Abstract
Aim: The expression of human neuropsin (KLK8) mRNA in normal and pathological skin samples was analysed and the results compared with those for tissue plasminogen activator (tPA) mRNA.
Methods: Northern blot and in situ hybridisation analyses of KLK8 mRNA in normal and lesional skin of patients with cutaneous diseases were performed.
Results: A weak signal for KLK8 mRNA and no signal for tPA mRNA was seen in normal skin on northern blot analysis. Weak signals for KLK8 were localised to the superficial cells beneath the cornified layer in normal skin on in situ hybridisation. Psoriasis vulgaris, seborrheic keratosis, lichen planus, and squamous cell carcinoma skin samples, which show severe hyperkeratosis, displayed a high density of KLK8 mRNA on northern and in situ hybridisation analyses. The signals were localised in granular and spinous layers of lesional skin in all hyperkeratic samples, including the area surrounding the horn pearls of squamous cell carcinoma. To examine the relation between mRNA expression and terminal differentiation, the expression of KLK8 mRNA was analysed in cell cultures. When keratinisation proceeded in high calcium medium, a correlative increase in the expression of KLK8 mRNA was observed.
Conclusion: The results are consistent with a role for this protease in the terminal differentiation of keratinocytes.
Keywords: neuropsin, KLK8, tissue plasminogen activator
The proliferation and differentiation of keratinocytes in the stratified epithelium are well organised processes regulated to maintain the homeostasis of the internal body as a barrier against environmental change.1 In both normal and pathological skin, the cell dynamics of keratinocytes might be controlled by cell to cell and cell to matrix interactions. Several proteases including metalloproteases, plasminogen activators/plasminogen system, stratum corneum trypsin-like serine protease, and neuropsin are involved in epidermal physiology—that is, migration, remodelling, and desquamation.2–6 These proteases might regulate a variety of molecules contained in the epidermal tissue, although the details of their functions are still vague.
Tissue plasminogen activator (tPA) and neuropsin are both suprabasal epidermal proteases involved in epidermal differentiation.2,7–10 Lesional epidermis from patients with psoriasis, pemphigus, bullous pemphigoid, Hailey-Hailey disease, and tumours contains raised concentrations of tPA,11–14 which is undetectable in normal epidermis.11–13 The increase of tPA in lesional skin suggests a role in the pathogenesis of cutaneous disease.9,10 Neuropsin is a new epidermal protease named after the neural tissue where the mRNA was first identified.15 By means of in situ hybridisation and immunohistochemical analyses, we revealed that neuropsin mRNA and protein are localised in the granular and spinous layers of mouse skin.2 A human homologue of neuropsin was recently cloned by Yoshida et al.16 The same gene was also independently cloned as tumour associated differentially expressed gene 14/ovasin from ovarian carcinoma.17 Fifteen kallikrein-like (KLK) genes homologous to kallikrein (pancreatic/renal kallikrein, human glandular kallikrein 2, and prostate specific antigen) were classified according to extensive sequence homology at the gene locus 19q13.3–13.4.16,18–20 Both classic and novel kallikrein genes were grouped into the KLK family and the human neuropsin gene is now referred to as KLK8.21
“Tissue plasminogen activator and neuropsin are both suprabasal epidermal proteases involved in epidermal differentiation”
In our previous study, we induced the expression of the neuropsin (mouse) gene in suprabasal epithelial cells by the application of a chemical tumour promoter.22 This means that the expression of this gene might correlate with extracellular environmental changes induced by a variety of epidermal diseases. Thus, in the present study, we focused on the expression of the human neuropsin gene, KLK8, in normal and pathological skin in comparison with the expression of tPA mRNA because these two mRNAs have a similar distribution pattern in the suprabasal layer of mouse and human skin.12–14,22
MATERIALS AND METHODS
Tissue samples
Samples of normal and pathological human skin were obtained from operative tissue after plastic surgery or biopsy with informed consent. Skin samples of psoriasis vulgaris were taken from the centre of typical psoriatic plaque lesions of three patients (two men and one woman, aged 26 to 40 years). Skin samples of seborrheic keratosis were taken from the operative tissue of four patients (one man and three women, aged 48 to 68 years). A skin sample of lichen planus was taken from the operative tissue of one patient (man, aged 35 years). Skin samples of squamous cell carcinoma (SCC) were taken from the operative tissue of four patients (three men and one woman, aged 68 to 77 years). Skin samples of basal cell carcinoma (BCC) were taken from the operative tissue of three patients (two men and one woman, aged 74 to 79 years). A skin sample of decubitus ulcer was taken from the operative tissue of one patient (woman, aged 30 years). Control samples, consisting of normal human skin from back, palm, scalp, and groin, were obtained during surgical operations for benign skin tumours or skin grafts (six samples from three men and three women, aged 23 to 78 years). Each sample was immediately frozen in powdered dry ice after the operation and stored in a deep freezer at −80°C until use.
Human keratinocyte culture
Human keratinocytes were cultured using a modification of the technique of Hashimoto et al.23 A skin sample was obtained from a patient during plastic surgery as described above. The normal skin was cut into pieces 3–5 mm2, and floated on dispase (1000 U/ml) in Dulbecco’s modified Eagle’s medium (DMEM; Nissui, Tokyo, Japan) overnight at 4°C. After the separation of epidermis from dermis by forceps, the epidermal sheets were rinsed with Ca2+ and Mg2+ free phosphate buffered saline and incubated in a 0.25% trypsin solution for 10 minutes at 37°C, and then the epidermis was teased free with forceps. Cells were suspended and cultured in low Ca2+ medium, namely: MCDB153 medium (Kyokuto, Tokyo, Japan) supplemented with 0.1mM CaCl2, insulin (2 × 10−7M), hydrocortisone (5 × 10−7M), ethanolamine (1 × 10−4M), phosphoethanolamine (1 × 10−4M), and bovine pituitary extracts (50 μg/ml). Keratinocytes were seeded on collagen coated dishes at a density of 5 × 105 cells/ml. The medium was changed the next day and every two days. After the keratinocytes became confluent, the medium was changed to high Ca2+ medium (MCDB153 medium supplemented as above but without 1.8mM CaCl2). Total RNA was collected at the stage of 70% confluence and 100% confluence in low Ca2+ medium, and at one day and three days of culture after the change to a high Ca2+ medium.
Northern blotting
Total RNA was isolated from frozen tissues or cultured cells by a single step extraction method. Samples of 30 μg of total RNA were electrophoresed on 1% agarose/formaldehyde gels, and vacuum transferred to nylon membranes. The membranes were hybridised for 18 hours at 42°C with a 32P labelled cDNA probe. The probe was prepared by random priming using a DNA fragment corresponding to nucleotides 431–779 of KLK8 cDNA.24 The membranes were washed at high stringency and exposed to Kodak XAR films (Kodak Japan, Tokyo, Japan). The blots were stripped and rehybridised with a human tPA probe (nucleotides 1197–1548 of tPA cDNA) and glyceralaldehyde 3-phosphate dehydrogenase (GAPDH) probe.
In situ hybridisation
In situ hybridisation histochemistry was performed according to Chen and colleagues15 and Kitayoshi et al.22 Fresh frozen sections (14 μm) of normal and pathological human skin samples were cut in a cryostat, then mounted on glass slides, and stored at −80°C until use. The sections were fixed with 4% formaldehyde in 0.1M phosphate buffer (pH 7.4), treated with 10 mg/ml protease K, and acetylated with acetic anhydride in 0.1M triethanolamine. After dehydration through an ascending series of alcohol, the sections were hybridised with 35S labelled cDNA probes at 55°C overnight.15 The sections were washed at 65°C in 50% formamide, 2× standard sodium citrate, and 5mM dithiothreitol. Subsequently, they were treated with RNase A then washed again at high stringency. After dehydration through an ascending alcohol series, they were immersed in Kodak NTB2 emulsion and exposed for four weeks. After development in D-19 developer, the sections were counterstained with haematoxylin and eosin and mounted with Entellan (Merck, Darmstadt, Germany). The sections were observed and photographed under a Nikon bright field microscope (Tokyo, Japan) and a low power dark field microscope (Nikon Engineering, Tokyo, Japan).
RESULTS
Expression of KLK8 and tPA mRNA in normal skin
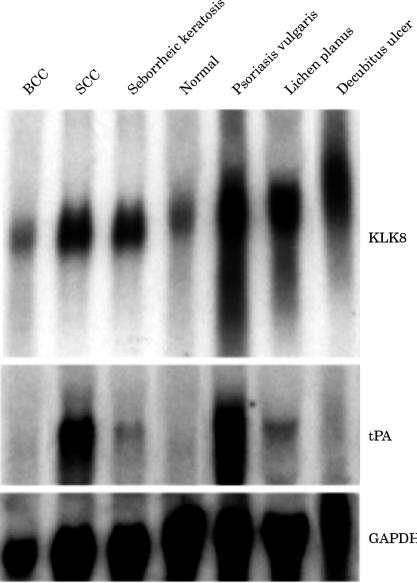
KLK8 mRNA was expressed at low density in normal skin from the scalp on northern analysis (fig 1 ▶). Skin from the groin and back showed similar band densities (data not shown). Consistent with this, in situ hybridisation revealed very faint hybridisation signals for the KLK8 probe in the superficial layer beneath the cornified cells (fig 2A ▶). In contrast, we did not detect tPA mRNA by northern analysis on rehybridisation of the stripped membrane with the tPA probe after dehybridisation of the KLK8 probe. The data agreed with the results of previous northern analyses.11–13
Figure 1.
Northern blot analysis of KLK8 mRNA in normal skin and the lesional skin of patients with cutaneous diseases. The same blot was rehybridised with the tissue plasminogen activator (tPA) probe and with a glyceralaldehyde 3-phosphate dehydrogenase (GAPDH) probe. Note the different band patterns between KLK8 and tPA mRNAs in the various skin samples. BCC, basal cell carcinoma; SCC, squamous cell carcinoma.
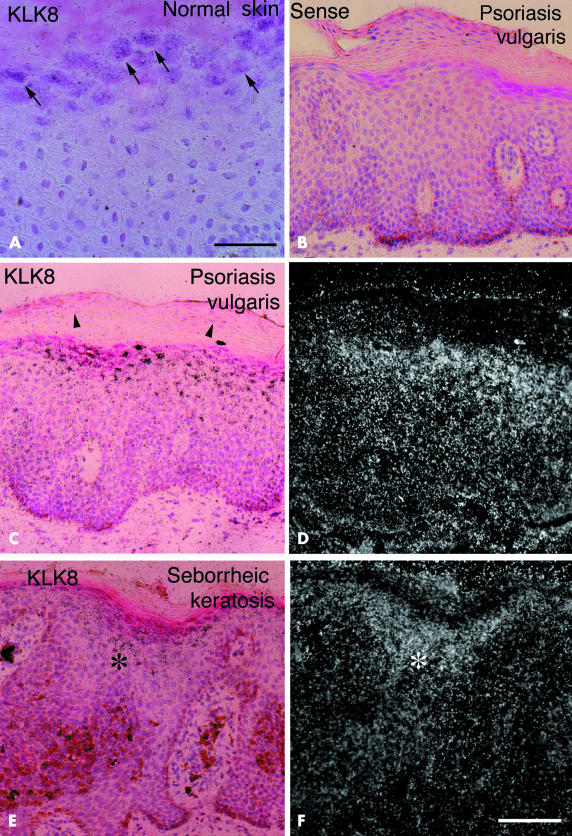
Figure 2.
In situ hybridisation for KLK8 mRNA in normal and pathological skin samples. (A) In normal skin, weak hybridisation signals were localised only in the superficial layer shown by arrows. No signals and strong hybridisation signals were found in the lesional skin samples of psoriasis vulgaris using (B) sense and (C) antisense probes for KLK8 mRNA, respectively. Parakeratotic cells localised in the cornified layer did not express KLK8 mRNA (arrowheads). (E) Strong hybridisation signals (asterisk) for KLK8 mRNA were found in lesional skin of seborrheic keratosis. (A, B, C, And E) are bright field micrographs of skin sections counterstained by haematoxylin and eosin; (D) and (F) are dark field micrographs of the same fields shown in (C) and (E), respectively. Scale bars: (A), 50 μm; (B–F), 100 μm.
Expression of KLK8 and tPA mRNA in pathological skin
An overview
No signals were detected in neighbouring control sections in psoriatic skin when the sense probe of the KLK8 gene was used (fig 2B ▶). The psoriatic tissue was used to examine the specificity of the probe, because strong signals were obtained with the antisense probe (fig 2C ▶). The result showed that the probe for KLK8 mRNA used in our study was highly specific.
We analysed several types of pathological skin to determine whether KLK8 and tPA mRNA is overexpressed or downregulated in various pathological disorders. We examined skin samples from patients with psoriasis vulgaris, seborrheic keratosis, lichen planus, decubitus ulcer, SCC, and BCC. In the northern analysis, the hybridisation band densities of KLK8 and tPA mRNA were similar among normal, psoriatic, SCC, and BCC skin (fig 1 ▶). Both genes were expressed strongly in psoriasis vulgaris and SCC. In contrast, the two mRNA species had low to no signal density in normal skin and skin from areas of BCC. However, in some pathological conditions—such as seborrheic keratosis, lichen planus, and the area surrounding decubitus ulcer—the expression patterns of the two mRNAs were very different. KLK8 mRNA was expressed extensively in seborrheic keratosis, lichen planus, and marginal skin of decubitus ulcer, whereas tPA mRNA was expressed at low signal density or not at all in these pathological samples (fig 1 ▶). Such a diverse expression pattern of both serine proteases in cutaneous diseases suggests that they are involved in separate epidermal functions.
Psoriasis vulgaris
The most intense hybridisation bands for KLK8 and tPA mRNA among all the samples were seen in the lesional skin samples of psoriasis vulgaris on northern analysis (fig 1 ▶). Our results for northern blotting of tPA mRNA were consistent with those of previous studies.9,25,26 As assessed by in situ hybridisation histochemistry, intense signals for KLK8 mRNA were localised in the granular and upper parts of spinous layers and weak to faint signals were found in the entire spinous layer (fig 2C,D ▶). The hybridisation signals were strongest in the eosinophilic cells of the granular layer beneath the cornified layer (fig 2C ▶). However, no signals were found in the horny cells and basal cells lining the basement membrane (fig 2C,D ▶). In addition, parakeratotic cells scattered in the thickened cornified layer did not contain KLK8 mRNA (arrowheads).
Seborrheic keratosis
Seborrheic keratosis is the benign epithelial tumour produced by the proliferation of keratinocytes.27 Skin samples of seborrheic keratosis expressed strong to moderate hybridisation bands on northern analysis (fig 1 ▶). In situ hybridisation histochemical analysis demonstrated that signals for KLK8 mRNA were concentrated in the basophilic cells of the granular and upper part of the spinous layer (fig 2E,F ▶). In contrast to the band for KLK8 mRNA, the band for tPA mRNA was very weak (fig 1 ▶).
Lichen planus
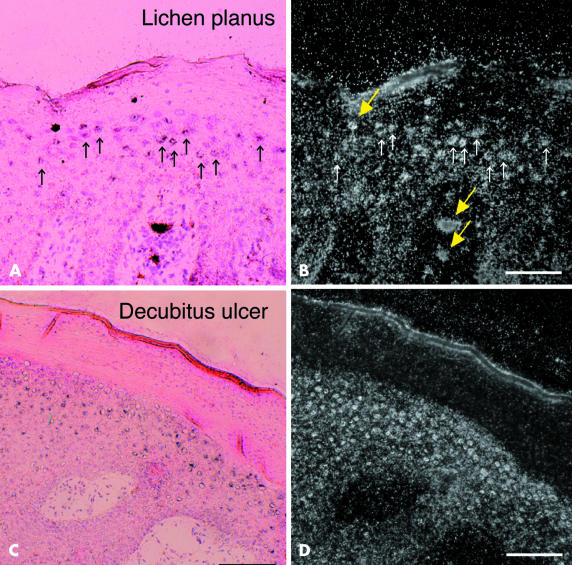
Lichen planus is characterised by orthohyperkeratosis in the suprabasal layer.27 A dense band for KLK8 mRNA was seen for lesional skin of lichen planus (fig 1 ▶). Strong hybridisation signals were seen under the bright field of the microscope (fig 3A,B ▶, small arrows). Although quite fine under bright field illumination, they could be seen better under dark field illumination (fig 3B ▶, small arrows). Cells labelled by the KLK8 probe were localised to the granular and upper part of the spinous layer (fig 3A,B ▶). In contrast, the expression of tPA mRNA was very weak for lichen planus (fig 1 ▶).
Figure 3.
In situ hybridisation for KLK8 mRNA in pathological skin. In situ hybridisation of KLK8 mRNA in the lesional skin of (A and B) lichen planus and (C and D) marginal skin around a decubitus ulcer. (A) And (C) are bright field micrographs of skin sections counterstained with haematoxylin and eosin; (B) and (D) are dark field micrographs of the same fields shown in (A) and (B), respectively. Silver grains were observed under light and dark field illumination (A–D). Small arrows in (A) and (B) represent cells labelled by the hybridisation probe. Thick arrows (yellow) represent artifacts (D). Scale bars: (A,B), 100 μm; (C,D), 200 μm.
Thickened epidermis surrounding decubitus ulcer
The marginal skin of a decubitus ulcer showed hyperkeratosis caused by chronic inflammation. The contracting thickened epidermis produced a dense band for KLK8 mRNA, which was a little weaker than that of psoriasis vulgaris. The signal was diffuse, presumably as a result of damaged mRNA from necrotic tissue. Diffuse hybridisation staining of GAPDH mRNA might support this assumption (fig 1 ▶). In situ hybridisation histochemistry for KLK8 mRNA clearly revealed numerous small keratinocytes with very fine silver grains in the suprabasal cells surrounding the decubitus ulcer (fig 3C,D ▶). Northern blot analysis for tPA mRNA did not detect a significant amount of mRNA in the tissue.
SCC
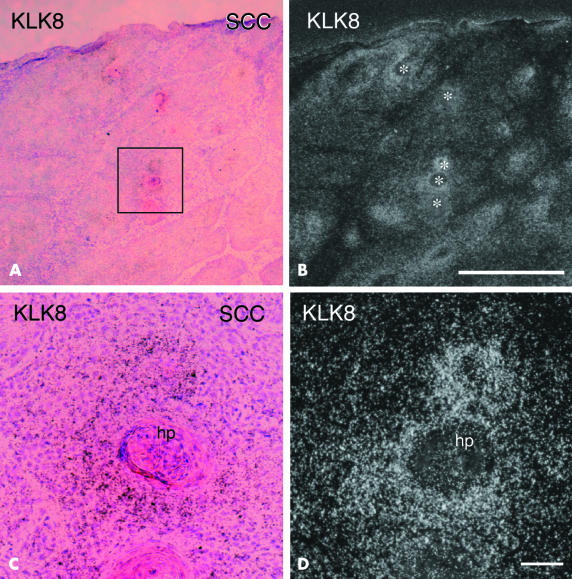
SCC presents as not only hyperkeratosis, but also an increased mitotic rate of cells.27 By northern blot analysis, pathological skin samples of SCC showed very dense staining both for KLK8 and tPA mRNA, whereas skin samples of BCC were stained only weakly for KLK8 mRNA and not at all for tPA mRNA (fig 1 ▶). In situ hybridisation analysis showed that signals for KLK8 mRNA were localised, with patch-like staining observed at low magnification, and each patch corresponded to a surrounding area of horn pearl (fig 4A,B ▶, white asterisks in B). At higher magnification, silver grains were clearly visible around horn pearls of SCC and were most condensed in the cells adjacent to the eosinophilic crystallised tissue (fig 4C,D ▶).
Figure 4.
In situ hybridisation of KLK8 mRNA in the lesional skin of squamous cell carcinoma (SCC). (A) Low power micrograph showing haematoxylin and eosin staining of highly differentiated SCC. (B) Dark field micrograph showing silver grains around horn pearls (asterisks). (C) And (D) are high power magnifications of the boxed area in (A) showing intense signals for KLK8 mRNA. (A) And (C) are bright field micrographs of skin sections counterstained by haematoxylin and eosin; (B) and (D) are dark field micrographs of the same fields shown in (A) and (C), respectively. Scale bars: (A,B), 1 mm; (C,D), 400 μm. hp, horn pearl.
Basal cell carcinoma
A weak northern band for KLK8 mRNA and no hybridisation band for tPA mRNA were seen in BCC. BCC consists of proliferating atypical basal cells and shows little (or no) sign of terminal differentiation.27 The band densities for both mRNA species were almost identical to those in normal skin (fig 1 ▶). KLK8 and tPA mRNA were both undetectable by in situ hybridisation histochemistry (data not shown).
Expression of KLK8 and tPA mRNA in cultured human keratinocytes
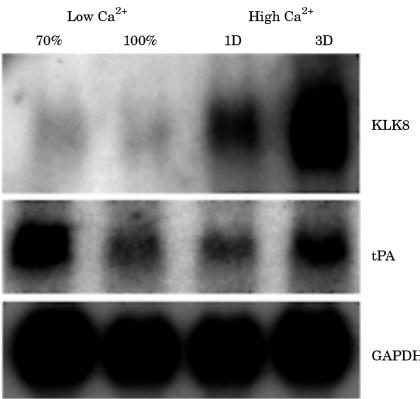
Normal human keratinocytes were cultured as described in the materials and methods section. The samples were collected at 70% and 100% confluence in low Ca2+ medium, after one day and three days of culture, respectively, in high Ca2+ medium. It is known that the extracellular Ca2+ concentration regulates the differentiation of keratinocytes; high Ca2+ medium induces the formation of large superficial squamous cells.28,29 We confirmed the differentiation of keratinocytes by morphological observation (data not shown). By means of northern blot analysis, KLK8 mRNA was weakly detected in 70% and 100% confluent cells in low Ca2+ medium, where proliferation is predominant (fig 5 ▶). After culture in the high Ca2+ medium for one or three days, KLK8 mRNA expression was greatly increased, particularly at three days (fig 1B ▶). However, the expression of tPA mRNA had a more complex pattern. The expression pattern of tPA mRNA was most extensive in 70% confluent cells after one day, and three days of culture in high Ca2+ medium. This observation suggests that KLK8 is involved in terminal differentiation.
Figure 5.
Northern blot analysis of KLK8 and tissue type plasminogen activator (tPA) mRNA in cultured human keratinocytes. Total RNA was collected at the stage of 70% confluence (70%) and 100% confluence (100%) in low Ca2+ medium (low Ca2+). Total RNA was also collected at one day (1D) and three days (3D) of culture after changing to high Ca2+ medium (high Ca2+). KLK8 mRNA expression increased greatly in parallel with the differentiation of keratinocytes. GAPDH, glyceralaldehyde 3-phosphate dehydrogenase.
DISCUSSION
When a chemical tumour promoter was topically applied to mouse skin, neuropsin/KLK8 mRNA expression in the suprabasal layers increased greatly.22 Therefore, abnormal proliferation or differentiation of keratinocytes might induce a regulatory disorder in the expression of the KLK8 gene. Expression patterns of the gene in pathological skin can provide clues to test the hypothesis that KLK8 is involved in the proliferation or differentiation of keratinocytes. In this study we therefore explored and compared the expression of KLK8 mRNA with the expression of tPA mRNA in skin from normal individuals and patients with cutaneous disorders. Expression was also examined in the cultured cell, which is considered comparable to cutaneous disorders.11 An increase in the expression of KLK8 mRNA was associated with hyperkeratinisation of epidermal tissue rather than proliferation of basal cells. There was some histopathological variation among the hyperkeratotic skin—namely: psoriasis vulgaris, seborrheic keratosis, lichen planus, and SCC. Lesional skin samples of psoriasis vulgaris and seborrheic keratosis showed hyperkeratosis associated with parakeratosis, severe acantosis, and elongation of the rete ridge. These symptoms are the result of incomplete differentiation of the keratinocytes in the superficial epidermis. In contrast, lichen planus is characterised by orthohyperkeratosis. SCC is characterised by hyperkeratosis with dyskeratosis. Lesional skin surrounding decubitus ulcer shows hyperkeratosis caused by chronic inflammation. All types of hyperkeratotic skin tested here showed pronounced increases in KLK8 mRNA regardless of parakeratosis, orthokeratosis, or dyskeratosis. Wrone-Smith and colleagues30 reported that keratinocytes derived from psoriatic plaques had abundant amounts of the cell survival protein Bcl-xL and were resistant to apoptosis. Parakeratotic keratinocytes, in which apoptosis might be slowed, did not express KLK8 mRNA. In contrast, although SCC had a higher apoptotic index,31 strong expression of KLK8 mRNA (similar to psoriasis) was observed, as was seen in our study. From these findings, it has been suggested that KLK8 does not participate in the apoptosis of keratinocytes. Alternatively, KLK8 might be involved in mechanical change involving cell to cell or cell to matrix interactions by modulating extracellular matrix components at desquamation. In fact, in our present study we found that those epidermal disorders with a pronounced increase in KLK8 expression all showed symptoms involving the desquamation process, such as squama, scale, Civatte body, and horn pearl. In contrast, lesional skin of BCC, which consists of proliferating atypical basal cells without hyperkeratosis, did not show an increase in KLK8 mRNA. This hypothesis is also supported by our previous ontogenetic observation that the expression of neuropsin/KLK8 protein was greatly increased concurrently with the construction of the cornified layer on embryonic days 15.5 to 16.5 in mouse skin.2 The in vitro differentiation model used in our present study, which showed that the expression of KLK8 mRNA was associated with terminal differentiation, supports this hypothesis.
“All types of hyperkeratotic skin tested here showed pronounced increases in KLK8 mRNA regardless of parakeratosis, orthokeratosis, or dyskeratosis”
The expression of tPA mRNA was greatly increased in cutaneous disorders, along with that of KLK8 mRNA, although the two patterns of expression differed considerably. Our northern blot data were consistent with previous investigations showing that tPA mRNA was raised in lesional skin from patients with psoriasis and SCC, although it was undetectable in normal and BCC lesional skin.11,13,14 In high Ca2+ medium, cultured keratinocytes immediately underwent terminal differentiation.32 Keratinocytes lose the ability to replicate their genes and to divide in high Ca2+ medium. Therefore, the expression of tPA mRNA is considerably reduced in cultured keratinocytes. Consequently, the expression pattern of tPA mRNA does not reflect the rate of terminal differentiation of keratinocytes. In pathological skin, the expression of tPA mRNA also appears to be independent of hyperkeratosis, unlike that of KLK8. Therefore, the function of tPA might be different to that of KLK8. Some investigators have suggested that tPA is involved in re-epithelialisation during wound repair and regeneration in pathological tissue.11 However, tPA mRNA expression was not raised in the area surrounding decubitus ulcer in our present study and this finding is incompatible with such a hypothesis.
Take home messages.
Normal skin displayed only weak signals for KLK8 mRNA and no signal for tissue plasminogen activator mRNA, whereas pathological skin samples that show severe hyperkeratosis had a high density of KLK8 mRNA
In cell culture the expression of KLK8 correlated with terminal differentiation
Thus, KLK8 may play a role in the terminal differentiation of keratinocytes and might be a suitable marker of the extent of differentiation in skin
In conclusion, the expression of neuropsin/KLK8 correlates with terminal differentiation, particularly desquamation. Therefore, the degree of expression of KLK8 mRNA in various cutaneous disorders might be a suitable marker of the extent of differentiation of skin.
Acknowledgments
This work was partly supported by Grants-in-Aid from the Ministry of Education, Science, Culture and Sports of Japan.
Abbreviations
BCC, basal cell carcinoma
GAPDH, glyceralaldehyde 3-phosphate dehydrogenase
KLK, kallikrein-like
SCC, squamous cell carcinoma
tPA, tissue plasminogen activator
REFERENCES
- 1.Zelickson AS. Ultrastructure of normal and abnormal skin. Philadelphia: Lea & Febiger, 1967.
- 2.Inoue N, Kuwae K, Ishida-Yamamoto A, et al. Expression of neuropsin in the keratinizing epithelial tissue—immunohistochemical analysis of wild-type and nude mice. J Invest Dermatol 1998;110:923–31. [DOI] [PubMed] [Google Scholar]
- 3.Jensen PJ, Lavker RM. Urokinase is a positive regulator of epidermal proliferation in vivo. J Invest Dermatol 1999;112:240–4. [DOI] [PubMed] [Google Scholar]
- 4.Weil M, Raff MC, Braga VM. Caspase activation in the terminal differentiation of human epidermal keratinocytes. Curr Biol 1999;9:361–4. [DOI] [PubMed] [Google Scholar]
- 5.Isseroff RR, Fusenig NE, Rifkin DB. Plasminogen activator in differentiating mouse keratinocytes. J Invest Dermatol 1983;80:217–22. [DOI] [PubMed] [Google Scholar]
- 6.Brattsand M, Egelrud T. Purification, molecular cloning, and expression of a human stratum corneum trypsin-like serine protease with possible function in desquamation. J Biol Chem 1999;274:30033–40. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z-L, Momota Y, Kato K, et al. Expression of neuropsin mRNA in the mouse embryo and the pregnant uterus. J Histochem Cytochem 1998;46:313–20. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu C, Yoshida S, Shibata M, et al. Characterization of recombinant and brain neuropsin, a plasticity-related serine protease. J Biol Chem 1998;273:11189–96. [DOI] [PubMed] [Google Scholar]
- 9.Grøndahl-Hansen J, Ralfkiaer E, Nielsen LS, et al. Immunohistochemical localization of urokinase- and tissue-type plasminogen activators in psoriatic skin. J Invest Dermatol 1987;88:28–32. [DOI] [PubMed] [Google Scholar]
- 10.Chen CS, Lyons-Giordano B, Lazarus GS, et al. Differential expression of plasminogen activators and their inhibitors in an organotypic skin coculture system. J Cell Sci 1993;106:45–53. [DOI] [PubMed] [Google Scholar]
- 11.Baird J, Lazarus GS, Vassalli J-D, et al. mRNA for tissue-type plasminogen activator is present in lesional epidermis from patients with psoriasis, pemphigus, or bullous pemphigoid, but is not detected in normal epidermis. J Invest Dermatol 1990;95:548–52. [DOI] [PubMed] [Google Scholar]
- 12.Sappino A-P, Belin D, Huarte J, et al. Differential protease expression by cutaneous squamous and basal cell carcinoma. J Clin Invest 1991;88:1073–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spiers EM, Lazarus GS, Lyons-Giordano B. Expression of plasminogen activator enzymes in psoriatic epidermis. J Invest Dermatol 1994;102:333–8. [DOI] [PubMed] [Google Scholar]
- 14.Spiers EM, Lazarus GS, Lyons-Giordano B. Expression of plasminogen activators in basal cell carcinoma. J Pathol 1996;178:290–6. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z-L, Yoshida S, Kato K, et al. Expression and activity-dependent changes of a novel limbic-serine protease gene in the hippocampus. J Neurosci 1995;15:5088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida S, Taniguchi M, Hirata A, et al. Sequence analysis and expression of human neuropsin cDNA and gene. Gene 1998;213:9–16. [DOI] [PubMed] [Google Scholar]
- 17.Underwood LJ, Tanimoto H, Wang Y, et al. Cloning of tumor-associated differentially expressed gene-14, a novel serine protease overexpressed by ovarian carcinoma. Cancer Res 1999;59:4435–9. [PubMed] [Google Scholar]
- 18.Diamandis EP, Yousef GM, Luo I, et al. The new human kallikrein gene family: implications in carcinogenesis. Trends Endocrinol Metab 2000;11:54–60. [DOI] [PubMed] [Google Scholar]
- 19.Gan L, Lee I, Smith R, et al. Sequencing and expression analysis of the serine protease gene cluster located in chromosome 19q13 region. Gene 2000;257:119–30. [DOI] [PubMed] [Google Scholar]
- 20.Harvey TJ, Hooper JD, Myers SA, et al. Tissue-specific expression patterns and fine mapping of the human kallikrein (KLK) locus on proximal 19q13.4. J Biol Chem 2000;275:37397–406. [DOI] [PubMed] [Google Scholar]
- 21.Diamandis EP, Yousef GM, Clements J, et al. New nomenclature for the human kallikrein gene family. Clin Chem 2000;46:1855–7. [PubMed] [Google Scholar]
- 22.Kitayoshi H, Inoue N, Kuwae K, et al. Effect of 12-O-tetradecanoyl-phorbol ester and incisional wounding on neuropsin mRNA and its protein expression in murine skin. Arch Dermatol Res 1999;291:333–8. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto K, Higashiyama S, Asada H, et al. Heparin-binding epidermal growth factor-like growth factor for human keratinocytes. J Biol Chem 1994;269:20060–6. [PubMed] [Google Scholar]
- 24.Yoshida S, Hirata A, Inoue N, et al. Assignment (1) of the neuropsin gene (Prss19) to mouse chromosome band 7B4 by in situ hybridization. Cytogenet Cell Genet 2000;88:97–8. [DOI] [PubMed] [Google Scholar]
- 25.Grøndahl-Hansen J, Lund LR, Ralfkiaer E, et al. Urokinase- and tissue-type plasminogen activators in keratinocytes during wound reepithelialization in vivo. J Invest Dermatol 1988;90:790–5. [DOI] [PubMed] [Google Scholar]
- 26.Lyons-Giordano B, Loskutoff D, Chen C-S, et al. Expression of plasminogen activator inhibitor type 2 in normal and psoriatic epidermis. Histochemistry 1994;101:105–12. [DOI] [PubMed] [Google Scholar]
- 27.Fitzpatrick TB, Johnson RA, Wolff K, et al. Color atlas and synopsis of clinical dermatology. New York: McGraw-Hill, 2001.
- 28.Hennings D, Michel C, Cheng P, et al. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell 1980;19:245–54. [DOI] [PubMed] [Google Scholar]
- 29.Nelson WG, Sun T-T. The 50- and 58-kdalton keratin classes as molecular markers for stratified squamous epithelia: cell culture studies. J Cell Biol 1983;97:244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wrone-Smith T, Mitra RS, Thompson CB, et al. Keratinocytes derived from psoriatic plaques are resistant to apoptosis compared with normal skin. Am J Pathol 1997;151:1321–9. [PMC free article] [PubMed] [Google Scholar]
- 31.Makino T, Tatebe S, Goto A, et al. Apoptosis and cellular proliferation in human epidermal squamous cell neoplasia. J Cutan Pathol 1998;25:136–42. [DOI] [PubMed] [Google Scholar]
- 32.Watt FM, Hudson DL, Lamb AG, et al. Mitogen induces calcium transients in both dividing and terminally differentiating keratinocytes. J Cell Sci 1991;99:397–405. [DOI] [PubMed] [Google Scholar]