Abstract
Aims: To determine the localisation and distribution of connective tissue growth factor (CCN2; CTGF) and transforming growth factor β type 1 (TGF-β1) in uterine tissues from cycling and early pregnant pigs.
Methods: In situ hybridisation and immunohistochemistry were used to localise CCN2 (CTGF) or TGF-β1 in uteri obtained from gilts on days 0, 5, 10, 12, 15, and 18 of the oestrous cycle or days 10, 12, 14, 16, 17, and 21 of gestation.
Results: In cycling animals, CCN2 (CTGF) mRNA and protein were abundant in luminal epithelial cells (LECs) and glandular epithelial cells (GECs), with lesser amounts in stromal fibroblasts and little or none in endothelial cells. A similar pattern of staining was seen up to day 10 of pregnancy, except that overall staining intensities for CCN2 (CTGF) mRNA or protein were higher and that stromal and endothelial cells were CCN2 (CTGF) positive. However, on days 12–17 there was a striking decrease in the amount of CCN2 (CTGF) in LECs at the utero–conceptus interface, which was associated with maternal stromal matrix reorganisation and the onset of subepithelial neovascularisation. This differential distribution of CCN2 (CTGF) was localised to those LECs that were in close proximity to or in apposition with trophoblast cells. This decrease in CCN2 (CTGF) staining was transient in nature and high amounts of CCN2 (CTGF) were again apparent in LECs on days 17–21, when endometrial neovascularisation and matrix remodelling were complete. The expression of uterine TGF-β1 was comparable to that of CCN2 (CTGF) at most stages of the oestrous cycle or early pregnancy. Pre-elongation blastocysts recovered on day 10 were positive for both CCN2 (CTGF) and TGF-β1 in the extra-embryonic trophectoderm, endoderm, and inner cell mass. On day 12, trophectoderm expressed low amounts of TGF-β1 mRNA and non-detectable amounts of TGF-β1 protein or CCN2 (CTGF) mRNA or protein. By days 17–21, the expression of both growth factors in the extra-embyronic/placental membranes increased and frequently exceeded that seen in LECs.
Conclusions: The pattern of CCN2 (CTGF) production during the initial attachment phase supports a role for this factor in stromal remodelling and neovascularisation, although alternative functions at later stages such as epithelial–epithelial interactions are also possible. In most major cell types in the uterus or utero–placental unit, CCN2 (CTGF) expression was highly correlated with that of TGF-β1, indicating that CCN2 (CTGF) may mediate some of the functions of TGF-β in the reproductive tract during the oestrous cycle and pregnancy. The data further highlight epithelium as an important source of CCN2 (CTGF) in the regulation of uterine function.
Keywords: placenta, angiogenesis, extracellular matrix, epithelium, endothelium, CCN2 (CTGF)
Connective tissue growth factor (CCN2; CTGF) is a member of the recently described CCN family, which contains five other members.1–4 Since the initial recognition of CCN2 (CTGF) as a fibroblast mitogen about a decade ago,5 the additional biological properties of CCN2 (CTGF) have been shown to include the stimulation of cell differentiation, adhesion, chemotaxis, migration, apoptosis, transdifferentiation, and extracellular matrix (ECM) production.1–4 CCN2 (CTGF) target and producer cells include fibroblasts, epithelial cells, endothelial cells, smooth muscle cells, and neuronal cells.3
The principal CCN2 (CTGF) transcript of 2.4 kb is induced in several cell types after treatment with transforming growth factor β (TGF-β) or serum, and is superinduced in the absence of de novo protein synthesis.6–8 Although TGF-β independent pathways of CCN2 (CTGF) expression have also been described,3 TGF-β dependent CCN2 (CTGF) gene expression has attracted considerable interest because there is a unique TGF-β response element in the CCN2 (CTGF) promoter.9,10 Thus, certain actions of TGF-β during embryogenesis, differentiation, and fibrotic disease may be indirect and the result of its induction and subsequent action of CCN2 (CTGF). CCN2 (CTGF) is overexpressed in fibrotic lesions of major organs and tissues, in the stromal compartment of certain tumours it is frequently coexpressed with TGF-β, and it is profibrogenic.1,3,9 A functional link between CCN2 (CTGF) and TGF-β is supported by the findings that antisense CCN2 (CTGF) or anti-CCN2 (CTGF) IgG are able to block TGF-β mediated anchorage independent growth,11 collagen synthesis,12 and apoptosis.13
Our interest in CCN2 (CTGF) arose through independent observations of the pig female reproductive tract, in which we demonstrated novel low mass forms of CCN2 (CTGF) (102–260 residues), which were stable C-terminal isoforms that were readily detectable in uterine luminal fluid.14–16 Subsequently, we isolated a full length pig CCN2 (CTGF) cDNA from endometrial tissues, which encoded a full length 349 residue protein, demonstrated a CCN2 (CTGF) transcript of 2.4 kb in pig endometrium, showed that the pig endometrial CCN2 (CTGF) primary translational product is of Mr 38 000, and showed that uterine luminal fluid contains proteases that rapidly convert 38 kDa CCN2 (CTGF) to lower mass forms.14,15,17 CCN2 (CTGF) is also produced by the mouse and human uterus, where it is localised primarily to luminal epithelial cells (LECs), glandular epithelial cells (GECs), and decidual cells.18,19 On the day of implantation in mice, staining for CCN2 (CTGF) in LECs is strongly reduced before its expression in the decidua.18 Recent evidence had shown that mouse uterine CCN2 (CTGF) synthesis is regulated by both oestrogen and progesterone and may involve TGF-β dependent and independent mechanisms.20
“Because most other published studies have focused on CCN2 (CTGF) related pathologies, it is essential that a more thorough investigation of uterine CCN2 (CTGF) should be undertaken to establish its role in normal tissue physiology”
The broad spectrum of biological activities of CCN2 (CTGF) support its role in diverse processes within the uterine tract, such as cell proliferation, differentiation, adhesion, chemotaxis, apoptosis, and angiogenesis. Because most other published studies have focused on CCN2 (CTGF) related pathologies, such as fibrosis, malignancy, and wound healing, it is essential that a more thorough investigation of uterine CCN2 (CTGF) should be undertaken to establish its role in normal tissue physiology. A central issue in CCN2 (CTGF) biology remains its induction by TGF-β and whether CCN2 (CTGF) mediates some of the activities that have previously been ascribed to TGF-β. Therefore, we have undertaken a detailed analysis of CCN2 (CTGF) and TGF-β type 1 (TGF-β1) at the utero–placental interface during early pregnancy in pigs, a species in which there is extensive remodelling of the extra-embryonic membranes and the formation of a loose diffuse non-invasive placenta. Our results show a high correlation between TGF-β1 and CCN2 (CTGF) expression at the feto–maternal junction and suggest that CCN2 (CTGF) is involved in endometrial ECM remodelling and angiogenesis during the crucial period of embryo attachment.
METHODS
Animals
Crossbred or Large White gilts between 7.5 and 10 months old were bred on their second cycle by observing them daily for oestrous behaviour and mating them at 12 and 24 hours after the onset of oestrus. The day of oestrus was designated as day 0. Pregnant animals were hysterectomised on days 10, 12, 14, 16, 17, and 21 of gestation (n = 2 or 3/group), whereas cyclic gilts were hysterectomised on days 0, 5, 10, 12, 15, and 18 of the oestrous cycle (n = 2 or 3/group), using previously described surgical procedures.21 Uterine horns from cycling animals were opened along the antimesometrial border and the endometrium was removed from the overlying myometrium using sterile scissors. Multiple sections of endometrium (∼ 0.5 cm) from the mesometrial region of the uterine horn were fixed in fresh 4% paraformaldehyde in phosphate buffered saline (PBS; pH 7.2). Uterine horns from pregnant animals on days 12 and beyond were dissected free of myometrium, cut into 5 cm segments, fixed in Histochoice (Amresco, Solon, Ohio, USA), and opened to locate embryonic material. Between three and six such segments from each pregnant animal were selected for further processing. Uteri from pregnant animals on day 10 were processed similarly, except that uterine horns were initially flushed with 10 ml PBS to recover blastocysts, which were also fixed using Histochoice. Specimens were placed in cassettes, processed through graded alcohol, cleared, and embedded in parablast. Blocks were cut at 4 μm and sections were mounted on to slides and dried using a hot plate. Slides were heat activated for one hour before dewaxing with two changes of xylene, followed by hydration through decreasing alcohol grades.
Immunohistochemistry for CCN2 (CTGF) and TGF-β1
A CCN2 (CTGF) peptide antiserum, made in rabbits against residues 81–94 of the pig CCN2 (CTGF) protein, was affinity purified and used at 10 μg/ml in PBS containing 2% bovine serum albumin (BSA) as previously described.22 For immunohistochemical detection of TGF-β1, sections were incubated with PBS/2% BSA containing 2 μg/ml anti-TGF-β1 IgG (Santa Cruz Biotechnology, Santa Cruz, Texas, USA), which has previously been used and validated for the detection of porcine TGF-β1, including its detection in uterine and embryonic tissues.23,24 Negative controls were equivalent concentrations of non-immune rabbit IgG. Details of the immunohistochemical procedures have been reported previously.18,20,22,25
In situ hybridisation for CCN2 (CTGF) or TGF-β1
The polymerase chain reaction of pig endometrial cDNA was used to generate a 339 bp porcine CCN2 (CTGF) DNA, corresponding to nucleotides 726 to 1065 of the full length 1047 bp pig CCN2 (CTGF) cDNA,14,17 using the primers 5′-gccgggatccatggaagagaacattaagaaggg-3' and 5'-ccagcggccgcgaatttaggccatgtctcc-3′, and a 884 bp porcine TGF-β1 DNA corresponding to nucleotides −278 to 606 of TGF-β1 mRNA using the primers 5′-tctcagacctgcctcagcttcc-3′ and 5′-tccggtgacatcaaaggacagcc-3′. The amplified products from each reaction were cloned into pCRII (Invitrogen Corp, Carlsbad, California, USA) and the resulting pCRII–pCCN2 (CTGF) or pCRII–pTGF-β1 plasmids cut with diagnostic restriction enzymes to verify insert directionality. Digoxygenin–UTP labelled RNA sense and antisense probes were made using a digoxygenin RNA labelling kit (Roche Molecular Diagnostics, Indianapolis, Indiana, USA), according to the manufacturer's instructions. pCRII–pCCN2 (CTGF) and pCRII–pTGF-β1 were linearised with EcoRV for SP6 generation of the sense probe and with Kpn I for T7 generation of the antisense probe. The prehybridisation, hybridisation, and post hybridisation steps were performed as described previously.20
RESULTS
CCN2 (CTGF) and TGF-β1 mRNA transcripts in pig uterine tissues were detected by in situ hybridisation using species specific probes, whereas their respective proteins were detected using affinity purified antibodies previously shown to react specifically with the respective ligand. Specificity of the signal obtained in each technique was verified through the use of sense probes and non-immune sera, none of which produced detectable staining (data not shown).
CCN2 (CTGF) and TGF-β1 production during the oestrous cycle
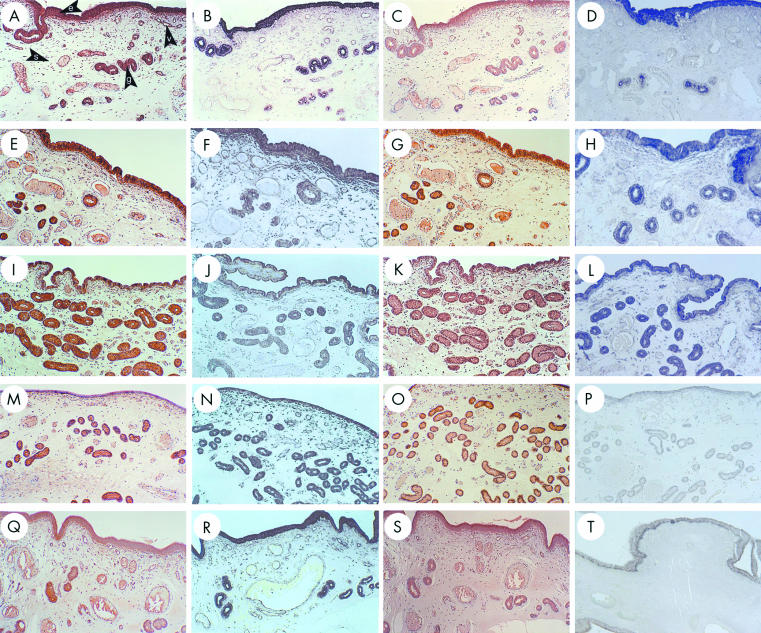
CCN2 (CTGF) mRNA and protein were abundant in LECs on days 0 and 5 of the oestrous cycle (fig 1A,B,E,F ▶). By day 10, amounts of CCN2 (CTGF) mRNA and protein appeared to be reduced in LECs, consistent with their phenotypic change from tall columnar to cuboidal cells, although the staining in GECs remained intense at this time point (fig 1I,J ▶). Over these time points, stromal cells and endothelial cells produced only low amounts of or no CCN2 (CTGF). The flattening of the luminal epithelium was most apparent on days 12 (data not shown) and 15 (fig 1M,N ▶) and, although LECs were still positive for CCN2 (CTGF), the staining intensity for both the protein and the mRNA was lower than that seen in GECs. At these later time points, CCN2 (CTGF) mRNA began to appear in stromal fibroblasts and to a lesser extent in the endothelial cells. CCN2 (CTGF) production was similarly distributed on day 18 when LEC height was restored (fig 1Q,R ▶).
Figure 1.
Connective tissue growth factor (CCN2; CTGF) and transforming growth factor β type 1 (TGF-β1) localisation in the pig uterine tract during the oestrous cycle. Uteri from cycling gilts on days 0 (A–D), 5 (E,F), 10 (I–L), 15 (M–P), and 18 (Q–T) were analysed for CCN2 (CTGF) protein (A,E,I,M,Q), CCN2 (CTGF) mRNA (B,F,J,N,R), TGF-β1 protein (C,G,K,O,S), or TGF-β1 mRNA (D,H,L,P,T). e, luminal epithelium; g, glandular epithelium; s, stroma; v, blood vessel.
The general distribution of uterine TGF-β1 during the oestrous cycle was comparable to that of CCN2 (CTGF), especially in terms of its relative abundance in LECs and GECs as compared with other cell types (fig 1C,D,G,H,K,L,O,P,S,T ▶). TGF-β1 mRNA was reduced in LECs and GECs beginning on day 12 (data not shown), a phenomenon that was most apparent on day 15 (fig 1P ▶). On Day 18, there was an increased synthesis of TGF-β1 mRNA in the basal region of LECs (fig 1T ▶). TGF-β1 protein was present in high amounts in LECs and GECs on days 5–15 (fig 1G,K,O ▶) and in lower amounts on days 0 and 18 (fig 1C,S ▶). Stromal cells and endothelial cells exhibited very weak or non-detectable staining for both TGF-β1 mRNA and protein.
CCN2 (CTGF) and TGF-β1 production during early pregnancy
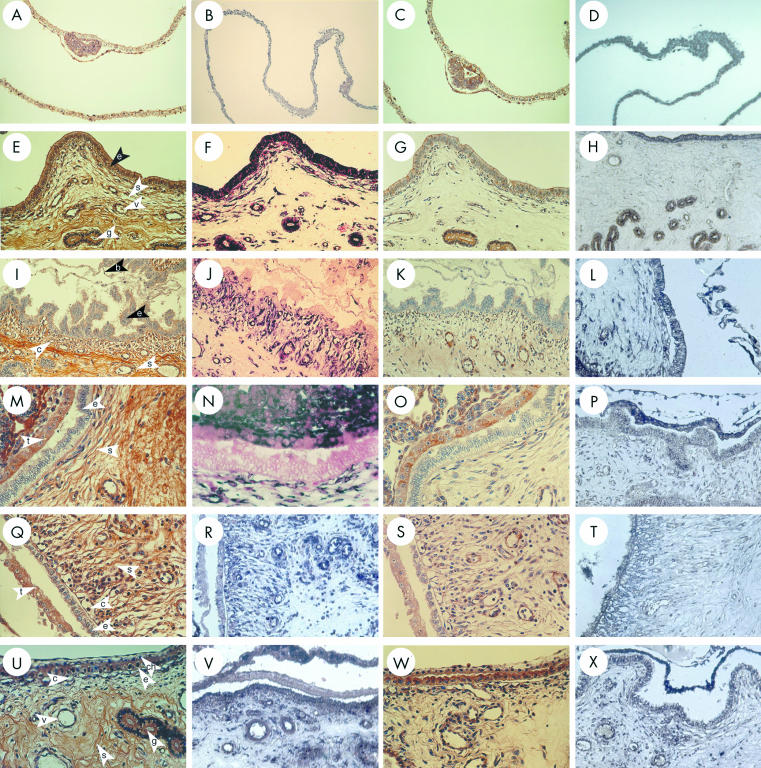
On day 10 of pregnancy, CCN2 (CTGF) and TGF-β1 mRNA and protein were detected in LECs and GECs, where they were present at higher amounts than on day 10 of the oestrous cycle (data not shown). Moreover, GECs exhibited a differential labelling, whereby proximal glands produced less CCN2 (CTGF) than distal glands. In addition, stromal elements, endothelium, and vascular smooth cells of the endometrial blood vessels and myometrium were intensely reactive (data not shown). Spherical non-elongated blastocysts recovered from uterine flushings on day 10 were strongly positive for both CCN2 (CTGF) and TGF-β1, which were colocalised to the extra-embryonic trophectoderm, endoderm, and inner cell mass (fig 2A–D ▶). On day 12 of pregnancy, regions of the uterus that were not intimately associated with the extra-embryonic membranes showed high amounts of CCN2 (CTGF) or TGF-β in LECs, GECs, stroma, and endothelium (fig 2E–H ▶). However, in contrast to these areas of intense staining, there was a dramatic and striking localised differential downregulation in LECs for both CCN2 (CTGF) and TGF-β1 when the luminal epithelial layer was in close proximity to or direct apposition with the extra-embryonic membranes. These differences were evident for the mRNA and protein for both growth factors and were characterised by a pronounced reduction—and in many cases a complete absence—of signal in the LECs (fig 2I–L ▶). On the maternal side, these regions of downregulated CCN2 (CTGF) or TGF-β1 expression were highly localised and correlated with pronounced ECM remodelling and collagen degradation in the underlying stroma, resulting in the development of a massive subepithelial blood capillary network (verified by triple staining; data not shown). On the conceptus side, trophoblast cells of the elongated blastocyst exhibited a similar downregulation of TGF-β1 mRNA, together with non-detectable amounts of TGF-β1 protein and CCN2 (CTGF) mRNA or protein.
Figure 2.
Connective tissue growth factor (CCN2; CTGF) and transforming growth factor β type 1 (TGF-β1) localisation in the pig uterine tract during early pregnancy. Pre-elongation blastocysts on day 10 (A–D) and uterine samples from pregnant gilts on days 12 (E–L), 17 (M–T), and 21 (U–X) were analysed for CCN2 (CTGF) protein (A, E,I,M,Q,U), CCN2 (CTGF) mRNA (B,F,J,N,R,V), TGF-β1 protein (C,G,K,O,S,W), or TGF-β1 mRNA (D,H,L,P,T,X). The use of the nuclear fast red counterstain confirms the presence (F) or absence (J,N) of CCN2 (CTGF) mRNA in LECs. b, blastocyst; c, capillary; ch, chorioallantois; e, luminal epithelium; g, glandular epithelium; s, stroma; t, trophectoderm; v, blood vessel.
The altered expression and localisation of CCN2 (CTGF) and TGF-β1 in the LECs was also seen on day 14 (data not shown) and day 17 (fig 2M–P ▶), by which time intimate apposition between the uterus and extra-embryonic membranes had been established. On day 17, CCN2 (CTGF) and TGF-β1 were produced at low or non-detectable amounts in LECs and at moderate to high amounts in the subepithelial stroma and endothelium. By this time, the extra-embryonic membranes stained partially or completely positive for both molecules. The stromal regions underlying the feto–maternal junction showed profound reorganisation of their extracellular and cellular elements, with new collagen architecture supporting an extensive subepithelial capillary network, the endothelial cells of which were positive for both CCN2 (CTGF) and TGF-β1 (fig 2Q–T ▶). By day 21, and with the establishment of a tight feto–maternal junction, the associated stromal reorganisation and vascularisation were largely complete. At this time, production of CCN2 (CTGF) and TGF-β1 by LECs was higher and approached the amount seen during the pre-attachment period (fig 2U–X ▶). CCN2 (CTGF) and TGF-β1 were also produced by cells of the vascular subepithelial stroma and placental membranes, the latter of which exhibited mRNA concentrations that exceeded those seen in LECs.
DISCUSSION
In the pig, maternal recognition of pregnancy (that is, the means by which the lifespan of functional copora lutea is prolonged by the presence of a conceptus) involves the production of oestrogen by the conceptus.26,27 This signal, which may exert its effect on endometrial prostaglandin release, is produced widely throughout the uterus by trophoblastic cells, following rapid elongation of pre-implantation blastocysts from small spheres (∼ 10 mm in diameter) to long filamentous thread-like structures (∼ 100 cm in length) on days 10–12.26,28 The process of elongation is accompanied by blastocyst migration and spacing and, moreover, establishes the boundaries for placentation.29 The porcine placenta is of the loose epitheliochorial type and is characterised by a simple apposition between the trophoblast and uterine epithelium, rather than the invasive implantation exhibited by blastocysts of certain other species, such as the mouse or human.29 As a result, the fetal and maternal blood systems in the placental unit of the pig remain separated throughout gestation, but are brought into close association by epithelial flattening and the establishment of a subepithelial vascular network.30 Ultrastructural studies have shown that the placental microvasculature on both the maternal and fetal side comprises a system of networks that indent the trophoblast and luminal epithelium, and thereby maximise the efficiency of maternal–fetal exchange.31–33
“We hypothesise that the normal high concentrations of CCN2 (CTGF) in luminal epithelial cells favour extracellular matrix production and stabilisation within the stroma”
The histological, biochemical, and functional changes that occur during blastocyst elongation, uterine receptivity, and placentation are driven and supported by constitutive or steroid regulated expression of molecules such as growth factors, carrier proteins, receptors, glycoproteins, adhesion molecules, and ECM proteins.34,35 Because CCN2 (CTGF) has diverse biological properties,3 predictions of its function in the uterus have been difficult to make. It is very clear from studies in the human, mouse, and pig that LECs and GECs are a principal site of CCN2 (CTGF) synthesis.3,19,25 However, we saw a clear downregulation of CCN2 (CTGF) production by LECs when trophoblastic cells were either in close proximity or undergoing apposition. Although CCN2 (CTGF) functions as a ligand for integrins and is an adhesion molecule for certain cell types in other systems,2,36–39 its downregulation by LECs at the time of blastocyst attachment argues against a primary role for LEC derived CCN2 (CTGF) in the attachment process. Because we found that the reduction in the expression of CCN2 (CTGF) by LECs was highly correlated with the processes of maternal stromal ECM reorganisation and the onset of neovascularisation, we hypothesise that the normal high concentrations of CCN2 (CTGF) in LECs favour ECM production and stabilisation within the stroma. The suppression of CCN2 (CTGF) synthesis by LECs during the attachment phase appears to favour the net stromal ECM degradation and reorganisation necessary to allow the development of a subepithelial endometrial vascular network required to support the non-invasive placenta. The pronounced re-expression of CCN2 (CTGF) by LECs after the establishment of maternal neovasculature presumably allows for net stromal ECM synthesis and stabilisation of the new vascular elements. This proposed role for CCN2 (CTGF) in regulating stromal reorganisation in the pig uterus may also occur in mice, in which implantation is preceded by a reduction in CCN2 (CTGF) production by LECs and is followed by CCN2 (CTGF) production at high concentrations by differentiating stromal cells.18 We have also reported similar localisation of CCN2 (CTGF) in LECs and decidua of the human uterus.19 Nonetheless, we should emphasise that alternative interpretations of the data are possible. For example, the suppression of epithelial CCN2 (CTGF) at the time of attachment may favour the diminution of the glycocalyx on the apical aspect of the epithelium, a process that is required for the transition to uterine receptivity.40 In addition, resynthesis of LEC derived CCN2 (CTGF) may facilitate epithelial–epithelial attachment at the apposition site, especially because integrins appear to be involved in this process,35,41 and are functional receptors for CCN2 (CTGF).2,36–38 Similarly, CTGF production by trophoblastic cells on days 17–21 may facilitate the attachment process. Whatever the precise role of CCN2 (CTGF) during the attachment/implantation phase, it probably plays a similar or conserved function among various mammalian species, irrespective of the type of placentation used.
Although CCN2 (CTGF) has been shown to be angiogenic in a variety of systems,36,42,43 the modulation of CCN2 (CTGF) production by LECs appears to support an angiogenic response indirectly through changes in ECM structure and organisation, rather than by acting as a direct stimulus for neovascularisation. However, a direct role for CCN2 (CTGF) in uterine angiogenesis may occur as a result of its increased expression by endometrial endothelium during the attachment period. Clearly, the net angiogenic response at the attachment site will reflect the coordinated actions of CCN2 (CTGF) and other molecules, such as vascular endothelial growth factor, fibroblast growth factors (FGFs), and platelet derived growth factor, which are produced in the vessel walls of porcine endometrial and placental vasculature.44–46 The expression of these factors in other cell types such as trophoblast, epithelial cells, and stromal cells has been interpreted as indicative of their involvement in other cellular processes such as attachment, differentiation, transport, or stromal reorganisation.44,47
In addition to exhibiting modifications for haemotrophic nutrition, the epitheliochorial placenta of the pig is also adapted for the uptake of nutrients from uterine secretions (histotrophe) throughout gestation. This is achieved via specialised structures called areolae, which comprise active phagocytic trophoblast cells overlying the mouths of uterine glands.48 The composition of uterine secretory fluids is also believed to be important during the “free living” phase of embryonic life, and is perhaps especially important during the period of blastocyst elongation.49 Numerous growth factors such as TGF-βs, FGFs, epidermal growth factors, and insulin-like growth factor have been identified in uterine secretory fluids during the peri-attachment phase.50–54 CCN2 (CTGF) is also a constituent of uterine secretions, where it exists as 10–20 kDa C-terminal isoforms that are stable bioactive proteolytic cleavage fragments of the full length 38 kDa CCN2 (CTGF) molecule.14–16 When compared with cycling animals, substantially increased amounts of CCN2 (CTGF) are present in uterine fluids on day 12 of pregnancy, whereas there are highly attenuated amounts around day 14–18 of pregnancy.15 Although the basis of these differences is not yet clear, the appearance of CCN2 (CTGF) in uterine fluids on day 12 may reflect its release from pre-attachment blastocysts, which appeared to lose their cell associated CCN2 (CTGF) staining between days 10 and 12. The reduced amount of CCN2 (CTGF) in uterine fluids thereafter could reflect its reduced synthesis by LECs during this period, its degradation, or its utilisation by the conceptus.
A central issue in CCN2 (CTGF) biology concerns the extent to which CCN2 (CTGF) expression is regulated by TGF-β.1,3,9 In this context, our data showed that CCN2 (CTGF) and TGF-β1 exhibit remarkable and comparable changes in their pattern of gene expression and protein localisation, especially during the formation of the utero–placental unit. Although we cannot exclude the possibility that the coexpression of TGF-β1 and CCN2 (CTGF) reflects their independent production in response to the same repertoire of stimuli, such as steroid hormones,20 these observations are clearly consistent with the premise that TGF-β1 is a principal cue for CCN2 (CTGF) production, at least in epithelial, endothelial, and stromal cells. If this is the case, then CCN2 (CTGF) is probably a mainstream mediator of many actions of TGF-β1 in the uterus. This is consistent with studies showing that CTGF mRNA is induced in cultured pig endometrial stromal cells after treatment with TGF-β (AK Wilson, unpublished data). The expression of TGF-β isoforms and their receptors at the maternal–conceptus interface of the pig has previously been reported to increase between days 10 and 14, although TGF-β proteins in uterine secretions are in their latent forms on days 10–11, but in their active forms thereafter, suggesting that components of the TGF-β system are important for epithelial–epithelial interactions during the period of blastocyst elongation.23,24,50 However, additional roles for TGF-β include immunosuppression,55 in addition to the regulation of adhesion molecules, integrins, and ECM proteins.56 A study of the TGF-β receptors in the mouse uterus during early pregnancy led to the conclusion that the TGF-β system is particularly important in the early phases of implantation.57 Functional evidence for this possibility was obtained from transgenic mice which had downregulated TGF-β receptors on days 3–5 and demonstrated a delayed implantation reaction.57 On days 1–4 of pregnancy in the mouse, TGF-β1 accumulates in the stroma after its initial synthesis by luminal and glandular epithelial cells.58 Therefore, it was proposed that epithelial derived TGF-β plays a role in stromal differentiation at the time of implantation,58 a role that we propose for CCN2 (CTGF) in both the mouse18 and the pig (these results). Thus CCN2 (CTGF) and TGF-β, together with other growth factors such as keratinocyte growth factor,59 may represent components of a molecular dialogue that contributes to the stromal–epithelial interactions that are important for the establishment of a receptive phase.35,60
Take home messages.
The pattern of CCN2 (CTGF) expression during the initial attachment phase supports a role for this factor in stromal remodelling and neovascularisation
Connective tissue growth factor (CCN2; CTGF) may also have other functions at later stages of pregnancy, such as epithelial–epithelial interactions
The expression of CCN2 (CTGF) correlated highly with that of transforming growth factor β type 1 (TGF-β1), suggesting that CCN2 (CTGF) mediates some of the functions of TGF-β in the reproductive tract during the oestrous cycle and pregnancy
Our results indicate that epithelium is an important source of CCN2 (CTGF) in the regulation of uterine function
“Our observations are clearly consistent with the premise that transforming growth factor β1 is a principal cue for CCN2 (CTGF) production, at least in epithelial, endothelial, and stromal cells”
In summary, CCN2 (CTGF) expression is highly correlated with that of TGF-β1 in endometrial epithelial cells, endothelial cells, and stromal fibroblasts during the oestrous cycle and early pregnancy, suggesting that pathways of TGF-β1 action in the uterus and utero–placental unit are CCN2 (CTGF) dependent. The pattern of CCN2 (CTGF) production during the initial attachment phase in the pig is consistent with a role for this factor in stromal remodelling and neovascularisation, although alternative functions such as epithelial–epithelial interactions at later stages are also possible. Collectively, these data demonstrate that epithelium is an important source of CCN2 (CTGF) in the mammalian uterus and support a role for epithelial CTGF in regulating stromal cell function.
Acknowledgments
We thank H Wooster for animal management and J Cooper DVM for veterinary assistance. This work was supported by USDA grant 98-35206-6430.
Abbreviations
BSA, bovine serum albumin
CCN2/CTGF, connective tissue growth factor
ECM, extracellular matrix
FGF, fibroblast growth factor
GECs, glandular epithelial cells
LECs, luminal epithelial cells
PBS, phosphate buffered saline
TGF-β1, transforming growth factor β type 1
REFERENCES
- 1.Brigstock DR. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev 1999;20:189–206. [DOI] [PubMed] [Google Scholar]
- 2.Lau LF, Lam SC. The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res 1999;248:44–57. [DOI] [PubMed] [Google Scholar]
- 3.Moussad EE, Brigstock DR. Connective tissue growth factor: what's in a name? Mol Genet Metab 2000;71:276–92. [DOI] [PubMed] [Google Scholar]
- 4.Perbal B. NOV (nephroblastoma overexpressed) and the CCN family of genes: structural and functional issues. Mol Pathol 2001;54:57–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradham DM, Igarashi A, Potter RL, et al. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol 1991;114:1285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Igarashi A, Okochi H, Bradham DM, et al. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell 1993;4:637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryseck RP, Macdonald-Bravo H, Mattei MG, et al. Structure, mapping, and expression of fisp-12, a growth factor-inducible gene encoding a secreted cysteine-rich protein. Cell Growth Differ 1991;2:225–33. [PubMed] [Google Scholar]
- 8.Brunner A, Chinn J, Neubauer M, et al. Identification of a gene family regulated by transforming growth factor-beta. DNA Cell Biol 1991;10:293–300. [DOI] [PubMed] [Google Scholar]
- 9.Grotendorst GR. Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev 1997;8:171–9. [DOI] [PubMed] [Google Scholar]
- 10.Grotendorst GR, Okochi H, Hayashi N. A novel transforming growth factor beta response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ 1996;7:469–80. [PubMed] [Google Scholar]
- 11.Kothapalli D, Frazier KS, Welply A, et al. Transforming growth factor beta induces anchorage-independent growth of NRK fibroblasts via a connective tissue growth factor-dependent signaling pathway. Cell Growth Differ 1997;8:61–8. [PubMed] [Google Scholar]
- 12.Duncan MR, Frazier KS, Abramson S, et al. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: down-regulation by cAMP. FASEB J 1999;13:1774–86. [PubMed] [Google Scholar]
- 13.Hishikawa K, Nakaki T, Fujii T. Transforming growth factor-beta(1) induces apoptosis via connective tissue growth factor in human aortic smooth muscle cells. Eur J Pharmacol 1999;385:287–90. [DOI] [PubMed] [Google Scholar]
- 14.Brigstock DR, Steffen CL, Kim GY, et al. Purification and characterization of novel heparin-binding growth factors in uterine secretory fluids. Identification as heparin-regulated Mr 10,000 forms of connective tissue growth factor. J Biol Chem 1997;272:20275–82. [DOI] [PubMed] [Google Scholar]
- 15.Ball DK, Surveyor GA, Diehl JR, et al. Characterization of 16- to 20-kilodalton (kDa) connective tissue growth factors (CTGFs) and demonstration of proteolytic activity for 38-kDa CTGF in pig uterine luminal flushings. Biol Reprod 1998;59:828–35. [DOI] [PubMed] [Google Scholar]
- 16.Brigstock DR. Purification and characterization of connective tissue growth factor (CTGF) using heparin-affinity chromatography. In: Aboul-Enein HY, ed. Analytical and preparative separation methods of biomacromolecules. New York: Marcel Dekker, 1999:397–414.
- 17.Harding PA, Surveyor GA, Brigstock DR. Characterization of pig connective tissue growth factor (CTGF) cDNA, mRNA and protein from uterine tissue. DNA Seq 1998;8:385–90. [DOI] [PubMed] [Google Scholar]
- 18.Surveyor GA, Wilson AK, Brigstock DR. Localization of connective tissue growth factor during the period of embryo implantation in the mouse. Biol Reprod 1998;59:1207–13. [DOI] [PubMed] [Google Scholar]
- 19.Uzumcu M, Homsi MF, Ball DK, et al. Localization of connective tissue growth factor in human uterine tissues. Mol Hum Reprod 2000;6:1093–8. [DOI] [PubMed] [Google Scholar]
- 20.Rageh MAE, Moussad EE, Wilson AK, et al. Steroidal regulation of connective tissue growth factor (CCN2;CTGF) synthesis in the mouse uterus. Mol Pathol 2001;54:338–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gries LK, Geisert RD, Zavy MT, et al. Uterine secretory alterations coincident with embryonic mortality in the gilt after exogenous estrogen administration. J Anim Sci 1989;67:276–84. [DOI] [PubMed] [Google Scholar]
- 22.Steffen CL, Ball-Mirth DK, Harding PA, et al. Characterization of cell-associated and soluble forms of connective tissue growth factor (CTGF) produced by fibroblast cells in vitro. Growth Factors 1998;15:199–213. [DOI] [PubMed] [Google Scholar]
- 23.Gupta A, Bazer FW, Jaeger LA. Differential expression of beta transforming growth factors (TGF beta 1, TGF beta 2, and TGF beta 3) and their receptors (type I and type II) in peri-implantation porcine conceptuses. Biol Reprod 1996;55:796–802. [DOI] [PubMed] [Google Scholar]
- 24.Gupta A, Ing NH, Bazer FW, et al. Beta transforming growth factors (TGFs) at the porcine conceptus–maternal interface. Part I: expression of TGFbeta1, TGFbeta2, and TGFbeta3 messenger ribonucleic acids. Biol Reprod 1998;59:905–10. [DOI] [PubMed] [Google Scholar]
- 25.Surveyor GA, Brigstock DR. Immunohistochemical localization of connective tissue growth factor (CTGF) in the mouse embryo between days 7.5 and 14.5 of gestation. Growth Factors 1999;17:115–24. [DOI] [PubMed] [Google Scholar]
- 26.Geisert RD, Zavy MT, Moffatt RJ, et al. Embryonic steroids and the establishment of pregnancy in pigs. J Reprod Fertil Suppl 1990;40:293–305. [PubMed] [Google Scholar]
- 27.Geisert RD, Short EC, Morgan GL. Establishment of pregnancy in domestic farm species. In: Zavy MT, Geisert RD, eds. Embryonic mortality in domestic species. Boca Raton: CRC Press, 1994:23–52.
- 28.Stroband HW, Van der Lende T. Embryonic and uterine development during early pregnancy in pigs. J Reprod Fertil Suppl 1990;40:261–77. [PubMed] [Google Scholar]
- 29.Geisert RD, Yelich JV. Regulation of conceptus development and attachment in pigs. J Reprod Fertil Suppl 1997;52:133–49. [PubMed] [Google Scholar]
- 30.Dantzer V. Electron microscopy of the initial stages of placentation in the pig. Anat Embryol 1985;172:281–93. [DOI] [PubMed] [Google Scholar]
- 31.Leiser R, Dantzer V. Structural and functional aspects of porcine placental microvasculature. Anat Embryol 1988;177:409–19. [DOI] [PubMed] [Google Scholar]
- 32.Friess AE, Sinowatz F, Skolek-Winnisch R, et al. Structure of the epitheliochorial porcine placenta. Bibl Anat 1982;22:140–3. [PubMed] [Google Scholar]
- 33.Dantzer V, Svendsen AM, Leiser R. Correlation between morphological events during the initial stages of placentation in the pig. In: Soma H, ed. Placenta: basic research for clinical application. Basel: Krager, 1991:188–99.
- 34.Rider V, Piva M. Role of growth factors of uterine and fetal-placental origin during pregnancy. In: Bazer FW, ed. The endocrinology of pregnancy. Totowa, NJ: Humana Press, 1997:83–124.
- 35.Burghardt RC, Bowen JA, Newton GR, et al. Extracellular matrix and the implantation cascade in pigs. J Reprod Fertil Suppl 1997;52:151–64. [PubMed] [Google Scholar]
- 36.Babic AM, Chen CC, Lau LF. Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin alphavbeta3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol Cell Biol 1999;19:2958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jedsadayanmata A, Chen CC, Kireeva ML, et al. Activation-dependent adhesion of human platelets to Cyr61 and Fisp12/mouse connective tissue growth factor is mediated through integrin alpha(IIb)beta(3). J Biol Chem 1999;274:24321–7. [DOI] [PubMed] [Google Scholar]
- 38.Chen CC, Chen N, Lau LF. The angiogenic factors cyr61 and connective tissue growth factor induce adhesive signaling in primary human skin fibroblasts. J Biol Chem 2001;276:10443–52. [DOI] [PubMed] [Google Scholar]
- 39.Kireeva ML, Latinkic BV, Kolesnikova TV, et al. Cyr61 and Fisp12 are both ECM-associated signaling molecules: activities, metabolism, and localization during development. Exp Cell Res 1997;233:63–77. [DOI] [PubMed] [Google Scholar]
- 40.Denker HW. Implantation: a cell biological paradox. J Exp Zool 1993;266:541–58. [DOI] [PubMed] [Google Scholar]
- 41.Bowen JA, Bazer FW, Burghardt RC. Spatial and temporal analyses of integrin and Muc-1 expression in porcine uterine epithelium and trophectoderm in vivo. Biol Reprod 1996;55:1098–106. [DOI] [PubMed] [Google Scholar]
- 42.Shimo T, Nakanishi T, Nishida T, et al. Connective tissue growth factor induces the proliferation, migration, and tube formation of vascular endothelial cells in vitro, and angiogenesis in vivo. J Biochem (Tokyo) 1999;126:137–45. [DOI] [PubMed] [Google Scholar]
- 43.Shimo T, Nakanishi T, Kimura Y, et al. Inhibition of endogenous expression of connective tissue growth factor by its antisense oligonucleotide and antisense RNA suppresses proliferation and migration of vascular endothelial cells. J Biochem (Tokyo) 1998;124:130–40. [DOI] [PubMed] [Google Scholar]
- 44.Winther H, Ahmed A, Dantzer V. Immunohistochemical localization of vascular endothelial growth factor (VEGF) and its two specific receptors, Flt-1 and KDR, in the porcine placenta and non-pregnant uterus. Placenta 1999;20:35–43. [DOI] [PubMed] [Google Scholar]
- 45.Katsahambas S, Hearn MT. Localization of basic fibroblast growth factor mRNA (FGF-2 mRNA) in the uterus of mated and unmated gilts. J Histochem Cytochem 1996;44:1289–301. [DOI] [PubMed] [Google Scholar]
- 46.Persson E, Rodriguez-Martinez H. Immunocytochemical localization of growth factors and intermediate filaments during the establishment of the porcine placenta. Microsc Res Tech 1997;38:165–75. [DOI] [PubMed] [Google Scholar]
- 47.Gupta A, Bazer FW, Jaeger LA. Immunolocalization of acidic and basic fibroblast growth factors in porcine uterine and conceptus tissues. Biol Reprod 1997;56:1527–36. [DOI] [PubMed] [Google Scholar]
- 48.Friess AE, Sinowatz F, Skolek-Winnisch R, et al. The placenta of the pig. II. The ultrastructure of the areolae. Anat Embryol 1981;163:43–53. [DOI] [PubMed] [Google Scholar]
- 49.Roberts RM, Bazer FW. The functions of uterine secretions. J Reprod Fertil 1988;82:875–92. [DOI] [PubMed] [Google Scholar]
- 50.Gupta A, Dekaney CM, Bazer FW, et al. Beta transforming growth factors (TGFbeta) at the porcine conceptus–maternal interface. Part II: uterine TGFbeta bioactivity and expression of immunoreactive TGFbetas (TGFbeta1, TGFbeta2, and TGFbeta3) and their receptors (type I and type II). Biol Reprod 1998;59:911–17. [DOI] [PubMed] [Google Scholar]
- 51.Brigstock DR, Kim GY, Steffen CL, et al. High molecular mass forms of epidermal growth factor in pig uterine secretions. J Reprod Fertil 1996;108:313–20. [DOI] [PubMed] [Google Scholar]
- 52.Kim GY, Besner GE, Steffen CL, et al. Purification of heparin-binding epidermal growth factor-like growth factor from pig uterine luminal flushings, and its production by endometrial tissues. Biol Reprod 1995;52:561–71. [DOI] [PubMed] [Google Scholar]
- 53.Brigstock DR, Heap RB, Brown KD. Polypeptide growth factors in uterine tissues and secretions. J Reprod Fertil 1989;85:747–58. [DOI] [PubMed] [Google Scholar]
- 54.Letcher R, Simmen RC, Bazer FW, et al. Insulin-like growth factor-I expression during early conceptus development in the pig. Biol Reprod 1989;41:1143–51. [DOI] [PubMed] [Google Scholar]
- 55.Segerson EC. Immunosuppressive activity of a porcine high-molecular weight uterine macromolecule is associated with transforming growth factor-beta. J Reprod Immunol 1995;29:47–60. [DOI] [PubMed] [Google Scholar]
- 56.Roberts AB, Heine UI, Flanders KC, et al. Transforming growth factor-beta. Major role in regulation of extracellular matrix. Ann N Y Acad Sci 1990;580:225–32. [DOI] [PubMed] [Google Scholar]
- 57.Das SK, Lim H, Wang J, et al. Inappropriate expression of human transforming growth factor (TGF)-alpha in the uterus of transgenic mouse causes downregulation of TGF-beta receptors and delays the blastocyst-attachment reaction. J Mol Endocrinol 1997;18:243–57. [DOI] [PubMed] [Google Scholar]
- 58.Tamada H, McMaster MT, Flanders KC, et al. Cell type-specific expression of transforming growth factor-beta 1 in the mouse uterus during the periimplantation period. Mol Endocrinol 1990;4:965–72. [DOI] [PubMed] [Google Scholar]
- 59.Ka H, Spencer TE, Johnson GA, et al. Keratinocyte growth factor: expression by endometrial epithelia of the porcine uterus. Biol Reprod 2000;62:1772–8. [DOI] [PubMed] [Google Scholar]
- 60.Cunha GR, Chung LW, Shannon JM, et al. Hormone-induced morphogenesis and growth: role of mesenchymal–epithelial interactions. Recent Prog Horm Res 1983;39:559–98. [DOI] [PubMed] [Google Scholar]