Abstract
Background
The incidence of non-tuberculous mycobacteria (NTM) has been rising globally, posing significant challenges for diagnosis and treatment, particularly in regions with high tuberculosis (TB) incidence. This study aims to investigate the epidemiological, demographic, and clinical characteristics of non-tuberculous mycobacterial lung disease (NTM-PD) in areas with a high incidence of TB.
Method
This study was conducted at the Affiliated Hospital of Zunyi Medical University from January 2017 to December 2021. A total of 6259 culture-positive specimens were analyzed. Screening was based on acid-fast staining, colony morphology, and p-nitrobenzoic acid detection, which identified 107 suspected NTM strains. Gene sequencing confirmed 51 NTM-positive cases.
Results
The predominant species identified were Mycobacterium abscessus (33.33%) and Mycobacterium intracellulare (27.45%). Several risk factors were associated with higher susceptibility to NTM-PD suspect, including bronchiectasis, low serum albumin levels (< 3.5 g/L), and male gender. The study found that although the isolation rate of NTM remained stable over the five-year period, drug resistance rates for the dominant species were notably high.
Conclusions
The findings highlight the need for clinicians in TB-endemic areas to carefully distinguish NTM infections from TB. The stable isolation rates of NTM, coupled with the high drug resistance of key species, underscore the importance of accurate diagnosis and tailored treatment strategies to manage NTM-PD effectively.
Introduction
Non-tuberculous mycobacteria (NTM) are a group of mycobacterial species distinct from Mycobacterium tuberculosis (MTB) and Mycobacterium leprae [1]. NTM are commonly found in environmental sources such as soil, water, and dust, and can act as opportunistic pathogens in humans [2].
Globally, the prevalence of NTM infections has been increasing, posing serious problems for diagnosis and treatment [3]. This increase is the result of several factors. First, exposure rates may increase due to environmental factors like air pollution and climate change. According to studies, NTM infection rates tend to increase in some areas as temperatures and humidity rise [4, 5]. Second, there has been a rise in the number of cases reported as a result of improvements in the detection of NTM infections brought about by developments in molecular diagnostic techniques [6]. Third, immunocompromised patients, including those with HIV/AIDS, organ transplant recipients are at higher risk for NTM infections [7]. Fourth, the rising prevalence of chronic pulmonary diseases like Chronic obstructive pulmonary disease(COPD), which impair lung function and increase infection risk, creates a favorable environment for NTM colonization and infection [8, 9].The incidence of NTM infections has been rising globally, particularly in regions with high TB prevalence [10]. In Guizhou Province, China, the TB incidence rate is between 160 and 200 cases per 100,000 population, reflecting the high prevalence of TB in this area [11]. This rise poses significant diagnostic and therapeutic challenges, as NTM and TB share overlapping clinical and radiological features, making it difficult to distinguish between the two without advanced laboratory techniques [12]. Misdiagnosis is a common issue due to their similar clinical presentation [13]. Recent advances, such as metagenomics next-generation sequencing (mNGS), have improved diagnostic accuracy by identifying multiple NTM species in mixed infections, which are challenging to diagnose through conventional methods [14].
In areas with a high burden of TB, such as Guizhou Province in China, acid-fast staining is the primary diagnostic method used to detect mycobacterial infections. However, this method does not differentiate between MTB and NTM, often leading to misdiagnosis [15]. Patients infected with NTM may often present clinical symptoms similar to those of multidrug-resistant TB (MDR-TB), leading to diagnostic confusion and potentially resulting in inappropriate treatment, as NTM is generally resistant to standard TB therapies [16]. NTMs are typically resistant to standard TB therapies, necessitating different treatment regimens [17]. Additionally, the delayed identification of NTM can contribute to prolonged morbidity and increased healthcare costs [2].
Previous studies have reported varying rates of NTM isolation across different regions of China and the world, with species distribution influenced by geographical and environmental factors. In northern China, Mycobacterium intracellulare is more prevalent, while Mycobacterium abscessus is commonly reported in southern regions [18]. A study conducted between 2017 and 2022 in Southwest China also shows a significant rise in NTM cases, with M. avium-intracellulare (MAC) and M. abscessus being the most common species isolated from respiratory tract samples [14]. Similarly, research from Southern China highlights the predominance of MAC, accounting for 44.5% of pulmonary NTM cases, while rapidly growing mycobacteria like M. abscessus are more common among younger, migrant populations [19]. In Eastern China, NTM accounts for 3.37% of all Mycobacterium isolates in smear-positive TB suspects, further complicating the diagnosis due to the potential for misdiagnosis as multidrug-resistant TB [20]. However, the epidemiological data on NTM in Guizhou Province, a region with a high TB burden, remains limited. In other regions, such as southern China, the proportion of NTM in suspected TB cases can reach 10% [16], which highlights the need for more targeted studies in Guizhou. Understanding the local patterns of NTM species and their clinical characteristics is crucial for developing effective diagnostic and treatment strategies [10]. Mycobacterium intracellulare and M. avium are among the most commonly isolated species in many regions [21], but geographical differences can influence the species distribution [22].
To address this, our study aims to investigate the epidemiological, demographic, and clinical characteristics of patients suspected of having NTM-PD, defined as those with two culture-positive results, radiographic evidence, and clinically relevant signs after excluding other diagnoses, in a high TB-endemic region. By conducting a five-year prospective surveillance at the Affiliated Hospital of Zunyi Medical University, we aim to provide a comprehensive analysis of the types of NTM species isolated, their drug resistance patterns, and associated risk factors.
Methods
Source of specimens
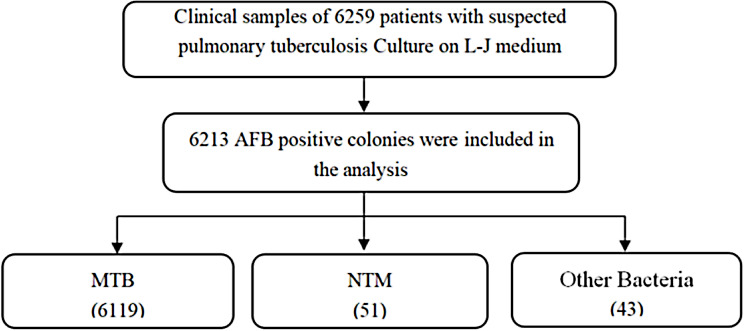
The Affiliated Hospital of Zunyi Medical University is a tertiary general hospital in Guizhou Province and one of the main medical centers in the healthcare system of Guizhou Province. The hospital was designated as the main medical institution in the regional referral system of MDR-TB in 2014, and specialized in treating MDR-TB patients diagnosed in the hospital and referred from three prefecture-level cities in northern Guizhou province (Bijie, Tongren, and Zunyi). A total of 6259 positive cultures were collected from the Affiliated Hospital of Zunyi Medical University between January 2017 and December 2021, and 6213 were positive by acid-fast staining. According to the colony morphology, growth time and p-nitrobenzoic acid test, 107 suspected NTM were screened for subsequent study. (Fig. 1)
Fig. 1.
Flowchart of NTM Strain Screening and Identification Proces
Reagent information
Acid-fast staining reagent, acid Roche medium, P-nitrobenzoic acid medium and solid drug sensitive medium were purchased from Zhuhai Bso Biotechnology Co., LTD.
NTM traditional bacterial type identification and drug sensitivity test
The laboratory of Respiratory Medicine has been certified by the Chinese Center for Disease Control and Prevention (CDC) to conduct MTB culture and drug susceptibility testing. Sputum samples from patients with clinically suspected pulmonary TB were cultured and tested for drug sensitivity by the respiratory medicine laboratory according to the protocol recommended by the World Health Organization (WHO). Suspected NTM isolates were screened from positive culture colonies of suspected TB patients for testing. The suspected NTM positive cultures were first subjected to acid-fast staining, positive colonies were subjected to PNB test, and then the positive colonies were subjected to sequencing analysis. After sequencing, the dominant NTM species were selected for drug sensitivity test to 14 anti-TB drugs.
Colony DNA extraction and sequencing
One or two bacterial colonies in logarithmic growth phase were scraped into the inner tube of a 1.5 ml EP, after which 200 µl of normal saline was added. After the screw lid was tightened, the bacterial colonies were sealed with sealant and placed in an 85℃water bath for 15 min for inactivation. The samples were packaged and sent to Qingk Biotechnology Co., LTD for sequencing (NTM specific primer sequences were: 16 S rRNA-P1: TGGAGAGTTTGATCTGG.
CTCAG, 16 S rRNA-P2: TACCGCGGCTGCTGGCAC). The sequencing data were submitted to www.ridom-rdna.de database for comparison, and the highest similarity (> 97%) were the result type. If the comparison did not correspond to any known mycobacterial species, it was likely a new mycobacterium or other kinds of bacteria, which were further analyzed on the NCBI (www.ncbi.nlm.nih.gov/BLAST).
Data analysis
Demographic and clinical factors of suspected NTM infections were tabulated, and conditional logistic regression (binary model) was used to investigate the associated factors of NTM-PD. In the model, NTM was used as the dependent variable (MTB and other bacterium = 0, NTM-PD = 1).
Results
Patient demographics and characteristics
The demographic analysis of the 51 NTM positive cases revealed key trends in terms of age, gender, and ethnicity. Among the 51 NTM-positive patients, 68.63% (35/51) were male, while 31.37% (16/51) were female. The majority (92.16%) of these NTM-PD suspect were of Han ethnicity (Table 1). Age distribution showed that patients ranged from 18 to 79 years (the mean age was 48 + 21.5 years). Middle-aged adults, particularly those aged 45–65 years, accounted for the largest proportion of cases (43.1%) (Fig. 2). This age and gender distribution aligns with global epidemiological data, which suggests that males and older individuals are more susceptible to NTM infections. These findings underscore the importance of targeting middle-aged males in future screening and prevention efforts.
Table 1.
Demographic and clinical characteristics associated with nontuberculous mycobacteria pulmonary diseases in ZunYi city
| Characteristics | NTM-PD suspect | TB + Others | OR (95% CI) |
P value | |||
|---|---|---|---|---|---|---|---|
| Gender | |||||||
| Men | 68.63% (35/51) | 62.50% (35/56) | 0.253 ~ 1.133 | 0.102 | |||
| Women | 31.37% (16/51) | 37.50% (21/56) | |||||
| Age group(years) | |||||||
| 18–40 | 16(31.37% (16/51) | 32.14% (18/56) | 0.967 ~ 1.006 | 0.180 | |||
| 41–65 | 43.14 (22/51) | 39.29% (22/56) | |||||
| 66–79 | 25.49% (13/51) | 28.57% (16/56) | |||||
| Ethnicity | |||||||
| Han | 92.16% (47/51) | 87.50% (49/56) | 0.290 ~ 2.443 | 0.752 | |||
| Other | 7.84% (4/51) | 12.50%(7/56) | |||||
| Sample | |||||||
| Sputum | 96.08% (49/51) | 91.07% (51/56) | 0.091 ~ 0.901 | 0.033 | |||
| Broncho Alveolar Lavage Fluid | 3.92% (2/51) | 3.57% (2/56) | |||||
| Pleural effusion | 0% (0/51) | 7.14% (4/56) | |||||
| History of Previous TB | |||||||
| Yes | 21.57% (11/51) | 17.86 (10/56) | 18.950 ~ 5.838 | 0.000 | |||
| No | 78.43% (40/51) | 82.14% (46/56) | |||||
| Comorbidities (yes v no) | |||||||
| Bronchiectasia | 31.37% (16/51) | 17.86% (10/56) | 0.037 ~ 2.241 | 0.623 | |||
| Diabetes | 1.96% (1/51) | 5.36% (3/56) | 0.106 ~ 6.421 | 0.235 | |||
| Hepatitis | 1.96% (1/51) | 3.57% (2/56) | 0.617 ~ 2.972 | 0.854 | |||
| COPD | 27.45% (14/51) | 26.79% (15/56) | 0.283 ~ 2.448 | 0.450 | |||
| Tumor | 7.84% (4/51) | 5.36% (3/56) | 0.035 ~ 2.172 | 0.739 | |||
| Autoimmunity disease | 1.96% (1/51) | 5.36% (3/56) | 0.110 ~ 6.136 | 0.221 | |||
| Chronic nephrosis | 1.96% (1/51) | 1.79% (1/56) | 0.037 ~ 2.241 | 0.848 | |||
| Clinical symptoms (yes v no) | |||||||
| Cough | 62.75% (32/51) | 55.36% (31/56) | 0.389 ~ 2.679 | 1.021 | |||
| Fever | 11.76% (6/51) | 17.86% (10/56) | 0.580 ~ 1.977 | 1.071 | |||
| Chest pain | 13.73% (7/51) | 8.93% (5/56) | 0.794 ~ 4.461 | 1.883 | |||
| Alb | |||||||
| < 35 | 49.02% (25/51) | 42.86% (24/56) | 0.964 ~ 0.996 | 0.017 | |||
| 35–50 | 43.14% (22/51) | 37.50% (21/56) | |||||
| > 50 | 7.84% (4/51) | 19.64% (11/56) | |||||
*p-values < 0.05 are shown in bold for comparisons between the NTM-PD suspect and control groups and Others other bacterium(NTM nontuberculosis mycobacteria); Alb albumin; OR odds ratio
Fig. 2.
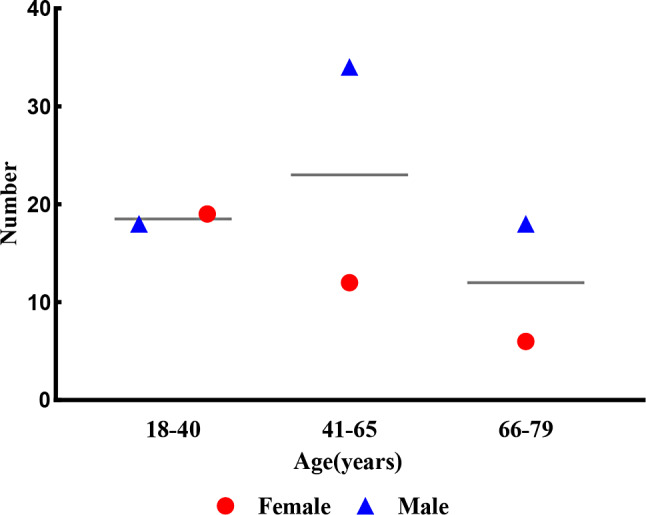
Age and gender distribution of NTM-positive patients, showing the predominance of middle-aged males
3 from 51 identified cases were treated. The decision to initiate therapy was based on clinical symptoms, radiological findings, and the patient’s overall health. Asymptomatic or stable patients with minimal disease were monitored closely without immediate treatment, while those with more severe or symptomatic infection received appropriate therapy based on species-specific guidelines.
Identification of NTM strains
The study identified a total of 107 suspected NTM colonies from 6259 culture-positive samples collected between January 2017 and December 2021. Following the initial acid-fast staining, colony morphology analysis, and p-nitrobenzoic acid (PNB) test, further confirmation using 16 S rRNA gene sequencing identified 51 NTM-positive cases. This represented 0.81% of the total cultures. Additionally, 13 cases of Mycobacterium tuberculosis (MTB) and 43 cases of other bacteria were detected (Fig. 3). These results highlight the low but stable prevalence of NTM infections in this TB-endemic region, reinforcing the need for specialized diagnostic methods to distinguish between NTM and MTB.
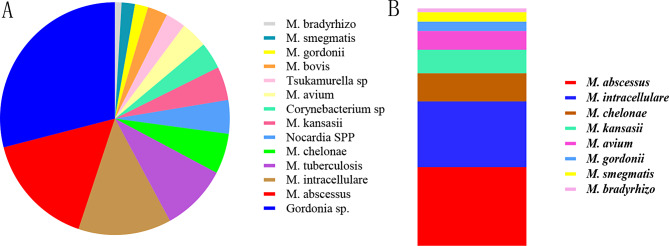
Fig. 3.
Species distribution of 107 suspected NTM cultures. A: Species distribution of 107 suspected NTM cultures. B: Species distribution of 51 NTM cultures. M.abscessus: Mycobacterium abscessus; M.intracellulare: Mycobacterium intracellulare; M.chelates: Mycobacterium chelates; M.kansasii: Mycobacterium kansasii; M.avium: Mycobacterium avium; M.Gordon: Mycobacterium Gordon; M.smegmatis: Mycobacterium smegmatis; M.bradyxanthum: Mycobacterium bradyxanthum
Species distribution of NTM
A closer look at the species distribution among the 51 NTM isolates revealed the dominance of Mycobacterium abscessus and Mycobacterium intracellulare. M. abscessus accounted for 33.33% (17/51) of the cases, followed by M. intracellulare (27.45%, 14/51), M. chelonae (11.76%, 6/51), and M. kansasii (9.80%, 5/51). Other species identified included Mycobacterium avium (7.84%), Mycobacterium gordonae (3.92%), Mycobacterium smegmatis (3.92%), and Mycobacterium bradyxanthum (1.96%) (Fig. 4). These results point to the clinical importance of M. abscessus and M. intracellulare as the leading causes of NTM-PD in this region, both of which are known for their high resistance to multiple antibiotics.
Fig. 4.
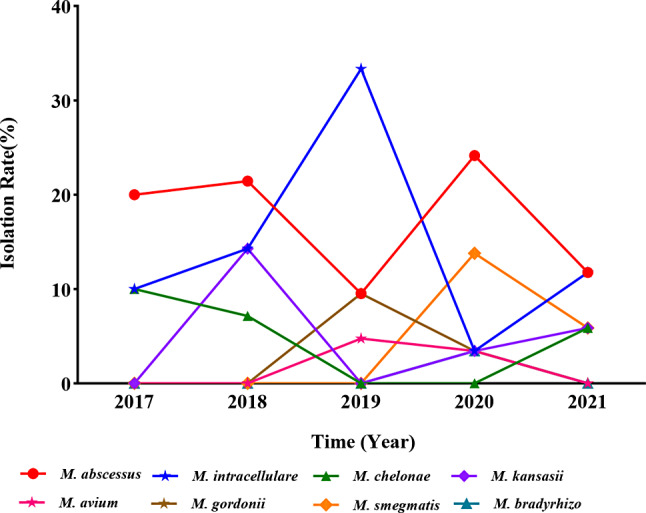
The isolation rates of NTM species fluctuated from 2017 to 2021
Trends in NTM isolation over five years
The isolation rates of NTM remained relatively stable over the five-year study period, though there were slight fluctuations. The highest isolation rate was recorded in 2019 at 1.18%, while the lowest rate occurred in 2021 at 0.77% (Fig. 5). This trend contrasts with the consistently high isolation rates of MTB, which remained above 99% throughout the study. The isolation rates of other bacteria, such as Gordonia spp. and Nocardia spp., showed more variability, peaking in 2020 at 0.82%.
Fig. 5.
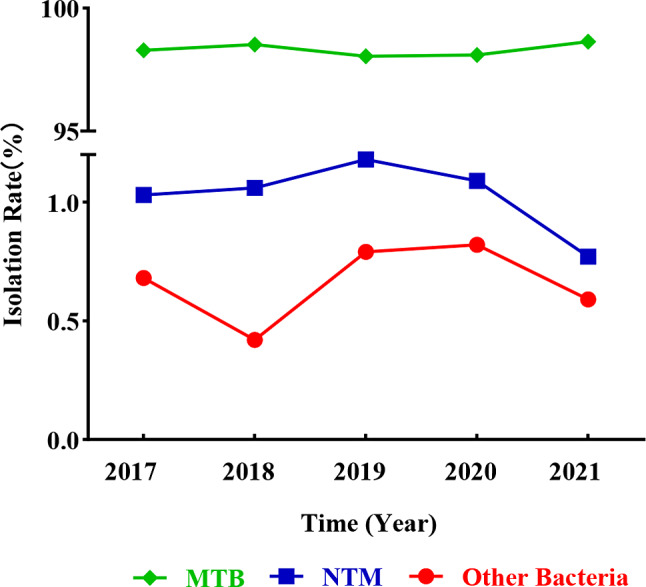
Annual trends in the isolation rates of NTM, MTB, and other bacteria. The isolation rates of NTM, MTB, and other bacteria fluctuated over five years. NTM: nontuberculous mycobacteria; MTB: Mycobacterium tuberculosis; Other Bacteria: Gordonia sp., Nocardia spp., Corynebacterium, Tsukamurella sp
Risk factors for NTM-PD
The analysis of patient risk factors revealed a strong association between NTM-PD and underlying respiratory conditions. Among the 51 NTM-positive patients, 31.37% (16/51) had bronchiectasis, 27.45% (14/51) had chronic obstructive pulmonary disease (COPD), and 7.84% (4/51) had a history of malignancy. Furthermore, 49.02% (25/51) of the patients had serum albumin levels below 35 g/L, and a significant proportion (62.75%) reported chronic cough and sputum production (Table 1). These findings are consistent with existing literature, which suggests that chronic lung conditions and malnutrition are major risk factors for NTM infections. The association between low serum albumin and poor clinical outcomes highlights the importance of addressing nutritional deficiencies in patients with NTM-PD suspect.
Discussion
This study offers the first comprehensive analysis of NTM-PD suspect in Guizhou Province, highlighting the stable prevalence of NTM infections and the growing drug resistance of dominant species like Mycobacterium abscessus and Mycobacterium intracellulare. These findings underscore the importance of species-specific drug susceptibility testing, as high resistance rates to key antibiotics, such as rifampicin and clarithromycin, make standard TB treatments ineffective for NTM infections. The study also emphasizes the need for improved diagnostic methods to differentiate NTM from TB, as misdiagnosis can lead to ineffective treatments and worsened outcomes for patients in TB-endemic areas.
The clinical characteristics of NTM-PD suspect patients in this region align with global patterns, where underlying respiratory conditions such as bronchiectasis and COPD are major risk factors. Additionally, malnutrition, particularly low serum albumin levels, was found to significantly increase the risk of disease progression and poorer outcomes. These factors suggest the importance of early diagnosis and targeted interventions for high-risk groups, especially middle-aged men with chronic respiratory diseases and malnutrition. By identifying these risk factors, clinicians can prioritize monitoring and treatment for those most vulnerable to severe NTM infections.
NTM and TB share similar clinical manifestations, including chronic cough, sputum production, chest pain, and radiological findings like lung cavities and nodules [23]. However, TB primarily affects immunocompromised or high-risk individuals, such as those with HIV or prior TB exposure, whereas NTM infections typically have a more gradual onset and are frequently found in patients with underlying chronic lung diseases, such as bronchiectasis [24]. NTM species, such as M. abscessus and M. intracellulare, need species-specific treatment plans because they are resistant to first-line TB drugs, unlike TB [25]. Although MDR-TB may need second-line medications, TB usually responds well to standard therapy and presents with systemic symptoms like weight loss and night sweats [26]. However, the treatment of NTM infections is more involved and takes longer; for example, M.abscessus frequently requires imipenem, macrolides, and aminoglycosides, while M. intracellulare is treated with rifampin and ethambutol [27].
For treatment to be effective, drug susceptibility testing is essential. Despite having a lower prevalence than MTB, NTM is still a persistent concern that is influenced by healthcare or environmental factors [28]. Our findings suggest that although the prevalence of NTM is lower than that of MTB, it remains a persistent concern in this region, with environmental or healthcare factors potentially influencing its annual fluctuations.
The drug resistance testing conducted in this study reveals significant challenges in the treatment of NTM infections. Mycobacterium abscessus, Mycobacterium intracellulare, and Mycobacterium chelonae isolates demonstrated high resistance to key antibiotics, particularly those commonly used in TB treatment regimens. Resistance was notably high to clarithromycin and rifampicin, two critical drugs in mycobacterial therapy. Additionally, resistance to first-line TB drugs such as isoniazid, streptomycin, and ethambutol further complicates treatment efforts. These findings highlight the inherent difficulties in managing NTM infections using standard TB protocols, which are often ineffective against NTM species. The results emphasize the importance of performing species-specific drug susceptibility testing to guide individualized and effective treatment strategies. Given the diverse resistance patterns among different NTM species, tailored antibiotic regimens are essential for improving patient outcomes and reducing the risk of prolonged or ineffective treatment. This study underscores the need for further research into alternative treatment options and the development of new antimicrobials specifically targeted at NTM pathogens.
Although this study did not include drug resistance data for MTB species, the growing concern of MDR-TB is well-documented in the literature, particularly in high TB burden regions. It is important to note that while we were unable to provide resistance data in this study, the increasing prevalence of MDR-TB and the impact of drug resistance on TB treatment outcomes remain significant public health concerns. Future studies with resistance data will be essential to better understand the evolving landscape of TB drug resistance and its implications for the management of co-infections with NTM.
This study highlights the urgent need for further research into new diagnostic techniques and biomarkers that could more accurately and rapidly detect NTM infections, particularly in resource-limited settings. Advanced diagnostic tools are essential to improving patient outcomes, as they would allow for timely identification and appropriate treatment of NTM infections. As the prevalence of drug-resistant NTM strains increases, there is a growing need for clinical strategies that can address these challenges and prevent the mismanagement of NTM infections in TB-endemic regions.
Acknowledgements
We thank all the members of the Laboratory for their assistance throughout this research.
Author contributions
Na-na Li led the investigation, data curation, funding acquisition, validated the findings, contributed to data visualization, drafted and edited the manuscript. Lulu Gao and Zhaojing Zong handled visualization. Mei Liu helped with data curation. Wan-min Zhang and Xiao-ke Zhang contributed to investigation. Ling Chen and Yuanbo Lan supervised the project, developed the methodology, secured funding, and reviewed the manuscript.
Funding
This work was supported by the GuiZhou Provincial Research Foundation for Basic Research, China (grant number Qiankehe jichu [2022]648, 2022]673), the Zunyi City Science and Technology Foundation (grant number Zun Shi Ke He HZ Zi [2019]77), and the Science and Technology Fund project of Guizhou Provincial Health Commission (grant number gzwkj2024-106).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval and consent to Participate
The research protocol for this study was reviewed and approved by the Medical Ethics Committee of the Affiliated Hospital of Zunyi Medical University (No: kll-2019-017). All procedures adhered to the ethical standards for conducting research involving human participants. Written informed consent was obtained from all patients, ensuring their voluntary participation and full understanding of the study’s purpose and procedures.
Consent for publication
All authors confirm that they have reviewed and approved the manuscript for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Na-na Li, Email: 252578718@qq.com.
Ling Chen, Email: lingjuncd@163.com.
Yuanbo Lan, Email: lee15085116731@sina.com.
References
- 1.Akbari Aghababa A, Nasiri MJ, Pakzad P, Mirsamadi ES. Delamanid and bedaquiline resistance patterns in Mycobacterium tuberculosis in Iran: a cross-sectional analysis. New Microbes new Infections 2024, 60–1:101437. [DOI] [PMC free article] [PubMed]
- 2.Kumar K, Ponnuswamy A, Capstick TG, Chen C, McCabe D, Hurst R, Morrison L, Moore F, Gallardo M, Keane J, et al. Non-tuberculous mycobacterial pulmonary disease (NTM-PD): epidemiology, diagnosis and multidisciplinary management. Clin Med. 2024;24:100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao Z, Hu H, Wang M, Li F, Tang H. Risk factors and Mental Health Status in patients with non-tuberculous mycobacterial lung disease: a single Center Retrospective Study. Front Public Health. 2022;10:912651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graudenz GS, Oliveira CH, Tribess A, Mendes C Jr., Latorre MR, Kalil J. Association of air-conditioning with respiratory symptoms in office workers in tropical climate. Indoor Air. 2005;15:62–6. [DOI] [PubMed] [Google Scholar]
- 5.Chen HH, Lin CH, Chao WC. Mortality association of nontuberculous mycobacterial infection requiring treatment in Taiwan: a population-based study. Ther Adv Respir Dis. 2022;16:17534666221103213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Chen H, Lin Y, Yang M, Zhao H, Hu J, Han D. Diagnosis of Non-tuberculous Mycobacterial Pulmonary Disease by Metagenomic Next-Generation sequencing on Bronchoalveolar Lavage Fluid. Infect drug Resist. 2023;16:4137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee Y, Song JW, Chae EJ, Lee HJ, Lee CW, Do KH, Seo JB, Kim MY, Lee JS, Song KS, Shim TS. CT findings of pulmonary non-tuberculous mycobacterial infection in non-AIDS immunocompromised patients: a case-controlled comparison with immunocompetent patients. Br J Radiol. 2013;86:20120209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeh JJ, Wang YC, Sung FC, Chou CY, Kao CH. Nontuberculosis mycobacterium disease is a risk factor for chronic obstructive pulmonary disease: a nationwide cohort study. Lung. 2014;192:403–11. [DOI] [PubMed] [Google Scholar]
- 9.Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: Pathogenesis and clinical significance. JPEN J Parenter Enter Nutr. 2019;43:181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore JE, Kruijshaar ME, Ormerod LP, Drobniewski F, Abubakar I. Increasing reports of non-tuberculous mycobacteria in England, Wales and Northern Ireland, 1995–2006. BMC Public Health. 2010;10:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q, Guo L, Wang J, Zhang L, Zhu W, Yuan Y, Li J. Spatial distribution of tuberculosis and its socioeconomic influencing factors in mainland China 2013–2016. Trop Med Int Health. 2019;24:1104–13. [DOI] [PubMed] [Google Scholar]
- 12.Lee WJ, Kang SM, Sung H, Won CH, Chang SE, Lee MW, Kim MN, Choi JH, Moon KC. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases. J Dermatol. 2010;37:965–72. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Varela E, Garcia-Basteiro AL, Santiago B, Wagner D, van Ingen J, Kampmann B. Non-tuberculous mycobacteria in children: muddying the waters of tuberculosis diagnosis. Lancet Respiratory Med. 2015;3:244–56. [DOI] [PubMed] [Google Scholar]
- 14.Wang DM, Liu H, Zheng YL, Xu YH, Liao Y. Epidemiology of Nontuberculous Mycobacteria in tuberculosis suspects, Southwest of China, 2017–2022. Front Cell Infect Microbiol. 2023;13:1282902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marchevsky AM, Damsker B, Green S, Tepper S. The clinicopathological spectrum of non-tuberculous mycobacterial osteoarticular infections. J bone Joint Surg Am Volume. 1985;67:925–9. [PubMed] [Google Scholar]
- 16.Piersimoni C, Scarparo C. Pulmonary infections associated with non-tuberculous mycobacteria in immunocompetent patients. Lancet Infect Dis. 2008;8:323–34. [DOI] [PubMed] [Google Scholar]
- 17.Wu UI, Holland SM. Host susceptibility to non-tuberculous mycobacterial infections. Lancet Infect Dis. 2015;15:968–80. [DOI] [PubMed] [Google Scholar]
- 18.McCallum AD, Watkin SW, Faccenda JF. Non-tuberculous mycobacterial infections in the Scottish Borders: identification, management and treatment outcomes–a retrospective review. J Royal Coll Physicians Edinb. 2011;41:294–303. [DOI] [PubMed] [Google Scholar]
- 19.Tan Y, Su B, Shu W, Cai X, Kuang S, Kuang H, Liu J, Pang Y. Epidemiology of pulmonary disease due to nontuberculous mycobacteria in Southern China, 2013–2016. BMC Pulm Med. 2018;18:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao Y, Chen C, Song H, Li G, Liu Q, Li Y, Zhu L, Martinez L, Lu W. The epidemiology and geographic distribution of nontuberculous mycobacteria clinical isolates from sputum samples in the eastern region of China. PLoS Negl Trop Dis. 2015;9:e0003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell CD, Claxton P, Doig C, Seagar AL, Rayner A, Laurenson IF. Non-tuberculous mycobacteria: a retrospective review of Scottish isolates from 2000 to 2010. Thorax. 2014;69:593–5. [DOI] [PubMed] [Google Scholar]
- 22.Yu XL, Lu L, Chen GZ, Liu ZG, Lei H, Song YZ, Zhang SL. Identification and characterization of non-tuberculous mycobacteria isolated from tuberculosis suspects in Southern-central China. PLoS ONE. 2014;9:e114353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka H, Asakura T, Okamori S, Furuuchi K, Yagi M, Nakayama Y, Kuramoto J, Yagi K, Hase I, Kamata H, et al. Distinctive clinical features of radiological pleuroparenchymal fibroelastosis with nontuberculous mycobacterial pulmonary disease: a multicenter retrospective cohort study. Int J Infect Dis. 2024;148:107233. [DOI] [PubMed] [Google Scholar]
- 24.Hsing SC, Weng SF, Cheng KC, Shieh JM, Chen CH, Chiang SR, Wang JJ. Increased risk of pulmonary tuberculosis in patients with previous non-tuberculous mycobacterial disease. Int J Tuberc Lung Dis. 2013;17:928–33. [DOI] [PubMed] [Google Scholar]
- 25.Binder AM, Adjemian J, Olivier KN, Prevots DR. Epidemiology of nontuberculous mycobacterial infections and associated chronic macrolide use among persons with cystic fibrosis. Am J Respir Crit Care Med. 2013;188:807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee M, Mok J, Kim DK, Shim TS, Koh WJ, Jeon D, Lee T, Lee SH, Kim JS, Park JS, et al. Delamanid, linezolid, levofloxacin, and pyrazinamide for the treatment of patients with fluoroquinolone-sensitive multidrug-resistant tuberculosis (treatment shortening of MDR-TB using existing and New drugs, MDR-END): study protocol for a phase II/III, multicenter, randomized, open-label clinical trial. Trials. 2019;20:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Portsmouth S, van Veenhuyzen D, Echols R, Machida M, Ferreira JCA, Ariyasu M, Tenke P, Nagata TD. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2018;18:1319–28. [DOI] [PubMed] [Google Scholar]
- 28.Sharma K, Sharma M, Ayyadurai N, Dogra M, Sharma A, Gupta V, Singh R, Gupta A. Evaluating Truenat Assay for the diagnosis of ocular tuberculosis and detection of Drug Resistance. Ocul Immunol Inflamm. 2024;32:976–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.