Abstract
Globally, oral cancer ranks among the top ten cancers, with a higher prevalence in lower-income countries, where risk factors such as tobacco use, excessive alcohol consumption, and poor oral hygiene are widespread. Metastasis plays a critical role in cancer progression. miR-21 is a crucial regulator of cancer metastasis, profoundly influencing cellular and molecular pathways that contribute to tumour aggressiveness. As a microRNA, miR-21 downregulates tumour suppressor genes, promoting cell proliferation, survival, invasion, and migration. Its role in epithelial-mesenchymal transition (EMT) further facilitates metastatic behaviour. miR-21 also modulates the tumour microenvironment by promoting angiogenesis and altering immune responses, thus enhancing cancer progression.
Moreover, miRNA − 21 influences the various signalling pathways like PI3K/ AKT, TGF-β, NF-κB, and STAT3, as well as involved in the cell fate mechanisms known as Autophagy and apoptosis. Clinically, elevated miR-21 levels are associated with poor prognosis, advanced tumour stages, and decreased survival rates, making it a valuable prognostic marker. Additionally, miR-21 expression levels can predict resistance to chemotherapy and targeted therapies, aiding in personalized treatment planning. Therapeutically, targeting miR-21 through anti-miR-21 oligonucleotides, small molecule inhibitors, and miRNA sponges shows promise in pre-clinical studies, potentially inhibiting tumour growth and improving sensitivity to existing treatments. Overall, miR-21’s multifaceted role in cancer biology, its prognostic and predictive value, and its potential as a therapeutic target highlight its significance in advancing cancer diagnosis, treatment, and patient outcomes. Further research and clinical trials are essential to exploit miR-21’s capabilities in oncology fully.
Keywords: MiRNA-21, Metastasis, EMT, mRNA degradation, And translation repression
Introduction
Oral cancer, a significant subset of head and neck cancers, encompasses malignant tumours that develop in various parts of the oral cavity, including the lips, tongue, cheeks, floor of the mouth, and the hard and soft palates. It is characterized by the uncontrolled proliferation of abnormal cells in these regions, often leading to severe morbidity and mortality if not diagnosed and treated early. Globally, oral cancer ranks among the top ten cancers, with a higher prevalence in developing countries, where risk factors such as tobacco use, excessive alcohol consumption, and poor oral hygiene are rampant [1]. In addition to these traditional risk factors, viral infections, particularly the human papillomavirus (HPV), and bacterial infections have significantly contributed to oral cancer development. High-risk HPV strains, especially HPV-16, are strongly associated with oropharyngeal cancers, as they promote oncogenesis by integrating into host DNA and disrupting tumour suppressor pathways. Szewczyk and their team 2024, examined 284 oral cancer patients from 2010 to 2021 to evaluate age-related differences in prevalence and treatment outcomes. Although older patients exhibited a higher prevalence of comorbidities and smoking, disease stage and treatment results remained comparable across age groups. These findings indicate that factors other than age, including inflammatory markers like PLR and NLR, may have a greater impact on prognosis [2].
Similarly, chronic bacterial infections, particularly with species like Porphyromonas gingivalis and Fusobacterium nucleatum, have been linked to inflammation-driven carcinogenesis in the oral cavity [3]. These pathogens can promote cancer through immune evasion, DNA damage, and the secretion of virulence factors that enhance tumour growth and invasion. The growing recognition of infectious agents as risk factors emphasizes the multifactorial nature of oral cancer, highlighting the importance of prevention and early detection strategies.
Oral cancer metastasis refers to the spread of malignant cells from the primary tumour site in the oral cavity to distant organs or tissues. This process significantly complicates the treatment and management of the disease, often resulting in a poorer prognosis and reduced survival rates. Understanding the mechanisms and pathways of metastasis is crucial for developing effective therapeutic strategies and improving patient outcomes. Metastasis significantly impacts human survival rates as metastatic cancers are often more challenging to treat than localized tumours [4]. While localized cancers can often be surgically removed or treated with localized therapies, metastatic cancer typically requires systemic treatments such as chemotherapy, targeted therapy, or immunotherapy, which may not be as effective and can have severe side effects [5].
The molecular mechanisms underlying metastasis are complex and involve multiple steps and various cellular and molecular changes. The process begins with local invasion, where cancer cells breach the basement membrane and invade surrounding tissues. The epithelial-mesenchymal transition (EMT) often facilitates this, a biological process that allows epithelial cells to acquire mesenchymal, migratory properties. During EMT, cells lose their epithelial characteristics, such as cell-cell adhesion, and gain increased motility, enabling them to invade adjacent tissues [6, 7].
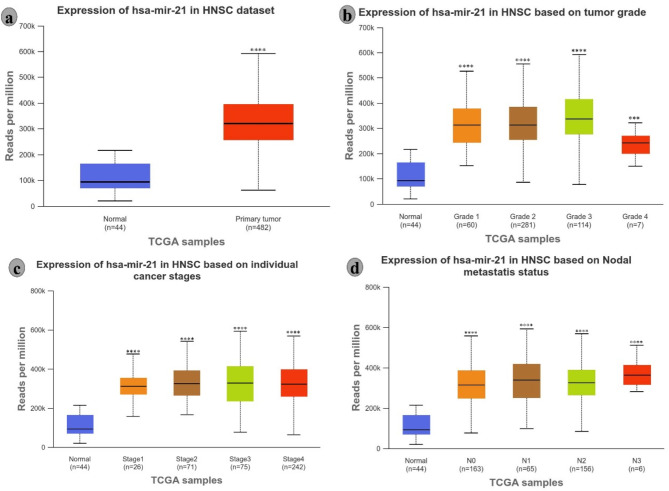
In recent decades, microRNAs (miRNAs) have been identified as key regulators in cancer progression, including oral cancer. These small, non-coding RNAs play crucial roles in gene expression regulation, influencing cellular processes such as proliferation, apoptosis, differentiation, and metastasis. In oral cancer, dysregulated miRNAs can act as oncogenes or tumour suppressors, depending on the specific target [8]. For instance, miR-21, an oncomiR, is frequently overexpressed in oral cancer, promoting tumour growth and metastasis by downregulating tumour suppressor genes, as Omics data are validated in Fig. 1. Other miRNAs, such as miR-34a and let-7, are often suppressed, leading to unchecked cell division and survival [9]. Identifying specific miRNA signatures in oral cancer has enhanced our understanding of the molecular mechanisms driving the disease and opened new avenues for diagnostic and therapeutic strategies. miRNAs can be non-invasive biomarkers for early detection, prognosis, and treatment response prediction. Moreover, therapeutic approaches targeting dysregulated miRNAs, such as miRNA mimics and antagomiRs, are being explored in pre-clinical studies, showing potential in reversing malignant phenotypes and improving treatment outcomes. Integrating miRNA-based therapies into clinical practice may offer a novel approach to combat oral cancer, particularly in cases resistant to conventional treatments.
Fig. 1.
miRNA-21 acts as an oncogenic miRNA in head and neck cancer. (a) Expression of miRNA-21 in normal tissue versus primary tumour, (b) Expression of miRNA-21 across different tumour grades, (c) Expression of miRNA-21 at individual cancer stages, and (d) miRNA-21 expression in nodal metastasis in head and neck cancer. Statistical significance for group comparisons was determined at ***p < 0.001 and **p < 0.0001
Understanding these molecular mechanisms is crucial for developing effective strategies to prevent and treat metastatic cancer. Current research focuses on identifying key regulators of metastasis, such as microRNAs like miR-21, which play significant roles in modulating the expression of genes involved in these processes [10]. By targeting these molecular pathways, new therapeutic approaches aim to inhibit the metastatic spread of cancer and improve survival rates for patients with advanced disease.
Domain structure and structural aspects of miR-21
miR-21 is a microRNA, a small non-coding RNA molecule approximately 22 nucleotides in length, that plays a crucial role in gene regulation. Unlike proteins, miRNAs do not have a traditional domain structure characterized by sequences of amino acids forming distinct functional and structural units [11]. Instead, the functional aspects miR-21 are determined by its sequence and the secondary structures it forms. miR-21 is transcribed as part of a primary miRNA (pri-miRNA) transcript, which is processed in the nucleus by the Drosha-DGCR8 microprocessor complex into a precursor miRNA (pre-miRNA). This pre-miRNA forms a characteristic stem-loop structure. The stem-loop structure of pre-miR-21 is critical for its recognition and further processing by the Dicer enzyme in the cytoplasm, which trims it into a mature miRNA duplex [12, 13].
The binding of miR-21 to its target mRNAs leads to either translational repression or mRNA degradation, depending on the degree of complementarity between miR-21 and the target mRNA [14]. The secondary structure of miR-21, characterized by its stem-loop configuration and the formation of the RISC-miRNA-mRNA complex, is essential for its regulatory functions [15]. These structural aspects allow miR-21 to modulate the expression of various genes involved in various cellular processes, including those critical for cancer progression and metastasis [16]. Thus, while miR-21 does not have domains in the traditional sense used to describe proteins, its functional architecture—comprising the pri-miRNA and pre-miRNA structures, the seed region for target binding, and its incorporation into the RISC—plays a pivotal role in its ability to regulate gene expression and influence cancer biology [17].
Physiological molecular and cellular functions of miR-21
miR-21 is one of the most studied microRNAs due to its involvement in various physiological and pathological processes, particularly in cancer. As a non-coding RNA, miR-21 regulates gene expression post-transcriptional levels, influencing several critical cellular functions [18].
Regulation of gene expression
miR-21 regulates gene expression primarily through its interaction with the 3’ untranslated regions (3’ UTRs) of target messenger RNAs (mRNAs) [11]. This process begins with the mature miR-21 being incorporated into the RNA-induced silencing complex (RISC), a multiprotein complex essential for RNA interference. Within this complex, miR-21 guides RISC to specific target mRNAs by binding to complementary sequences in their 3’ UTRs [19]. Its seed region mediates the binding of miR-21 to these regions, a short sequence of 6–8 nucleotides at the 5’ end of the miRNA. The seed region is highly conserved and critical for the specificity of target recognition, as it pairs with complementary bases in the target mRNA [20]. When miR-21 pairs with its target mRNA with perfect or near-perfect complementarity, RISC facilitates the endonucleolytic cleavage of the mRNA. This cleavage is a precise cut within the mRNA strand, initiated by the Argonaute (AGO) proteins within the RISC. Following cleavage, the mRNA is rapidly degraded by cellular exonucleases, substantially reducing the mRNA levels [21]. This degradation process effectively silences gene expression by removing the mRNA template required for protein synthesis, thereby downregulating the production of the encoded protein [22].
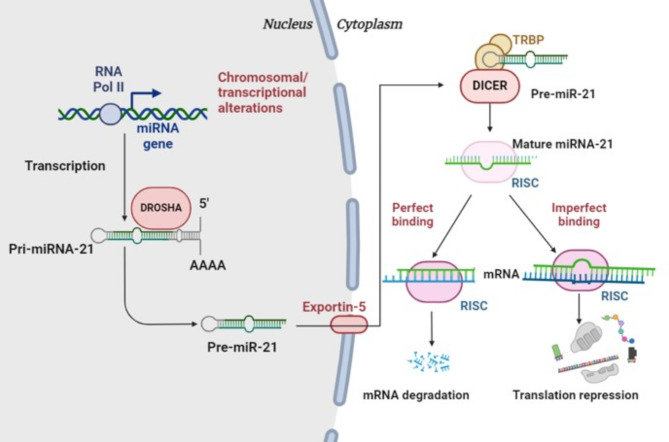
In cases where miR-21 binds to its target mRNA with imperfect complementarity, the silencing mechanism shifts to translational repression. In this scenario, RISC remains bound to the target mRNA without cleaving it. The binding of RISC to the mRNA hinders the translation process by blocking the assembly and movement of ribosomes along the mRNA strand [23]. This obstruction prevents the ribosomes from effectively synthesizing proteins from the mRNA, decreasing protein production despite intact mRNA [24]. The presence of miR-21-bound RISC on the mRNA can also recruit other factors that suppress translation or promote deadenylation and subsequent degradation of the mRNA, adding another layer of gene expression regulation [25, 26]. Figure 2 represent the ability miR-21 to employ both mRNA degradation and translational repression allows for a versatile and robust regulation of gene expression,. This dual mechanism enables miR-21 to fine-tune the expression of a wide range of target genes involved in critical cellular functions such as growth, apoptosis, and differentiation [27]. In cancer, miR-21 regulatory actions contribute significantly to tumorigenesis and metastasis by downregulating tumour suppressor genes and promoting oncogenic pathways. Understanding these silencing mechanisms provides valuable insights into miR-21’s role in cellular physiology and its potential as a therapeutic target for various diseases, especially cancer.
Fig. 2.
Physiological Process of miR-21. miR-21 is initially transcribed as a primary miRNA (pri-miR-21) transcript. This pri-miR-21 is processed in the nucleus by the microprocessor complex, which includes the enzyme DROSHA, to produce a precursor miRNA (pre-miR-21). The pre-miR-21 is then exported to the cytoplasm by a transporter protein, exportin-5. In the cytoplasm, the enzyme DICER further processes pre-miR-21 into mature miR-21. The mature miR-21 is incorporated into the RNA-induced silencing complex (RISC). Once bound to RISC, mature miR-21 can interact with target mRNAs. If miR-21 binds perfectly or near-perfectly to its target mRNA, it can lead to degradation. However, if the binding is imperfect, it results in translational repression, reducing the production of the target protein without degrading the mRNA
miR-21 influence various signaling pathway in tumor promotion
miR-21, a microRNA, has been extensively studied due to its significant role in cancer biology, particularly in tumour metastasis. Its physiological functions are mediated through the post-transcriptional regulation of target genes that control key cellular processes such as proliferation, apoptosis, invasion, and migration. Firstly, miR-21 promotes cell proliferation and survival by targeting and downregulating several tumour suppressor genes. Recently, Jaksic Karisik et al., 2025, indicate the miR-21 influence in the oral cancer stem cell to cancer progression [28]. Here, we discuss how role of miRNA − 21 in oral tumour progression by regulating several signaling pathways.
PI3K/AKT signaling
The PI3K/AKT signalling pathway is one of the most critical pathways involved in cell survival, growth, and proliferation, and its dysregulation is frequently observed in various cancers, including oral cancer [29]. miRNA-21 plays a significant role in activating this pathway through its interaction with PTEN (Phosphatase and Tensin Homolog), a well-known tumour suppressor. PTEN acts as a negative regulator of the PI3K/AKT pathway by dephosphorylating PIP3 (phosphatidylinositol 3,4,5-trisphosphate) back to PIP2 (phosphatidylinositol 4,5-bisphosphate), thereby preventing the activation of AKT [30]. When miRNA-21 is overexpressed, it binds to the mRNA of PTEN, leading to its degradation or translational repression. This downregulation of PTEN results in the accumulation of PIP3, which then facilitates the recruitment and activation of AKT (also known as Protein Kinase B) [31].
Once activated, AKT phosphorylates a range of downstream targets, promoting cell survival, growth, and proliferation. For instance, AKT inhibits pro-apoptotic factors such as BAD (Bcl-2-associated death promoter) and caspase-9, thereby preventing apoptosis and allowing cancer cells to evade programmed cell death. Additionally, AKT activation enhances protein synthesis and cell growth by activating mTOR (mechanistic target of rapamycin) signalling. Moreover, AKT can promote cell cycle progression by modulating the activity of GSK-3β (glycogen synthase kinase 3 beta) and cyclin D1 [32].
The hyperactivation of the PI3K/AKT pathway due to miRNA-21-mediated PTEN downregulation leads to uncontrolled cell proliferation and survival, which are hallmarks of cancer progression. This pathway facilitates tumour growth and contributes to the invasive and metastatic potential of oral cancer cells. The persistent activation of AKT signalling can further drive resistance to apoptosis, even in the presence of therapeutic agents, making oral cancer more aggressive and challenging to treat [33]. Thus, miRNA-21’s regulation of the PI3K/AKT pathway through PTEN suppression plays a pivotal role in the malignant behaviour of oral cancer, underscoring its potential as a therapeutic target.
TGF-β/ Smad signaling
Transforming Growth Factor-beta (TGF-β) signalling is a complex pathway that plays a dual role in cancer, acting as a tumour suppressor in the early stages of cancer development but promoting tumour progression and metastasis in later stages [34]. In oral cancer, miRNA-21 has been shown to modulate TGF-β signalling, significantly influencing the epithelial-to-mesenchymal transition (EMT) process. EMT is a biological process through which epithelial cells lose their cell polarity and adhesion properties, transforming into mesenchymal cells with enhanced migratory and invasive capabilities. This transition is critical for cancer cells to acquire invasive and metastatic properties, enabling them to spread to distant organs [35].
miRNA-21 influences TGF-β signalling by targeting various components of this pathway. For instance, it can downregulate the expression of Smad7, an inhibitory Smad protein that usually acts as a negative regulator of TGF-β signalling. By suppressing Smad7, miRNA-21 enhances the activity of the TGF-β/Smad signalling axis, leading to the increased expression of EMT-related transcription factors such as Snail, Slug, and Twist. These transcription factors play pivotal roles in repressing epithelial markers like E-cadherin while upregulating mesenchymal markers such as N-cadherin and vimentin. The loss of E-cadherin is significant, as it leads to the dissolution of cell-cell junctions, a key event in the initiation of EMT [36].
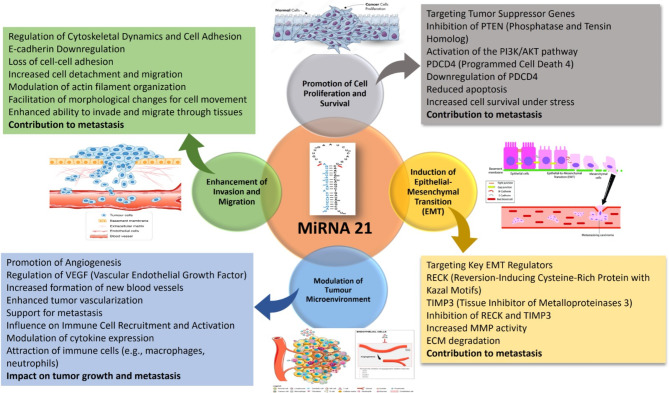
Moreover, miR-21 promotes EMT by targeting several key regulators of this process. For instance, miR-21 downregulates the expression of RECK (reversion-inducing cysteine-rich protein with Kazal motifs) [37] and TIMP3 (tissue inhibitor of metalloproteinases 3), both of which are inhibitors of matrix metalloproteinases (MMPs). The regulation of MMP activity is complicated, involving transcriptional supervision, activation of latent proenzymes, and suppression via tissue inhibitors of metalloproteases. Dysregulation of MMP expression and activity is a hallmark of cancer progression [38]. By inhibiting RECK and TIMP3, miR-21 enhances MMP activity, thereby promoting ECM degradation and enabling cancer cell invasion (Fig. 3) [39, 40].
Fig. 3.
Overview of the MiRNA-21 role in cancer progression. MiRNA − 21 induces Epithelial-mesenchymal Transition via inhibiting TIMP3 and RECK, Increasing MMPs activity. MiRNA-21 Modulate the tumour Microenvironments to enhance the cancer cell metastasis, increase the tumour vascularization, and promote the angiogenesis. MiRNA-21 coregulates with the PI3K/AKT pathway to improve cell proliferation, inhibit PTEN expression, and downregulate PDCD4. TIMP3- tissue inhibitors of metalloproteinases, PTEN- Phosphatase and TENsin homolog, RECK- reversion-inducing cysteine-rich protein with Kazal motifs, PI3K - phosphoinositide 3-kinase, PDCD4- programmed cell death 4, EMT- epithelial-mesenchymal transition
In oral cancer, the ability of miRNA-21 to modulate TGF-β signalling and promote EMT is particularly concerning, as it facilitates the transition of cancer cells from a relatively benign state to one that is highly invasive and capable of metastasis [41]. This makes miRNA-21 a critical player in the progression of oral cancer, linking its overexpression to poor prognosis and increased metastatic potential. Targeting miRNA-21 or its downstream effects on TGF-β signalling could represent a promising therapeutic strategy to inhibit EMT and reduce the invasiveness of oral cancer.
NF-κB signalling pathway
The NF-κB (Nuclear Factor kappa-light-chain-enhancer of activated B cells) signalling pathway is a crucial regulator of inflammation, immune responses, cell survival, and proliferation. In cancer, including oral cancer, this pathway often becomes aberrantly activated, contributing to tumour development and progression [42]. miRNA-21 is known to play a significant role in activating the NF-κB pathway. Typically, NF-κB remains inactive in the cytoplasm, bound to its inhibitor, IκB. Upon activation by various stimuli, IκB is phosphorylated and degraded, releasing NF-κB to translocate into the nucleus, which induces the expression of genes involved in inflammation, survival, and cell proliferation [43].
miRNA-21 facilitates the activation of NF-κB by targeting and downregulating multiple negative regulators of this pathway. For instance, miRNA-21 can suppress the expression of PTEN \, which is known to inhibit NF-κB activity. With these inhibitors downregulated, NF-κB signalling becomes hyperactivated, leading to the persistent expression of pro-inflammatory cytokines and growth-promoting factors. This continuous activation of NF-κB creates a microenvironment that favours cancer cell survival, proliferation, and invasion [44].
The overexpression of miRNA-21 and subsequent activation of NF-κB contribute significantly to the tumour’s aggressive behaviour. NF-κB-driven inflammation promotes cancer cell survival and recruits immune cells that release further pro-tumorigenic factors, creating a feedback loop that enhances tumour growth and resistance to therapy. Additionally, NF-κB activation is strongly associated with resistance to apoptosis [45].
Moreover, the pro-inflammatory environment generated by NF-κB activation is conducive to tumour progression and metastasis. Chronic inflammation induced by NF-κB can lead to increased matrix metalloproteinases (MMPs) production, which degrades the extracellular matrix and facilitates cancer cell invasion into surrounding tissues and distant organs [46]. The convergence of inflammation, cell survival, and proliferation driven by NF-κB makes this pathway a central player in the malignancy and progression of oral cancer.
STAT3 signaling pathway
STAT3 (Signal Transducer and Activator of Transcription 3) signalling pathway. STAT3 is a transcription factor commonly activated in various cancers and associated with processes such as cell proliferation, survival, angiogenesis, and immune evasion [47]. miR-21 indirectly enhances STAT3 activity by targeting negative regulators of this pathway, such as PTEN and SOCS (Suppressor of Cytokine Signaling) proteins [48]. Specifically, miR-21 downregulates SOCS3, a key inhibitor of STAT3 activation. By suppressing SOCS3, miR-21 removes the inhibitory feedback mechanism that would otherwise limit STAT3 activity, leading to its continuous activation. Activated STAT3 translocates to the nucleus, promoting gene expression in tumour growth, survival, and metastasis, including those that regulate anti-apoptotic proteins like Bcl-2 and Mcl-1 [49].
In addition, the elevated activity of STAT3 driven by miR-21 contributes to the development of an inflammatory tumour microenvironment, further supporting cancer progression. This activation of STAT3 also facilitates epithelial-mesenchymal transition (EMT), enhancing cancer cells’ migratory and invasive capabilities. Overall, the upregulation of miR-21 promotes a pro-tumorigenic environment by sustaining STAT3 activity, making it a key player in cancer progression and a potential target for therapeutic intervention in various malignancies, including gastric cancer [50].
Role of MiRNA 21 in cell fate
MiRNA 21 influences autophagy
miRNA-21 plays a complex and critical role in the regulation of Autophagy, a cellular process essential for maintaining cellular homeostasis by degrading and recycling damaged organelles and proteins. Autophagy has a dual role in cancer, acting as a tumour suppressor and a promoter of cancer cell survival under stress conditions. In the context of cancer, including oral cancer, miRNA-21 is frequently overexpressed and has been found to influence Autophagy in ways that support tumour progression [27].
miRNA-21 can inhibit Autophagy by targeting essential autophagy-related genes, such as PTEN and Beclin-1 (BECN1). PTEN, a tumour suppressor, is a positive regulator of Autophagy through its inhibition of the PI3K/AKT/mTOR pathway, which usually suppresses Autophagy. When miRNA-21 downregulates PTEN, it activates the PI3K/AKT/mTOR pathway, thereby inhibiting autophagy [51]. Similarly, miRNA-21 targets Beclin-1, a core component of the autophagy initiation complex. By reducing Beclin-1 levels, miRNA-21 further disrupts the formation of autophagosomes, the vesicles responsible for degrading cellular components [52].
The dysregulation of Autophagy driven by miRNA-21 contributes to several aspects of cancer progression, including resistance to chemotherapy. In cancer, for example, the suppression of Autophagy by miRNA-21 has been linked to increased resistance to chemotherapeutic agents, as autophagy inhibition can prevent the degradation of pro-survival factors [53]. This dual regulatory role of miRNA-21 in Autophagy highlights its significance in the tumour microenvironment, where it can switch between promoting and inhibiting Autophagy depending on the cellular components, ultimately supporting cancer cell survival and growth.
Apoptosis
miRNA-21 plays a pivotal role in regulating apoptosis, the programmed cell death process crucial for maintaining cellular balance and eliminating damaged or abnormal cells. In cancer, miRNA-21 is often overexpressed and acts as an anti-apoptotic factor, allowing cancer cells to evade death and continue proliferating. It exerts influence by targeting and downregulating key pro-apoptotic genes and signaling pathways, disrupting the normal apoptotic processes [54].
One of the primary targets of miRNA-21 is PDCD4 (Programmed Cell Death 4), a tumour suppressor gene that promotes apoptosis. By binding to the 3’-UTR of PDCD4 mRNA, miRNA-21 suppresses its expression, reducing pro-apoptotic signalling and enhancing cancer cell survival. Another critical target is PTEN (Phosphatase and Tensin Homolog), which negatively regulates the PI3K/AKT pathway. When PTEN is downregulated by miRNA-21, the AKT pathway becomes hyperactivated, inhibiting pro-apoptotic factors such as BAD and caspase-9, further blocking apoptosis [55].
Additionally, miRNA-21 influences the expression of Bcl-2, an anti-apoptotic protein that prevents mitochondrial outer membrane permeabilization (MOMP), a critical step in the intrinsic apoptotic pathway. By increasing Bcl-2 levels, miRNA-21 enhances the survival of cancer cells, even in the face of cellular stress or DNA damage. This resistance to apoptosis allows for unchecked cell growth and contributes to the development of more aggressive and treatment-resistant cancer phenotypes [56].
The ability miRNA-21 to inhibit apoptosis is a significant factor in its oncogenic role. By targeting multiple components of the apoptotic machinery, miRNA-21 effectively creates a cellular environment where cancer cells can thrive, resist therapy, and avoid death. This anti-apoptotic effect is one of the significant reasons why miRNA-21 is associated with poor prognosis in many cancers [57]. Therapeutically targeting miRNA-21 to restore apoptotic pathways represents a promising approach to sensitize cancer cells to treatment and limit tumour progression.
Potential of miR-21 as a predictive, prognostic, or therapeutic target
miR-21 has garnered significant attention in the field of oncology. Its overexpression is associated with various cancers progression via modulating several signalling pathways, including breast [58], lung [59], colorectal [60] and pancreatic cancers [61], making it a critical biomarker for cancer diagnosis and treatment as listed in Table 1.
Table 1.
MiR-21 role in various Cancer progression
| Sl.No. | Type of Cancer | Study Type | Signalling Pathway | Molecular Mechanism | Reference |
|---|---|---|---|---|---|
| 1 | Breast Cancer | In vitro and in vivo studies | PI3K/Akt, PTEN | miR-21 downregulates PTEN, activating PI3K/Akt signaling and promoting metastasis | [73] |
| 2 | Breast Cancer | Clinical studies | TGF-β/Smad, MMPs | miR-21 enhances MMP expression via the TGF-β/Smad signaling pathway | [74] |
| 3 | Breast Cancer | In vitro studies | EGFR, Ras, MAPK | miR-21 regulates the Ras/MAPK pathway to promote migration and invasion | [75] |
| 4 | Breast Cancer | Clinical, Bioinformatics | miR-21-3p, Tumor Suppressive Pathways | Hsa-miR-21-3p is associated with breast cancer patient survival and targets genes in tumour suppressive pathways. | [76] |
| 5 | Breast Cancer | Meta-analysis | PTEN/Akt | miR-21 targets PTEN to activate the Akt pathway in breast cancer | [77] |
| 6 | Colorectal Cancer | Clinical studies | TGF-β/Smad, MMPs | miR-21 enhances MMP expression via the TGF-β/Smad signaling pathway | [78] |
| 7 | Lung Cancer | In vitro studies | EGFR, Ras, MAPK | miR-21 regulates the Ras/MAPK pathway to promote migration and invasion | [79] |
| 8 | Lung Cancer (HBE cells) | In vitro | HIF-1α, miR-21, Akt/NF-κB | miR-21, regulated by HIF-1α, promotes the malignant transformation of HBE cells via the Akt/NF-κB pathway when induced by cigarette smoke extract. | [80] |
| 9 | Non-Small Cell Lung Cancer (NSCLC) | In vitro, Clinical | miR-21, p53 (R175H, R248Q) | miR-21 associates with mutant p53 sites R175H and R248Q, impacting clinicopathological features and prognosis of NSCLC. | [81] |
| 10 | Prostate Cancer | In vitro studies | PTEN/Akt | miR-21 targets PTEN to activate the Akt pathway in prostate cancer | [82] |
| 11 | Gastric Cancer | Animal model studies | STAT 3 | miR-21 target for solid malignancies characterized by excessive Stat3 activity | [50] |
| 12 | Oral Cancer | In vitro studies | PTEN | miR-21 and PTEN loop influences OSCC cell proliferation, invasion, and apoptosis | [83] |
| 13 | Multiple | In vitro, In vivo | miR-21, miR-7, Caspase-mediated apoptosis | miRzip-21 targets tumour proliferation, migration, and invasion and increases apoptosis. Combined miR-21 downregulation and miR-7 upregulation reduce tumour burden and improve survival. | [84] |
| 14 | Hepatocellular Carcinoma (HCC) | In vitro, In vivo | Akt/PTEN, Autophagy | miR-21 induces sorafenib resistance by downregulating PTEN and inhibiting Autophagy. Targeting miR-21 restores sensitivity to sorafenib. | [85] |
| 15 | Hepatocellular Carcinoma (HCC) | In vitro, In vivo | AKT/ERK, PTEN, hSulf-1 | miR-21 suppresses PTEN and hSulf-1 expression, promoting HCC progression through the AKT/ERK pathways. | [86] |
| 16 | Gastric Cancer | Systems Biology | miR-21, Hub Genes | Based on systems biology analysis, microRNA-21 exerts therapeutic effects on differentially expressed hub genes in gastric cancer. | [87] |
| 17 | Glioblastoma | In vitro, In vivo | miR-21, Tumor-Suppressive Pathways | MicroRNA-21 targets a network of key tumour-suppressive pathways in glioblastoma cells, affecting their progression and behaviour. | [88] |
| 18 | Pancreatic Cancer | Animal model studies | KRAS, PDAC | miR-21 mediates KRAS activation in pancreatic adenocarcinoma | [89] |
miR-21 can serve as a predictive biomarker, providing valuable insights into how a patient might respond to specific therapies. High levels of miR-21 have been correlated with resistance to chemotherapy and radiotherapy in several cancer types. For instance, in breast cancer, elevated miR-21 levels are linked to resistance to trastuzumab [62] and tamoxifen [63], common therapies used for treating HER2-positive and hormone receptor-positive breast cancers [64]. By assessing miR-21 levels before treatment, clinicians can predict the likelihood of a patient’s response to these therapies and adjust treatment plans accordingly. This predictive capability can help personalize cancer treatment, optimize therapeutic efficacy, and minimize unnecessary side effects [65].
The prognostic value miR-21 lies in its ability to predict cancer progression and patient outcomes. High miR-21 expression levels are often associated with poor prognosis, including increased tumour aggressiveness, higher metastatic potential, and reduced overall survival in Head and neck cancer [66]. For example, in colorectal cancer, elevated miR-21 levels have been linked to advanced tumour stages, lymph node metastasis, and shorter survival times [58]. Similarly, in non-small cell lung cancer (NSCLC), high miR-21 expression is correlated with lower survival rates and higher recurrence rates [67]. By evaluating miR-21 expression levels, clinicians can gain insights into the likely course of the disease, helping in risk stratification and informing decisions about the intensity and type of follow-up care required.
Targeting miR-21 therapeutically holds promise for cancer treatment due to its role in promoting oncogenesis and metastasis. Strategies to inhibit miR-21 include the use of anti-miR-21 oligonucleotides [68], small molecules [69], and miRNA sponges [70]. Anti-miR-21 oligonucleotides are synthetic sequences designed to bind to miR-21 and prevent it from interacting with its target mRNAs. This inhibition can restore the expression of tumour suppressor genes and impede cancer cell proliferation, invasion, and survival [71]. Pre-clinical studies have shown that anti-miR-21 therapies can reduce tumour growth and enhance sensitivity to chemotherapy in various cancer models [27].
Additionally, small molecule inhibitors can be designed to disrupt the biogenesis and function of miR-21. For instance, small molecules inhibiting the Drosha or Dicer enzymes involved in miR-21 processing can reduce miR-21 levels in cancer cell levels [72]. miRNA sponges, another therapeutic approach, are RNA molecules engineered to contain multiple binding sites for miR-21. These sponges can sequester miR-21, preventing it from interacting with its natural targets thereby mitigating its oncogenic effects. However, there is no FDA approval still now, in future more research in clinical studies and drug discovery against miRNA-21 are needed.
Conclusion
Taken together, miR-21 has extensive involvement in cancer metastasis, influencing numerous cellular and molecular pathways that contribute to the aggressive behaviour of cancer cells. As a critical regulator of gene expression, miR-21 promotes oncogenesis by targeting and downregulating tumour suppressor genes, thereby enhancing cell proliferation, survival, invasion, and migration. Its involvement in epithelial-mesenchymal transition (EMT) further underscores its significance in facilitating metastasis, and combined with its potential therapeutic target, it underscores its significance in cancer biology. Continued research into miR-21 will further elucidate its mechanisms and refine therapeutic strategies, paving the way for improved clinical outcomes for cancer patients.
Acknowledgements
The authors express their thanks and gratitude to AlMaarefa University, Riyadh, Saudi Arabia for the support to publish this article.
Author contributions
MP and DH: Conceptualization, Formal analysis, Investigation, Writing-original draft. MF: Formal analysis, Writing - review & editing. MIK: Conceptualization, Formal analysis, Project administration, Resources, Supervision, Writing - review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
None.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not required.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reference
- 1.Mohanprasanth A, Saravanan M. Advancements in cancer research: 3D Bioprinting as an optimal oral tumor model. Oral Oncol Rep. 2024;100174. 10.1016/j.oor.2024.100174.
- 2.Szewczyk M, Pazdrowski J, Golusiński P, Więckowska B, Golusiński W. Oral cancer in young adults: should we approach these patients differently? Front Oncol. 2024;14:1297752. 10.3389/fonc.2024.1297752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saravanan M, Carmelin DS, Mohanprasanth A, Arockiaraj J. Comment on oral Microbiome and risk of incident head and neck cancer: A nested case-control study. Oral Oncol. 2024;154:106858. 10.1016/j.oraloncology.2024.106858. [DOI] [PubMed] [Google Scholar]
- 4.Castaneda M, den Hollander P, Kuburich NA, Rosen JM, Mani SA. Mechanisms of cancer metastasis. Sem Cancer Biol. 2022;87:17–31. 10.1016/j.semcancer.2022.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Debela DT, Muzazu SG, Heraro KD, Ndalama MT, Mesele BW, Haile DC, Kitui SK, Manyazewal T. New approaches and procedures for cancer treatment: current perspectives. SAGE Open Med. 2021;9:20503121211034366. 10.1177/20503121211034366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almagro J, Messal HA, Elosegui-Artola A, van Rheenen J, Behrens A. Tissue architecture in tumor initiation and progression. Trends cancer. 2022;8(6):494–505. 10.1016/j.trecan.2022.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Investig. 2009;119(6):1420–8. 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otmani K, Lewalle P. Tumor suppressor MiRNA in Cancer cells and the tumor microenvironment: mechanism of deregulation and clinical implications. Front Oncol. 2021;11:708765. 10.3389/fonc.2021.708765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stahlhut C, Slack FJ. Combinatorial action of MicroRNAs let-7 and miR-34 effectively synergizes with erlotinib to suppress Non-small cell lung Cancer cell proliferation. Cell Cycle (Georgetown Tex). 2015;14(13):2171–80. 10.1080/15384101.2014.1003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bautista-Sánchez D, Arriaga-Canon C, Pedroza-Torres A, De La Rosa-Velázquez IA, González-Barrios R, Contreras-Espinosa L, Montiel-Manríquez R, Castro-Hernández C, Fragoso-Ontiveros V, Álvarez-Gómez RM, Herrera LA. The promising role of miR-21 as a Cancer biomarker and its importance in RNA-Based therapeutics. Mol Therapy Nucleic Acids. 2020;20:409–20. 10.1016/j.omtn.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Brien J, Hayder H, Zayed Y, Peng C. Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol. 2018;9:402. 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bofill-De Ros X, Vang Ørom UA. Recent progress in MiRNA biogenesis and decay. RNA Biol. 2024;21(1):1–8. 10.1080/15476286.2023.2288741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rice GM, Shivashankar V, Ma EJ, Baryza JL, Nutiu R. Functional atlas of primary MiRNA maturation by the microprocessor. Mol Cell. 2020;80(5):892–e9024. 10.1016/j.molcel.2020.10.028. [DOI] [PubMed] [Google Scholar]
- 14.Naeli P, Winter T, Hackett AP, Alboushi L, Jafarnejad SM. The intricate balance between microRNA-induced mRNA decay and translational repression. FEBS J. 2023;290(10):2508–24. 10.1111/febs.16422. [DOI] [PubMed] [Google Scholar]
- 15.Ying SY, Chang DC, Lin SL. The MicroRNA (miRNA): overview of the RNA genes that modulate gene function. Mol Biotechnol. 2008;38(3):257–68. 10.1007/s12033-007-9013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhim J, Baek W, Seo Y, Kim JH. From molecular mechanisms to therapeutics: Understanding MicroRNA-21 in Cancer. Cells. 2022;11(18):2791. 10.3390/cells11182791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Catalanotto C, Cogoni C, Zardo G. MicroRNA in control of gene expression: an overview of nuclear functions. Int J Mol Sci. 2016;17(10):1712. 10.3390/ijms17101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ratti M, Lampis A, Ghidini M, Salati M, Mirchev MB, Valeri N, Hahne JC. MicroRNAs (miRNAs) and long Non-Coding RNAs (lncRNAs) as new tools for Cancer therapy: first steps from bench to bedside. Target Oncol. 2020;15(3):261–78. 10.1007/s11523-020-00717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macfarlane LA, Murphy PR. MicroRNA: biogenesis, function and role in Cancer. Curr Genom. 2010;11(7):537–61. 10.2174/138920210793175895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellwanger DC, Büttner FA, Mewes HW, Stümpflen V. The sufficient minimal set of MiRNA seed types. Bioinf (Oxford England). 2011;27(10):1346–50. 10.1093/bioinformatics/btr149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jungers CF, Djuranovic S. Modulation of miRISC-Mediated gene Silencing in eukaryotes. Front Mol Biosci. 2022;9:832916. 10.3389/fmolb.2022.832916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin S, Tang X, Chen Y, Chen K, Fan N, Xiao W, Zheng Q, Li G, Teng Y, Wu M, Song X. mRNA-based therapeutics: powerful and versatile tools to combat diseases. Signal Transduct Target Therapy. 2022;7(1):166. 10.1038/s41392-022-01007-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwakawa HO, Tomari Y. Life of RISC: formation, action, and degradation of RNA-induced Silencing complex. Mol Cell. 2022;82(1):30–43. 10.1016/j.molcel.2021.11.026. [DOI] [PubMed] [Google Scholar]
- 24.Joazeiro CAP. Mechanisms and functions of ribosome-associated protein quality control. Nat Rev Mol Cell Biol. 2019;20(6):368–83. 10.1038/s41580-019-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eulalio A, Huntzinger E, Nishihara T, Rehwinkel J, Fauser M, Izaurralde E. Deadenylation is a widespread effect of MiRNA regulation. RNA (New York N Y). 2009;15(1):21–32. 10.1261/rna.1399509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braun JE, Huntzinger E, Izaurralde E. A molecular link between MiRISCs and deadenylases provides new insight into the mechanism of gene Silencing by MicroRNAs. Cold Spring Harb Perspect Biol. 2012;4(12):a012328. 10.1101/cshperspect.a012328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashemi M, Mirdamadi MSA, Talebi Y, Khaniabad N, Banaei G, Daneii P, Gholami S, Ghorbani A, Tavakolpournegari A, Farsani ZM, Zarrabi A, Nabavi N, Zandieh MA, Rashidi M, Taheriazam A, Entezari M, Khan H. Pre-clinical and clinical importance of miR-21 in human cancers: tumorigenesis, therapy response, delivery approaches and targeting agents. Pharmacol Res. 2023;187:106568. 10.1016/j.phrs.2022.106568. [DOI] [PubMed] [Google Scholar]
- 28.Jaksic Karisik M, Lazarevic M, Mitic D, Milosevic Markovic M, Riberti N, Jelovac D, Milasin J. MicroRNA-21 as a regulator of Cancer stem cell properties in oral Cancer. Cells. 2025;14(2):91. 10.3390/cells14020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rascio F, Spadaccino F, Rocchetti MT, Castellano G, Stallone G, Netti GS, Ranieri E. The pathogenic role of PI3K/AKT pathway in Cancer onset and drug resistance: an updated review. Cancers. 2021;13(16):3949. 10.3390/cancers13163949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li W, Zhang T, Guo L, Huang L. Regulation of PTEN expression by non-coding RNAs. J Experimental Clin cancer Research: CR. 2018;37(1):223. 10.1186/s13046-018-0898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergez-Hernández F, Irigoyen-Arredondo M, Martínez-Camberos A. A systematic review of mechanisms of PTEN gene down-regulation mediated by MiRNA in prostate cancer. Heliyon. 2024;10(15):e34950. 10.1016/j.heliyon.2024.e34950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu R, Chen Y, Liu G, Li C, Song Y, Cao Z, Li W, Hu J, Lu C, Liu Y. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020;11(9):797. 10.1038/s41419-020-02998-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glaviano A, Foo ASC, Lam HY, Yap KCH, Jacot W, Jones RH, Eng H, Nair MG, Makvandi P, Geoerger B, Kulke MH, Baird RD, Prabhu JS, Carbone D, Pecoraro C, Teh DBL, Sethi G, Cavalieri V, Lin KH, Javidi-Sharifi NR, Kumar AP. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol Cancer. 2023;22(1):138. 10.1186/s12943-023-01827-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giarratana AO, Prendergast CM, Salvatore MM, Capaccione KM. TGF-β signaling: critical nexus of fibrogenesis and cancer. J Translational Med. 2024;22(1):594. 10.1186/s12967-024-05411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abba ML, Patil N, Leupold JH, Allgayer H. MicroRNA regulation of epithelial to mesenchymal transition. J Clin Med. 2016;5(1):8. 10.3390/jcm5010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu X, Hong P, Wang Z, Tang Z, Li K. MicroRNAs in transforming growth Factor-Beta signaling pathway associated with fibrosis involving different systems of the human body. Front Mol Biosci. 2021;8:707461. 10.3389/fmolb.2021.707461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russell JJ, Grisanti LA, Brown SM, Bailey CA, Bender SB, Chandrasekar B. Reversion inducing cysteine rich protein with Kazal motifs and cardiovascular diseases: the recklessness of adverse remodeling. Cell Signal. 2021;83:109993. 10.1016/j.cellsig.2021.109993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srinivasan S, Mohanprasanth A, Nadeem A, Saravanan M. Exploring the anti-cancer and antimetastatic effect of Silymarin against lung cancer. Toxicol Rep. 2024;13:101746. 10.1016/j.toxrep.2024.101746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Lin Y, Jiang T, Gao C, Wang D, Wang X, Wei Y, Liu T, Zhu L, Wang P, Qi F. Up-regulation of TIMP-3 and RECK decrease the invasion and metastasis ability of colon cancer. Arab J Gastroenterology: Official Publication Pan-Arab Association Gastroenterol. 2019;20(3):127–34. 10.1016/j.ajg.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Gabriely G, Wurdinger T, Kesari S, Esau CC, Burchard J, Linsley PS, Krichevsky AM. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol Cell Biol. 2008;28(17):5369–80. 10.1128/MCB.00479-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li C, Song L, Zhang Z, Bai XX, Cui MF, Ma LJ. MicroRNA-21 promotes TGF-β1-induced epithelial-mesenchymal transition in gastric cancer through upregulating PTEN expression. Oncotarget. 2016;7(41):66989–7003. 10.18632/oncotarget.11888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang T, Ma C, Zhang Z, Zhang H, Hu H. NF-κB signaling in inflammation and cancer. MedComm. 2021;2(4):618–53. 10.1002/mco2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Markopoulos GS, Roupakia E, Tokamani M, Alabasi G, Sandaltzopoulos R, Marcu KB, Kolettas E. Roles of NF-κB signaling in the regulation of MiRNAs impacting on inflammation in Cancer. Biomedicines. 2018;6(2):40. 10.3390/biomedicines6020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sai X, Qin C, Zhang Z, Yu H, Bian T. A miRNA-21-Mediated PTEN/Akt/NF-κB Axis promotes chronic obstructive pulmonary disease pathogenesis. Int J Chronic Obstr Pulm Dis. 2024;19:1141–51. 10.2147/COPD.S453593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma X, Buscaglia B, Barker LE, J. R., Li Y. MicroRNAs in NF-kappaB signaling. J Mol Cell Biol. 2011;3(3):159–66. 10.1093/jmcb/mjr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guan C, Zhou X, Li H, Ma X, Zhuang J. NF-κB inhibitors gifted by nature: the anticancer promise of polyphenol compounds. Biomed pharmacotherapy = Biomedecine Pharmacotherapie. 2022;156:113951. 10.1016/j.biopha.2022.113951. [DOI] [PubMed] [Google Scholar]
- 47.Tošić I, Frank DA. STAT3 as a mediator of oncogenic cellular metabolism: pathogenic and therapeutic implications. Neoplasia (New York N Y). 2021;23(12):1167–78. 10.1016/j.neo.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sajjadi-Dokht M, Mohamad M, Sulaiman Rahman TA, Suliman Maashi H, Danshina M, Shomali S, Solali N, Marofi S, Zeinalzadeh F, Akbari E, Adili M, Aslaminabad A, Farshdousti Hagh R, M., Jarahian M. MicroRNAs and JAK/STAT3 signaling: A new promising therapeutic axis in blood cancers. Genes Dis. 2021;9(4):849–67. 10.1016/j.gendis.2021.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang L, Xu X, Li J, Yang C. Interaction between MicroRNAs and Myeloid-Derived suppressor cells in tumor microenvironment. Front Immunol. 2022;13:883683. 10.3389/fimmu.2022.883683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tse J, Pierce T, Carli ALE, Alorro MG, Thiem S, Marcusson EG, Ernst M, Buchert M. Onco-miR-21 promotes Stat3-Dependent gastric Cancer progression. Cancers. 2022;14(2):264. 10.3390/cancers14020264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shan C, Chen X, Cai H, Hao X, Li J, Zhang Y, Gao J, Zhou Z, Li X, Liu C, Li P, Wang K. The emerging roles of Autophagy-Related MicroRNAs in Cancer. Int J Biol Sci. 2021;17(1):134–50. 10.7150/ijbs.50773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Su Z, Yang Z, Xu Y, Chen Y, Yu Q. MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget. 2015;6(11):8474–90. 10.18632/oncotarget.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lei Y, Chen L, Liu J, Zhong Y, Deng L. The MicroRNA-Based strategies to combat Cancer chemoresistance via regulating autophagy. Front Oncol. 2022;12:841625. 10.3389/fonc.2022.841625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buscaglia LE, Li Y. Apoptosis and the target genes of microRNA-21. Chin J cancer. 2011;30(6):371–80. 10.5732/cjc.011.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH. Programmed cell death 4 (PDCD4) is an important functional target of the MicroRNA miR-21 in breast cancer cells. J Biol Chem. 2008;283(2):1026–33. 10.1074/jbc.M707224200. [DOI] [PubMed] [Google Scholar]
- 56.Wu H, Wang J, Ma H, Xiao Z, Dong X. MicroRNA-21 inhibits mitochondria-mediated apoptosis in keloid. Oncotarget. 2017;8(54):92914–25. 10.18632/oncotarget.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abdul Manap AS, Wisham AA, Wong FW, Najmi A, Ng HR, Z. F., Diba RS. Mapping the function of MicroRNAs as a critical regulator of tumor-immune cell communication in breast cancer and potential treatment strategies. Front Cell Dev Biology. 2024;12:1390704. 10.3389/fcell.2024.1390704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang H, Tan Z, Hu H, Liu H, Wu T, Zheng C, Wang X, Luo Z, Wang J, Liu S, Lu Z, Tu J. microRNA-21 promotes breast cancer proliferation and metastasis by targeting LZTFL1. BMC Cancer. 2019;19(1):738. 10.1186/s12885-019-5951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li H, Zhao J, Jia X, Zhang Y, Du Y, Li H, Ma L, Huang J. miR-21 promotes growth, invasion and migration of lung cancer cells by AKT/P-AKT/cleaved-caspase 3/MMP-2/MMP-9 signaling pathway. Int J Clin Exp Pathol. 2020;13(4):692–700. [PMC free article] [PubMed] [Google Scholar]
- 60.You C, Jin L, Xu Q, Shen B, Jiao X, Huang X. Expression of miR-21 and miR-138 in colon cancer and its effect on cell proliferation and prognosis. Oncol Lett. 2019;17(2):2271–7. 10.3892/ol.2018.9864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ali A, Jamieson NB, Khan IN, Chang D, Giovannetti E, Funel N, Frampton AE, Morton J, Sansom O, Evans TRJ, Duthie F, McKay CJ, Samra J, Gill AJ, Biankin A, Oien KA. Prognostic implications of microRNA-21 overexpression in pancreatic ductal adenocarcinoma: an international multicenter study of 686 patients. Am J cancer Res. 2022;12(12):5668–83. [PMC free article] [PubMed] [Google Scholar]
- 62.Gong C, Yao Y, Wang Y, Liu B, Wu W, Chen J, Su F, Yao H, Song E. Up-regulation of miR-21 mediates resistance to trastuzumab therapy for breast cancer. J Biol Chem. 2011;286(21):19127–37. 10.1074/jbc.M110.216887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amiruddin A, Massi MN, Islam AA, Patellongi I, Pratama MY, Sutandyo N, Natzir R, Hatta M, Latar M, N. H., Wahid S. microRNA-221 and Tamoxifen resistance in luminal-subtype breast cancer patients: A case-control study. Annals Med Surg (2012). 2021;73:103092. 10.1016/j.amsu.2021.103092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fogazzi V, Kapahnke M, Cataldo A, Plantamura I, Tagliabue E, Di Cosimo S, Cosentino G, Iorio MV. The role of MicroRNAs in HER2-Positive breast cancer: where we are and future prospective. Cancers. 2022;14(21):5326. 10.3390/cancers14215326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang K, Wang S, Cheng Y, Tian Y, Hou J. Role of miRNA-21 in the diagnosis and prediction of treatment efficacy of primary central nervous system lymphoma. Oncol Lett. 2019;17(3):3475–81. 10.3892/ol.2019.9941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Irimie-Aghiorghiesei AI, Pop-Bica C, Pintea S, Braicu C, Cojocneanu R, Zimța AA, Gulei D, Slabý O, Berindan-Neagoe I. Prognostic value of MiR-21: an updated Meta-Analysis in head and neck squamous cell carcinoma (HNSCC). J Clin Med. 2019;8(12):2041. 10.3390/jcm8122041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pop-Bica C, Pintea S, Magdo L, Cojocneanu R, Gulei D, Ferracin M, Berindan-Neagoe I. The clinical utility of miR-21 and let-7 in Non-small cell lung Cancer (NSCLC). A systematic review and Meta-Analysis. Front Oncol. 2020;10:516850. 10.3389/fonc.2020.516850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Javanmard SH, Vaseghi G, Ghasemi A, Rafiee L, Ferns GA, Esfahani HN, Nedaeinia R. Therapeutic Inhibition of microRNA-21 (miR-21) using locked-nucleic acid (LNA)-anti-miR and its effects on the biological behaviors of melanoma cancer cells in pre-clinical studies. Cancer Cell Int. 2020;20:384. 10.1186/s12935-020-01394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gumireddy K, Young DD, Xiong X, Hogenesch JB, Huang Q, Deiters A. Small-molecule inhibitors of Microrna miR-21 function. Angew Chem Int Ed Engl. 2008;47(39):7482–4. 10.1002/anie.200801555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jie J, Liu D, Wang Y, Wu Q, Wu T, Fang R. Generation of MiRNA sponge constructs targeting multiple MiRNAs. J Clin Lab Anal. 2022;36(7):e24527. 10.1002/jcla.24527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ding T, Cui P, Zhou Y, Chen C, Zhao J, Wang H, Guo M, He Z, Xu L. Antisense oligonucleotides against miR-21 inhibit the growth and metastasis of colorectal carcinoma via the DUSP8 pathway. Mol Therapy Nucleic Acids. 2018;13:244–55. 10.1016/j.omtn.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shortridge MD, Chaubey B, Zhang HJ, Pavelitz T, Vidadala V, Tang C, Olsen GL, Calin GA, Varani G. Drug-Like small molecules that inhibit expression of the oncogenic MicroRNA-21. ACS Chem Biol. 2023;18(2):237–50. 10.1021/acschembio.2c00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Han M, Liu M, Wang Y, Chen X, Xu J, Sun Y, Zhao L, Qu H, Fan Y, Wu C. Antagonism of miR-21 reverses epithelial-mesenchymal transition and cancer stem cell phenotype through AKT/ERK1/2 inactivation by targeting PTEN. PLoS ONE. 2012;7(6):e39520. 10.1371/journal.pone.0039520. Epub 2012 Jun 25. PMID: 22761812; PMCID: PMC3382593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang C, Liu K, Li T, Fang J, Ding Y, Sun L, Tu T, Jiang X, Du S, Hu J, Zhu W, Chen H, Sun X. miR-21: A gene of dual regulation in breast cancer. Int J Oncol. 2016;48(1):161–72. 10.3892/ijo.2015.3232. [DOI] [PubMed] [Google Scholar]
- 75.Yan LX, Wu QN, Zhang Y, Li YY, Liao DZ, Hou JH, Fu J, Zeng MS, Yun JP, Wu QL, Zeng YX, Shao JY. Knockdown of miR-21 in human breast cancer cell lines inhibits proliferation, in vitro migration and in vivo tumor growth. Breast cancer Research: BCR. 2011;13(1):R2. 10.1186/bcr2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Amirfallah A, Knutsdottir H, Arason A, Hilmarsdottir B, Johannsson OT, Agnarsson BA, Barkardottir RB, Reynisdottir I. Hsa-miR-21-3p associates with breast cancer patient survival and targets genes in tumor suppressive pathways. PLoS ONE. 2021;16(11):e0260327. 10.1371/journal.pone.0260327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fragni M, Bonini SA, Bettinsoli P, Bodei S, Generali D, Bottini A, Spano PF, Memo M, Sigala S. The miR-21/PTEN/Akt signaling pathway is involved in the anti-tumoral effects of Zoledronic acid in human breast cancer cell lines. Naunyn Schmiedebergs Arch Pharmacol. 2016;389(5):529–38. 10.1007/s00210-016-1224-8. [DOI] [PubMed] [Google Scholar]
- 78.Kern HB, Niemeyer BF, Parrish JK, Kerr CA, Yaghi NK, Prescott JD, Gutierrez-Hartmann A, Jedlicka P. Control of MicroRNA-21 expression in colorectal cancer cells by oncogenic epidermal growth factor/ras signaling and Ets transcription factors. DNA Cell Biol. 2012;31(8):1403–11. 10.1089/dna.2011.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kunz M, Göttlich C, Walles T, Nietzer S, Dandekar G, Dandekar T. MicroRNA-21 versus microRNA-34: lung cancer promoting and inhibitory MicroRNAs analyzed in Silico and in vitro and their clinical impact. Tumour Biology: J Int Soc Oncodevelopmental Biology Med. 2017;39(7):1010428317706430. 10.1177/1010428317706430. [DOI] [PubMed] [Google Scholar]
- 80.Lu L, Xu H, Yang P, Xue J, Chen C, Sun Q, Yang Q, Lu J, Shi A, Liu Q. Involvement of HIF-1α-regulated miR-21, acting via the Akt/NF-κB pathway, in malignant transformation of HBE cells induced by cigarette smoke extract. Toxicol Lett. 2018;289:14–21. 10.1016/j.toxlet.2018.02.027. [DOI] [PubMed] [Google Scholar]
- 81.Zhou Y, Guo D, Zhang Y. Association of MicroRNA-21 with p53 at mutant sites R175H and R248Q, clinicopathological features, and prognosis of NSCLC. Mol Therapy Oncolytics. 2020;19:208–17. 10.1016/j.omto.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang Y, Guo JX, Shao ZQ. miR-21 targets and inhibits tumor suppressor gene PTEN to promote prostate cancer cell proliferation and invasion: an experimental study. Asian Pac J Trop Med. 2017;10(1):87–91. 10.1016/j.apjtm.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 83.Zheng Y, Xie J, Jiang F, Li Y, Chang G, Ma H. Inhibition of miR-21 promotes cell apoptosis in oral squamous cell carcinoma by upregulating PTEN. Oncol Rep. 2018;40(5):2798–805. 10.3892/or.2018.6663. [DOI] [PubMed] [Google Scholar]
- 84.Bhere D, Arghiani N, Lechtich ER, Yao Y, Alsaab S, Bei F, Matin MM, Shah K. Simultaneous downregulation of miR-21 and upregulation of miR-7 has anti-tumor efficacy. Sci Rep. 2020;10(1):1779. 10.1038/s41598-020-58072-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.He C, Dong X, Zhai B, Jiang X, Dong D, Li B, Jiang H, Xu S, Sun X. MiR-21 mediates Sorafenib resistance of hepatocellular carcinoma cells by inhibiting autophagy via the PTEN/Akt pathway. Oncotarget. 2015;6(30):28867–81. 10.18632/oncotarget.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bao L, Yan Y, Xu C, Ji W, Shen S, Xu G, Zeng Y, Sun B, Qian H, Chen L, Wu M, Su C, Chen J. MicroRNA-21 suppresses PTEN and hSulf-1 expression and promotes hepatocellular carcinoma progression through AKT/ERK pathways. Cancer Lett. 2013;337(2):226–36. 10.1016/j.canlet.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 87.Kalajahi HG, Yari A, Amini M, Catal T, Ahmadpour Youshanlui M, Pourbagherian O, Zhmurov CS, Mokhtarzadeh A. Therapeutic effect of microRNA-21 on differentially expressed hub genes in gastric cancer based on systems biology. Sci Rep. 2023;13(1):21906. 10.1038/s41598-023-49225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Papagiannakopoulos T, Shapiro A, Kosik KS. MicroRNA-21 targets a network of key tumor-suppressive pathways in glioblastoma cells. Cancer Res. 2008;68(19):8164–72. 10.1158/0008-5472.CAN-08-1305. [DOI] [PubMed] [Google Scholar]
- 89.Yu SN, Ma YH, Zhao WG, Jin XL, Yang HY, Liu PP, Chen J. KRAS-related non-coding RNAs in pancreatic ductal adenocarcinoma. Chronic Dis Translational Med. 2016;2(4):215–22. 10.1016/j.cdtm.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.