5000, and the difference in the expression of mRNA transcripts was calculated relative to the β actin standards. Results of analysis for tPA are shown in the bar graph (C). The numbers under the bar graph correspond to the number used in A and B.
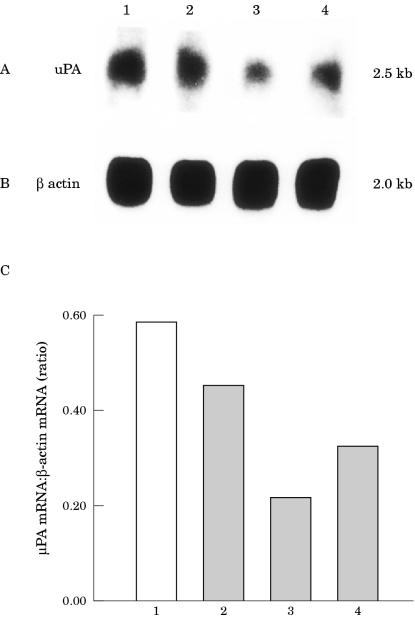
We also assessed uPA expression in the EMT6 cells. Northern blot analysis was carried out on poly (A+) RNA derived from cells from the clones expressing antisense IGF-IR and on poly (A+) RNA derived from cells from the clone transfected with the control vector. We found that uPA was expressed by all of the cell samples (fig 4A ▶). The expression of uPA mRNA in the IGF-IR antisense transfected cells was reduced by 25% in clone H, by 65% in clone J, and by 45% in clone O relative to that in the cells from the clone carrying the control CMV construct (fig 4C ▶). The filter was rehybridised with a cDNA probe for chicken β actin to verify the integrity and amount of poly (A+) RNA in the samples (fig 4B ▶).
Figure 4.
Expression of urokinase type plasminogen (uPA) activator in EMT6 murine mammary carcinoma cells by northern blot analysis of mRNA from transfected EMT6. (A) Poly (A+) RNA (8 μg/lane) derived from cells from the cytomegalovirus control transfected clone (lane 1) and from cells from clones H (lane2), J (lane 3), and O (lane 4) carrying the antisense insulin-like growth factor I receptor (IGF-IR) construct was analysed using a uPA cDNA hybridisation probe. (B) Rehybridisation of the same filter was performed with a β actin probe. The mRNA signals were scanned using the laser densitometer SciScan 5000, and the difference in the expression of mRNA transcripts was calculated relative to the β actin standards. Results of analysis for uPA are shown in the bar graph (C). The numbers under the bar graph correspond to the numbers used in A and B.
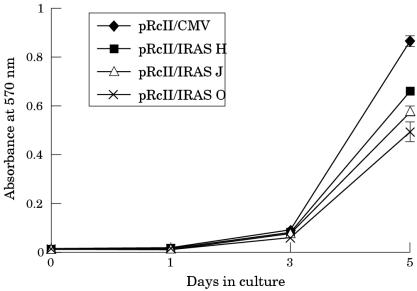
A series of assays was performed to characterise the proliferative capabilities of the EMT6 cells after treatment with the antisense IGF-IR construct. The proliferation rate of the cells from clone H, clone J, and clone O (fig 5A ▶) carrying the antisense IGF-IR construct was significantly inhibited on day 5 when compared with the proliferation rate of cells from the clone carrying the construct lacking the IGF-IR insert (fig 5A ▶). In addition to slower growth rates, large numbers of non-adherent cells were present in cultures of the clones carrying the IGF-IR antisense construct compared with the cultures of the clone carrying the control construct.
Figure 5.
Effect of treatment with an insulin-like growth factor I receptor (IGF-IR) antisense construct on the proliferation rate of EMT6 murine mammary carcinoma cells. Cell proliferation was measured using an MTT based assay. Data points are means (SE) of six wells. The treatment of EMT6 cells with an antisense IGF-IR construct inhibits cell proliferation in three clonal populations, H (pRcII/IRAS H; squares), J (pRcII/IRAS J; triangles), and O (pRcII/IRAS O; crosses) compared with cells from the clone carrying the construct lacking the IGF-IR insert (pRcII/CMV; diamonds).
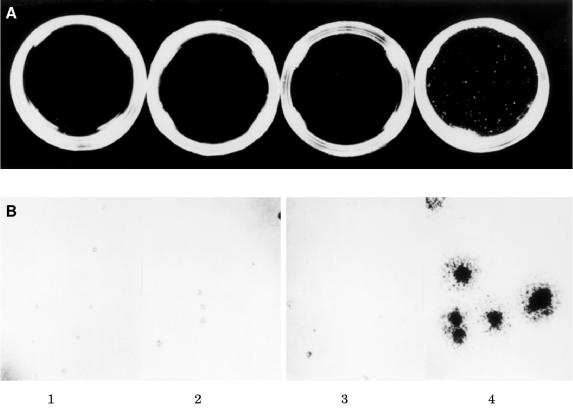
Anchorage independent growth in the semisolid medium of soft agar is a strong indicator of the transformed phenotype and a more stringent test of mitogenic capacity because several cycles of cell division are required to form detectable colonies. Therefore, a series of soft agar assays was performed to assess the ability of EMT6 cells treated with antisense IGF-IR to form colonies in soft agar. Colony formation in soft agar was completely abrogated in cells from the H, J, and O clones carrying the antisense IGF-IR construct (fig 6A ▶). However, cells from the clone carrying the construct lacking the antisense IGF-IR insert (figure 6A ▶) exhibited avid clonogenic growth under identical culture conditions. There was no detectable colony formation by cells carrying the antisense IGF-IR construct, whereas the cells transfected with the control vector displayed significant colony formation after 14 days in culture (table 1 ▶).
Figure 6.
Soft agar colonisation of EMT6 murine mammary carcinoma cells. Assays were performed in parallel on all cells under identical culture conditions. (A) Photographs are from a soft agar assay carried out on cells from the H, J, and O clones (panels 1, 2, and 3, respectively), carrying the antisense insulin-like growth factor I receptor (IGF-IR) construct and cells carrying the cytomegalovirus construct lacking the antisense IGF-IR insert (panel 4). Original magnification, ×1. (B) High power photographs of sample fields taken from assays depicted above. Original magnification, ×40.
Table 1.
Anchorage independent colony formation by EMT6 murine mammary carcinoma cells carrying an antisense IGF-IR expression plasmid
| Treatment | No. of colonies |
| pRcII/IRAS H | 0 |
| pRcII/IRAS J | 0 |
| pRcII/IRAS O | 0 |
| pRcII/CMV | 660 (53) |
Triplicate plates were seeded with 1 × 103 cells each and colonies were counted 14 days later. Each number represents the average of triplicate determinations expressed as mean (SE).
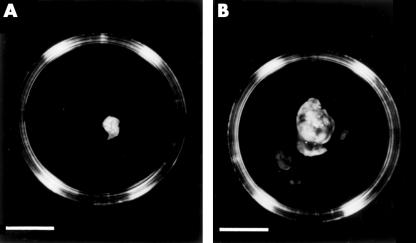
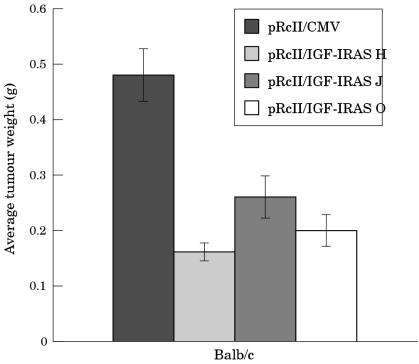
To assess whether treatment of the EMT6 murine mammary carcinoma cells with antisense IGF-IR affects the tumorigenic potential of the cells in vivo, assays were carried out in female syngeneic mice. Mice (six/group) were injected with cells from the antisense IGF-IR H, J, or O clones or cells from the control clone carrying the construct lacking the antisense IGF-IR insert. A dramatic inhibition in tumorigenesis was seen in the mice injected with cells from the H, J, and O clones. In contrast, the animals injected with cells from the clone carrying the construct lacking the antisense IGF-IR insert developed large tumours. Figure 7A ▶ shows a typical small tumour removed from a mouse injected with cells carrying the antisense IGF-IR construct on day 14 after injection; mice injected with cells carrying the construct lacking the antisense IGF-IR insert developed large tumours by day 14 post injection (fig 7B ▶). All of the mice injected with cells from the H, J, and O clones developed tumours; however, the tumours were significantly smaller than those that formed after injection of the control cells (fig 8 ▶).
Figure 7.
Tumour growth in syngeneic mice. (A) Representative tumour obtained from a mouse injected with cells carrying the antisense insulin-like growth factor I receptor (IGF-IR) construct. Bar = 10 mm. (B) Representative tumour obtained from a mouse injected with cells carrying the cytomegalovirus construct and not the antisense IGF-IR insert. Bar = 10 mm.
Figure 8.
Suppression of tumorigenesis in syngeneic mice injected with EMT6 murine mammary carcinoma cells carrying antisense insulin-like growth factor I receptor (IGF-IR). Female mice were injected subcutaneously in the region of the thoracic mammary fat pad with cells from the H (light grey bars), J (medium grey bars), and O (white bars) clones in addition to cells from the clone carrying the cytomegalovirus construct lacking the antisense IGF-IR insert (dark grey bars). The mice were killed 14 days after injection, the tumours were excised, and the tumour weights were determined. The data represent the mean (SE) for each group of mice.
DISCUSSION
We have characterised cells from the EMT6 murine mammary carcinoma cell line for the expression of IGF-IR, tPA, and uPA mRNA and for the production of IGF-IR protein. In addition, we have established that the syngeneic Balb/c mouse provides a suitable in vivo model for studying the role of IGF-IR in tumorigenesis. We then applied an antisense gene therapy strategy that targets the IGF-IR to define mechanisms that are involved in the growth of this cell line. In addition, we have shown that the treatment of EMT6 cells with antisense IGF-IR reduces IGF-IR protein as well as the mRNA expression of the plasminogen activators, tPA and uPA.
“We have established that the syngeneic Balb/c mouse provides a suitable in vivo model for studying the role of type I insulin-like growth factor receptor in tumorigenesis”
IGF-IR is produced by normal and malignant cells and is a crucial coordinator of cell cycle progression, cell survival, and the establishment and maintenance of the transformed phenotype.26 IGF-IRs are overexpressed in virtually all breast cancer cell lines, in which they are believed to enhance growth and inhibit apoptosis.27–29 In our study, we have established that cells from the murine mammary carcinoma cell line, EMT6, express transcripts for IGF-IR in addition to IGF-IR protein. Several reports, including those from our group, have shown that one mechanism by which gene therapy strategies that target IGF-I or IGF-IR in rat glioblastoma cells inhibit tumour growth is by the stimulation of a cellular immune response.30–34 The EMT6 mouse mammary cell tumour has been shown to be a useful model system for studying another member of the growth factor family, transforming growth factor β,23,35 and has also been useful in studies that investigated the role of the immune system in host–tumour interactions in vivo.36–38 Therefore, we plan to use the EMT6 model system to elucidate further the role of antisense IGF-IR in an immune competent animal.
Because EMT6 cells express IGF-IR transcripts we then used this model system to study the role of IGF-IR in murine mammary tumorigenesis. We carried out a series of experiments to determine whether the treatment of EMT6 cells by an antisense IGF-IR gene targeting approach could change the malignant phenotype of the murine breast cancer cells. We extended the findings of others, in addition to studies by our group, which showed that targeting the IGF-IR by both monoclonal antibodies directed against IGF-IR and antisense strategies inhibits breast cancer cell growth. The monoclonal antibody α-IR-3 has been used to block the IGF-IR and can successfully inhibit the growth of human breast cancer cells, MDA-MB-231.39 A recent report from our laboratory showed that the treatment of MDA-MB-435s cells with an antisense IGF-IR expression construct suppressed the growth of this metastatic human breast cancer cell line.11 We were unable to show a quantitative decrease in IGF-IR expression at the mRNA level by northern blot analysis of the antisense IGF-IR EMT6 cell clones; however, small changes in mRNA values may not be detectable using this method. In contrast, we found a decrease in IGF-IR protein concentrations in cells from the antisense IGF-IR clones using flow cytometry, in addition to a significant inhibition in the cell proliferation rate. It has been shown that a small increment in the number of receptors for each cell, well within the physiological range, can modulate the mitogenic and transforming activities of the IGF-IR in mouse embryo fibroblast 3T3-like cells.40 Although it was not in the scope of our study to assess the number of IGF-IR binding sites on the EMT6 cells, it is likely that the treatment of the cells with our antisense IGF-IR construct resulted in a reduction in the number of binding sites for IGF-IR. Indeed, our results indicate that the reduction in the IGF-IR protein concentration in EMT6 cells treated with an antisense IGF-IR construct has profound effects on the mitogenic and transforming activities of the IGF-IR.
“The use of antisense type I insulin-like growth factor receptor for cancer treatment in humans has far reaching implications”
The abolition of anchorage independent growth in soft agar by cells from the H, J, and O clones suggests that the transformed phenotype has been reversed after treatment with the antisense IGF-IR expression construct. Several laboratories (including our group) have reported that the treatment of cancer cells with antisense IGF-IR results in the reversal of the transformed phenotype.11,31,41–44 We found that the antisense strategy was more effective in inhibiting the growth of EMT6 cells in soft agar than in the cell proliferation assay. This is in agreement with a report showing that the IGF-IR has a more profound influence on the transformed phenotype than on cellular proliferation for several tumour cell lines, including MCF-7 human breast cancer cells.45
We showed that there was a dramatic reduction in tumour size when cells from the H, J, and O clones carrying antisense IGF-IR were injected in the region of the mammary fat pad in syngeneic mice. A recent report from our group demonstrated that human breast cancer cells treated with a construct carrying antisense IGF-IR have a delay in the formation of tumours and significant inhibition of tumour growth in immune compromised mice.11 A previous report from our laboratory showed that the treatment of rat C6 glioblastoma cells with a transfection vector that was constructed to drive transcription of the homopurine (AG) sequence 3` to the termination codon of the IGF-IR that can form a potential triple helix resulted in dramatic inhibition of tumour growth in vivo.34 In addition, the downregulation of IGF-IR by either antisense or a potential triple helix approach resulted in upregulation of the cell surface expression of major histocompatability complex I, suggesting that there may be an immune system component involved in the suppression of tumorigenesis. A study by Resnicoff et al showed that the injection of C6 cells expressing antisense IGF-IR RNA into syngeneic animals provided protection against tumour induction by C6 wild-type cells and, in fact, caused complete regression of established C6 wild-type tumours.30 Furthermore, the direct injection of an antisense IGF-IR expression vector into established neuroblastoma tumours growing in syngeneic mice resulted in a pronounced inhibition of tumour growth, with complete and durable tumour regression in half of the animals.46 Therefore, the mechanisms by which gene therapy strategies targeting the IGF-IR inhibit the growth of some tumour cells may be through a decrease in receptor number as well as by yet undefined immune components. Indeed, the use of antisense IGF-IR for cancer treatment in humans has far reaching implications. A recently completed phase I study of 10 terminally ill patients with malignant glioblastoma treated with an 18 mer oligonucleotide that targets the sequence of IGF-I mRNA resulted in a dramatic long term response for two patients, an objective but transient response for four patients, and no response in four others.47 This study demonstrates the importance of using syngeneic animal models to provide information that can then translate to potential treatments for cancer in humans.
Take home messages.
The type I insulin-like growth factor receptor (IGF-IR) plays an important role in the progression, transformation, and tumorigenesis of EMT6 murine mammary carcinoma cells
The suppression of IGF-IR mRNA in EMT6 cells decreases the expression of tissue-type and urokinase-type plasminogen activator
EMT6 cells and the syngeneic mouse provide a suitable model for studying the role of IGF-IR in breast tumour progression
In our present study, we found a reduction in the expression of the plasminogen activators, tPA and uPA, in antisense IGF-IR transfected EMT6 cells. These serine proteases are involved in tumour invasion in vivo.48 Indeed, both tPA and uPA play crucial roles in tumour growth and metastasis.19 IGF-I induces tPA activity in developing astrocytes.49 Breast cancer recurrence is more likely if tumours express high amounts of IGF-IR,50 whereas early relapse is associated with the overexpression of uPA.51 A recent report has shown that in serum starved MDA-MB-231 human breast cancer cells uPA mRNA increased in expression over time in the presence of IGF-I.21 These investigators also showed that the inhibition of IGF-IR by the stable expression of a dominant negative inhibitor of IGF-IR, 486stop, suppressed invasion and decreased the expression of uPA mRNA in MDA-MB-231 cells. Moreover, uPA overproduction results in increased skeletal metastasis by human prostate cancer cells.52 uPA expressed by PA-III prostate cancer cells has been implicated in the osteoblastic process through its protease action by hydrolysing IGF binding proteins, which results in increased bioavailability of IGFs.53 A report from our group showed that PA-III rat prostate cancer cells transfected with an antisense IGF-IR construct have a decreased expression of tPA and uPA.20
In conclusion, our data have shown that the IGF-IR plays a role in proliferation, transformation, tumorigenesis, and growth progression in a murine mammary carcinoma cell line. In addition, we have established a correlation between IGF-IR and plasminogen activators in the murine EMT6 cells. The EMT6 murine mammary carcinoma cell line will provide an in vitro/in vivo model for studying the role of the IGF/plasminogen system in invasion and metastasis. Moreover, we have provided data showing that the EMT6 cell line and its syngeneic mouse can be useful for studying the role of the IGF-IR in tumorigenesis and the host immune response.
Acknowledgments
This work was supported by a grant from the Department of Defense, DMAD17–95–1–5020 to J Ilan. The flow cytometric analysis was carried out in the Comprehensive Cancer Centers core flow cytometry facility supported by NCI grant, CA43703.
Abbreviations
CMV, cytomegalovirus
FBS, fetal bovine serum
IGF-IR, type I insulin-like growth factor receptor
αMEM, minimum essential medium α
PBS, phosphate buffered saline
PE, phycoerythrin
SDS, sodium dodecyl sulfate
SSC, saline sodium citrate
tPA, tissue-type plasminogen activator
uPA, urokinase-type plasminogen activator
REFERENCES
- 1.Rasmussen AA, Cullen KJ. Paracrine/autocrine regulation of breast cancer by the insulin-like growth factors. Breast Cancer Res Treat 1998;47:219–33. [DOI] [PubMed] [Google Scholar]
- 2.Baserga R, Rubin R. Cell cycle and growth control. Critical Rev Eukaryot Gene Expr 1993;3:47–61. [PubMed] [Google Scholar]
- 3.Resnicoff M, Burgaud J-L, Rotman HL, et al. Correlation between apoptosis, tumorigenesis, and levels of insulin-like growth factor I receptors. Cancer Res 1995;55:3739–41. [PubMed] [Google Scholar]
- 4.Baserga R, Hongo A, Rubini M, et al. The IGF-I receptor in cell growth, transformation and apoptosis. Biochim Biophys Acta 1997;1332:F105–26. [DOI] [PubMed] [Google Scholar]
- 5.Werner H, Le Roith D. The insulin-like growth factor-I receptor signaling pathways are important for tumorigenesis and inhibition of apoptosis. Crit Rev Oncog 1997;8:71–92. [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Otin C, Diamandis EP. Breast and prostate cancer: an analysis of common epidemiological, genetic, and biochemical features. Endocr Rev 1998;19:365–96. [DOI] [PubMed] [Google Scholar]
- 7.Ellis MJ, Jenkins J, Hanfelt J, et al. Insulin-like growth factors in human breast cancer. Breast Cancer Res Treat 1998;52:175–84. [DOI] [PubMed] [Google Scholar]
- 8.Sumacz E, Guvakova MA, Nolan MK, et al. Type I insulin-like growth factor receptor function in breast cancer. Breast Cancer Res Treat 1998;47:255–67. [DOI] [PubMed] [Google Scholar]
- 9.Arteaga CL, Kitten LJ, Coronado EB, et al. Blockade of the type I somatomedin receptor inhibits growth of human breast cancer cells in athymic mice. J Clin Invest 1989;84:1418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuenschwander S, Roberts CT, LeRoith D. Growth inhibition of MCF-7 breast cancer cells by stable expression of an insulin-like growth factor I receptor antisense ribonucleic acid. Endocrinology 1995;136:4298–303. [DOI] [PubMed] [Google Scholar]
- 11.Chernicky CL, Yi L, Tan H, et al. Treatment of human breast cancer cells with antisense RNA to the type I insulin-like growth factor receptor inhibits cell growth, suppresses tumorigenesis, alters the metastatic potential, and prolongs survival in vivo. Cancer Gene Ther 2000;7:384–95. [DOI] [PubMed] [Google Scholar]
- 12.Guvakova M, Surmacz E. Overexpressed IGF-I receptors reduce estrogen growth requirements, enhance survival, and promote E-cadherin mediated cell–cell adhesion in human breast cancer cells. Exp Cell Res 1997;231:149–62. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita J, Ogawa S, Yamashita S, et al. Differential biological significance of tissue-type and urokinase-type plasminogen activator in human breast cancer. Br J Cancer 1993;68:524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colombi M, Bellotti D, De Petro, et al. Plasminogen activators in nude mice xenotransplanted with human tumorigenic cells. Invasion Metastasis 1995;15:22–33. [PubMed] [Google Scholar]
- 15.Hildenbrand R, Dilger I, Horlin A, et al. Urokinase plasminogen activator induces angiogenesis and tumor vessel invasion in breast cancer. Pathol Res Pract 1995;191:403–9. [DOI] [PubMed] [Google Scholar]
- 16.Schmitt M, Harbeck N, Thomssen C, et al. Clinical impact of the plasminogen activation system in tumor invasion and metastasis: prognostic relevance and target for therapy. Thromb Haemost 1997;78:285–96. [PubMed] [Google Scholar]
- 17.Bugge TH, Lund LR, Kombrinck KK, et al. Reduced metastasis of polyoma virus middle T antigen-induced mammary cancer in plasminogen-deficient mice. Oncogene 1998;16:3097–104. [DOI] [PubMed] [Google Scholar]
- 18.De Petro G, Vartio T, Salonen EM, et al. Tissue type plasminogen activator, but not urokinase, exerts transformation-enhancing activity. Int J Cancer 1984;33:563–7. [DOI] [PubMed] [Google Scholar]
- 19.Columbi M, Barlati S, Magdelenat H, et al. Relationship between multiple forms of plasminogen activator in human breast tumors and plasma and the presence of metastases in lymph nodes. Cancer Res 1984;44:2971–5. [PubMed] [Google Scholar]
- 20.Burfeind P, Chernicky CL, Rininsland F, et al. Antisense RNA to the type I insulin-like growth factor receptor suppresses tumor growth and prevents invasion by rat prostate cancer cells in vivo. Proc Natl Acad Sci U S A 1996;93:7263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunn SE, Torres JV, Nihei N, et al. The insulin-like growth factor-1 elevates urokinase-type plasminogen activator-1 in human breast cancer cells: a new avenue for breast cancer therapy. Mol Carcinog 2000;27:10–17. [DOI] [PubMed] [Google Scholar]
- 22.Rockwell S, Kallman RF, Fajardo LF. Characteristics of a serially transplanted mouse mammary tumor and its tissue-culture-adapted derivative. J Natl Cancer Inst 1972;49:735–49. [PubMed] [Google Scholar]
- 23.McEarchern JA, Besselsen DG, Akporiaye ET. Interferon gamma and antisense transforming growth factor beta transgenes synergize to enhance the immumogenicity of a murine mammary carcinoma. Cancer Immunol Immunother 1999;48:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feinberg AP, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 1983;132:6–13. [DOI] [PubMed] [Google Scholar]
- 25.Hansen MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods 1989;119:203–10. [DOI] [PubMed] [Google Scholar]
- 26.Rubin R, Baserga R. Biology of disease: insulin-like growth factor-I receptor: its role in cell proliferation, apoptosis, and tumorigenicity. Lab Invest 1995;73:311–31. [PubMed] [Google Scholar]
- 27.Cullen KJ, Yee D, Sly WS, et al. Insulin-like growth factor receptor expression and function in human breast cancer. Cancer Res 1990;50:48–53. [PubMed] [Google Scholar]
- 28.Peyrat JP, Bonneterre J. Type I IGF-I receptor in human breast disease. Breast Cancer Res Treat 1992;22:59–68. [DOI] [PubMed] [Google Scholar]
- 29.Pezzino V, Papa V Milazzo G, et al. Insulin-like growth factor-I (IGF-I) receptors in breast cancer. Ann N Y Acad Sci 1996;784:189–201. [DOI] [PubMed] [Google Scholar]
- 30.Resnicoff M, Sell C, Rubini M, et al. Rat glioblastoma cells expressing an antisense RNA to the insulin-like growth factor-1 (IGF-1) receptor are nontumorigenic and induce regression of wild-type tumors. Cancer Res 1994;54:2218–22. [PubMed] [Google Scholar]
- 31.Resnicoff M, Li W, Basak S, et al. Inhibition of rat C6 glioblastoma tumor growth by expression of insulin-like growth factor I receptor antisense mRNA. Cancer Immunol Immunother 1996;42:64–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trojan J, Blossey BK, Johnson TR, et al. Loss of tumorigenicity of rat glioblastoma directed by episome-based antisense cDNA transcription of insulin-like growth factor I. Proc Natl Acad Sci U S A 1992;89:4874–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trojan J, Johnson TR, Rudin SD, et al. Treatment and prevention of rat glioblastoma by immunogenic C6 cells expressing antisense insulin-like growth factor I RNA. Science 1993;259:94–7. [DOI] [PubMed] [Google Scholar]
- 34.Rininsland F, Johnson TR, Chernicky CL, et al. Suppression of insulin-like growth factor type I receptor by a triple-helix strategy inhibits IGF-I transcription and tumorigenic potential of rat C6 glioblastoma cells. Proc Natl Acad Sci U S A 1997;94:5854–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McAdam AJ, Felcher A, Woods ML, et al. Transfection of transforming growth factor-β producing tumor EMT6 with interleukin-2 elicits tumor rejection and tumor reactive cytotoxic T-lymphocytes. J Immunother 1994;15:155–64. [DOI] [PubMed] [Google Scholar]
- 36.Akporiaye ET, Stewart SJ, Stevenson AP, et al. A gelatin sponge model for studying tumor growth: flow cytometric analysis and quantitation of leukocytes and tumor cells in the EMT6 mouse tumor. Cancer Res 1985;45:6457–62. [PubMed] [Google Scholar]
- 37.Kurt RA, Park JA, Panelli MC, et al. T lymphocytes infiltrating sites of tumor rejection and progression display identical V beta usage but different cytotoxic activities. J Immunol 1995;154:3969–74. [PubMed] [Google Scholar]
- 38.Panelli MC, Wang E, Shen S, et al. Interferon γ (IFNγ) gene transfer of an EMT6 tumor that is poorly responsive to IFNγ stimulation: increase in tumor immunogenicity is accompanied by induction of a mouse class II transactivator and class II MHC. Cancer Immunol Immunother 1996;42:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arteaga CI, Osborne CK. Growth inhibition of human breast cancer cells in vitro with an antibody against the type I somatomedin receptor. Cancer Res 1989;49:6237–41. [PubMed] [Google Scholar]
- 40.Rubini M, Hongo A, D'Ambrosio C, et al. The IGF-I receptor in mitogenesis and transformation of mouse embryo cells: role of receptor number. Exp Cell Res 1997;230:284–92. [DOI] [PubMed] [Google Scholar]
- 41.Resnicoff M, Ambrose A, Coppola D, et al. Insulin-like growth factor-1 and its receptor mediate the autocrine proliferation of ovarian carcinoma cell lines. Lab Invest 1993;69:756–60. [PubMed] [Google Scholar]
- 42.Resnicoff M, Coppola D, Sell C, et al. Growth inhibition of human melanoma cells in nude mice by antisense strategies to the type 1 insulin-like growth factor receptor. Cancer Res 1994;54:4848–50. [PubMed] [Google Scholar]
- 43.Shapiro D, Jones BG, Shapiro LH, et al. Antisense-mediated reduction in insulin-like growth factor-I receptor expression suppresses the malignant phenotype of a human alveolar rhabdomyosarcoma. J Clin Invest 1994;94:1235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ambrose D, Resnicoff M, Coppola D, et al. Growth regulation of human glioblastoma T98G cells by insulin-like growth factor I (IGF-I) and the IGF-I receptor. J Cell Physiol 1994;159:92–100. [DOI] [PubMed] [Google Scholar]
- 45.Baserga R, Sell C, Porcu P, et al. The role of the IGF-I receptor in the growth and transformation of mammalian cells. Cell Prolif 1994;27:63–71. [DOI] [PubMed] [Google Scholar]
- 46.Liu X, Turbyville T, Fritz A, et al. Inhibition of insulin-like growth factor I receptor expression in neuroblastoma cells induces the regression of established tumors in mice. Cancer Res 1998;58:5432–8. [PubMed] [Google Scholar]
- 47.Bauman N. Blocking IGF may be effective anti-cancer strategy. Urology Times 1999;27:7. [Google Scholar]
- 48.Mignatti P, Rifkin DB. Biology and biochemistry of proteinases in tumor invasion. Physiol Rev 1993;73:161–95. [DOI] [PubMed] [Google Scholar]
- 49.Tranque P, Naftolin F, Robbins R. Differential regulation of astrocyte plasminogen activators by insulin-like growth factor-I and epidermal growth factor. Endocrinology 1994;134:2602–13. [DOI] [PubMed] [Google Scholar]
- 50.Turner BC, Haffty BG, Narayanan L, et al. Insulin-like growth factor-1 receptor overexpression mediates cellular radioresistance and local breast cancer recurrence after lumpectomy and radiation. Cancer Res 1997;57:3079–83. [PubMed] [Google Scholar]
- 51.Janicke F, Schmitt M, Ulm K, et al. Urokinase-type plasminogen activator antigen and early relapse in breast cancer. Lancet 1989;28:1049. [DOI] [PubMed] [Google Scholar]
- 52.Achbarou A, Kaiser S, Tremblay G, et al. Urokinase overproduction results in increased skeletal metastasis by prostate cancer cells in vivo. Cancer Res 1994;54:2372–7. [PubMed] [Google Scholar]
- 53.Koutsilieris M, Frenette G, Lazure C, et al. Urokinase-type plasminogen activator: a paracrine factor regulating the bioavailability of IGFs in PA-III cell-induced osteoblastic metastases. Anticancer Res 1993;13:481–6. [PubMed] [Google Scholar]