Abstract
Aims/Background: Total phosphoglycerate mutase (PGM) activity in serum has been shown to be increased in acute myocardial infarction with the same time course as creatine kinase (CK) activity. However, the increase in the muscle (MM) and in the cardiac (MB) PGM isoenzymes was not as high as expected. The present study was undertaken to characterise PGM inactivation by serum and to compare it with serum CK inactivation.
Methods: The PGM and the CK activities of extracts of human heart, skeletal muscle, and brain were determined spectrophotometrically after incubation with different media, namely: plasma, whole serum, dialysed serum, heated serum, serum ultrafiltrate, urate solution, and buffer solution.
Results: Type MM PGM was inactivated by plasma, whole serum, heated serum, dialysed serum, and serum ultrafiltrate. Inactivation in dialysed serum was reduced by EDTA and largely reversed by thiol agents. Inactivation in serum ultrafiltrate was not prevented by EDTA and only partially reversed by dithiothreitol. The muscle and type BB CK isoenzymes were inactivated in all the tested media. The incubation of human and rabbit skeletal muscle PGM and CK in urate solution showed that urate does not affect mutase activity under conditions that inactivate CK.
Conclusions: These results confirm the mechanisms of CK inactivation proposed by others and show that the type M PGM subunit is inactivated by two different mechanisms, which appear to involve the thiol groups of the enzyme. One mechanism is caused by either a protein component or a protein bound serum component and involves calcium ions and/or another chelatable metal ion. The other mechanism is caused by a lower molecular weight serum component and is metal ion independent.
Keywords: creatine kinase, inactivation by serum, phosphoglycerate mutase
Phosphoglycerate mutase (D-phosphoglycerate 2,3-phosphomutase; EC 5.4.2.1; PGM) is a glycolytic enzyme present in mammalian cells in large amounts that catalyses the interconversion of 3-phosphoglycerate and 2-phosphoglycerate in the presence of the cofactor 2,3-bisphosphoglycerate.1 In mammals there are three PGM isoenzymes, which are derived from the homodimeric and heterodimeric combinations of two different subunits (types M and B). These isoenzymes have a similar distribution and developmental transition to creatine kinase (ATP:creatine N-phosphotransferase; EC 2.7.3.2; CK) isoenzymes. In adult mammals, skeletal muscle and mature sperm cells possess almost exclusively type MM PGM, whereas the BB isoenzyme is found in most other tissues. All three isoenzymes (MM, BB, and MB) are present in substantial amounts in myocardial tissue only.2–4
We have shown that total serum PGM activity increases in acute myocardial infarction with the same time course as CK activity. However, the increase in MM PGM and MB PGM activities in the patients was not as high as expected. Therefore, it was suggested that PGM isoenzymes, after release into the blood, are somewhat inactivated.5 PGM inactivation would also explain the findings in patients with Duchenne muscular dystrophy. It has been reported that in the plasma of these patients both CK and PGM activities increase, although the increase in PGM activity is not as striking as that seen for CK.6,7 It is known that CK is also inactivated by plasma and serum,8–24 and uric acid is one serum component that has been shown to participate in this inactivation.10,11,15 Our present study was undertaken to characterise the inactivation of PGM by serum and to ascertain whether or not urate is involved.
“We have shown that total serum phosphoglycerate mutase activity increases in acute myocardial infarction with the same time course as creatine kinase activity”
MATERIALS AND METHODS
Materials
Enzymes, substrates, cofactors, and biochemicals were purchased from either Boehringer (Mannheim, Germany) or Sigma (St Louis, Missouri, USA). β Mercaptoethanol was from Merck (Darmstadt, Germany) and bovine serum albumin was from Calbiochem (La Jolla, California, USA). Other chemicals were reagent grade. Cellulose acetate strips (TITAN III ISO-VIS 94 × 76 mm) were from Helena Laboratories (Beaumont, Texas, USA). Paper wicks (Wathman number 3) were from Wathman Ltd (Maidstone, UK).
Serum and tissue samples
Serum and plasma from healthy donors were provided by the laboratory of clinical biochemistry of the Hospital Clinic i Provincial, Barcelona, Spain. Specimens from human heart and skeletal muscle were obtained during necropsy within 24 hours after death. Brain samples were provided by the brain bank of the Hospital Clinic i Provincial. Commercial rabbit muscle PGM and CK were obtained from Boehringer.
Tissue extraction
Tissue extracts were prepared by homogenisation in 3 vol (wt/vol) of cold 20mM Tris/HCl buffer, pH 7.5, containing 1mM EDTA and 1mM β mercaptoethanol with a Polytron homogeniser (Luzern, Switzerland) (position 5, 20 seconds). Cellular debris was removed by centrifugation at 4°C for 60 minutes at 25 000 ×g and the supernatants were used for the assay of enzyme activities and isoenzymes.
Enzyme and protein assay
PGM activity was measured spectrophotometrically at 30°C, by coupling the formation of 2-phosphoglycerate from 3-phosphoglycerate with the enolase, pyruvate kinase, and lactate dehydrogenase catalysed reactions.25 The reaction mixture contained 100mM Tris/HCl, 0.5mM EDTA, 100mM KCl, 10mM MgCl2, 1.5mM ADP, 2 mM 3-phosphoglycerate, 10μM 2,3-bisphosphoglycerate, 0.2mM NADH, enolase (0.3 U/ml), pyruvate kinase (0.15 U/ml), and lactate dehydrogenase (0.5 U/ml), pH 7.4.
CK activity was measured spectrophotometrically at 30°C, essentially as recommended by the International Federation of Clinical Chemistry,26 but without N-acetylcysteine in the assay mixture. The assay is based on the formation of ATP linked to the production of NADHP via hexokinase and glucose-6-phosphate dehydrogenase. The reaction mixture contained 100mM imidazole acetate, 2mM EDTA, 10mM magnesium acetate, 2mM ADP, 5mM AMP, 20mM D-glucose, 2mM NADP, 30mM phosphocreatine, hexokinase (3 U/ml), and glucose-6-phosphate dehydrogenase (2 U/ml), pH 6.7. Protein was determined by the method of Bradford,27 using bovine serum albumin as a standard.
Isoenzyme analysis
Electrophoretic evaluation of PGM isozymes was performed as described previously.28 Cellulose acetate strips were presoaked in sodium barbital buffer (50mM, pH 8.6) and contact with the electrophoresis buffer was made using Whatman number 3 paper wicks. The samples (0.5 μl) were applied by means of a commercial applicator kit and electrophoresis was performed for 60 minutes at 4°C and 250 V.
The bands with PGM activity were stained using a mixture containing 10mM Tris/HCl, 10mM MgSO4, 20mM KCl, 15mM AMP, 2mM ADP, 0.5mM NADP+, 11mM glucose, 5mM 3-phosphoglycerate, 50μM 2,3-bisphosphoglycerate, 2.4mM 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, 0.13mM phenazine methosulfate, 8 mg/ml agar noble, 0.2 U/ml enolase, 0.8 U/ml pyruvate kinase, 0.5 U/ml hexokinase, and 1.4 U/ml glucose 6-phosphate dehydrogenase, pH 8.0. The staining mixture (12 ml, freshly prepared) was quickly placed over the cellulose acetate strip and incubated at 37°C for 30 minutes, in the dark. The reaction was stopped by washing the strip with 5% acetic acid, the solid layer of staining mixture was removed, and the strip was gently washed. The strip was photographed with a Polaroid MP4 land camera and the photograph was scanned at 500 nm with a Shimadzu CS-900 densitometer.
RESULTS
Effects of serum components on the stability of PGM and CK isoenzymes
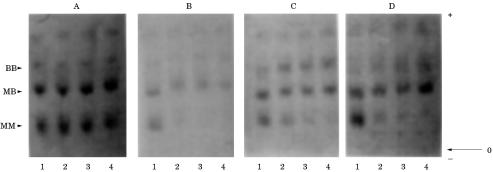
To detect the effects of human plasma and serum on the stability of the MM, MB, and BB PGM isoenzymes, extracts of human heart were incubated in whole plasma and serum, and with heated serum at 37°C and at 0°C. In all cases, incubation at 37°C produced a progressive decrease of the total PGM activity and a progressive inactivation of the MM PGM isoenzyme (fig 1 ▶; table 1 ▶). In contrast, incubation at 0°C had no effect. The addition of dithiothreitol (DTT) at the end of the various incubation periods at 37°C partially restored the lost total and MM PGM enzymatic activity (data not shown).
Figure 1.
Electrophoretogram of phosphoglycerate mutase (PGM) isoenzymes in extracts of human heart incubated in plasma and serum. (A) Plasma at 0°C; (B) plasma at 37°C: lane 1, 0 minutes; lane 2, 12 hours; lane 3, 24 hours; lane 4, 48 hours. (C) Serum at 37°C; (D) preheated serum (60°C, 20 minutes) at 37°C: lane 1, 0 minutes; lane 2, four hours; lane 3, eight hours; lane 4, 24 hours.
Table 1.
Stability of phosphoglycerate mutase (PGM) isoenzymes in human heart extract incubated in plasma and serum
| % Activity of isoenzymes | |||||
| Incubation medium | Time | Total activity | MM | MB | BB |
| Plasma at 0°C | 0 minutes | 100 | 39 | 40 | 21 |
| 12 hours | 100 | 40 | 43 | 17 | |
| 24 hours | 100 | 39 | 41 | 20 | |
| 48 hours | 100 | 35 | 54 | 11 | |
| Plasma at 37°C | 0 minutes | 100 | 50 | 36 | 14 |
| 12 hours | 57 | 20 | 63 | 17 | |
| 24 hours | 35 | 3 | 74 | 23 | |
| 48 hours | 23 | 0 | 51 | 49 | |
| Serum at 37°C | 0 minutes | 100 | 52 | 43 | 5 |
| 4 hours | 47 | 34 | 44 | 22 | |
| 24 hours | 35 | 23 | 58 | 19 | |
| 48 hours | 22 | 12 | 64 | 24 | |
| Preheated serum (60°C, 30 minutes) at 37°C | 0 minutes | 100 | 56 | 39 | 5 |
| 4 hours | – | 28 | 61 | 11 | |
| 24 hours | – | 18 | 68 | 14 | |
| 48 hours | 22 | 11 | 71 | 18 | |
The activity of the isoenzymes is expressed as a percentage of the total activity on electrophoretogram.
To compare the effects of the components of human serum on the stability of the PGM and CK isoenzymes extracts of human skeletal muscle and brain were incubated at 37°C in Tris buffer solution (pH 7.5), in whole serum, in dialysed serum, in heated serum, and in serum ultrafiltrate. Incubations were performed in the absence and in the presence of EDTA to differentiate the metal ion dependent and the metal ion independent effects. PGM and CK activities were measured at intervals, directly and after preincubation with DTT to detect any effects resulting from the oxidation of the SH groups of the enzymes.
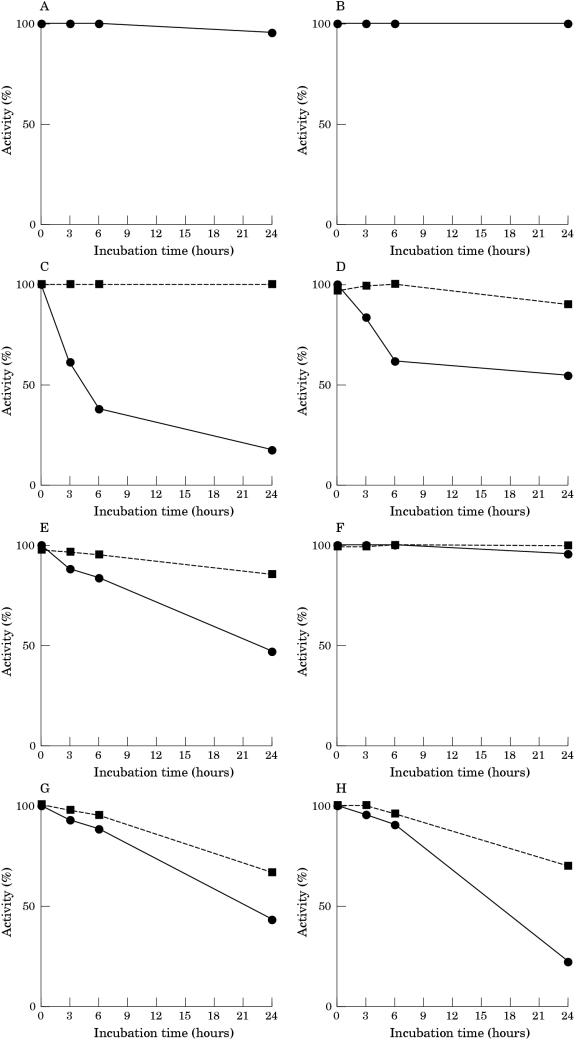
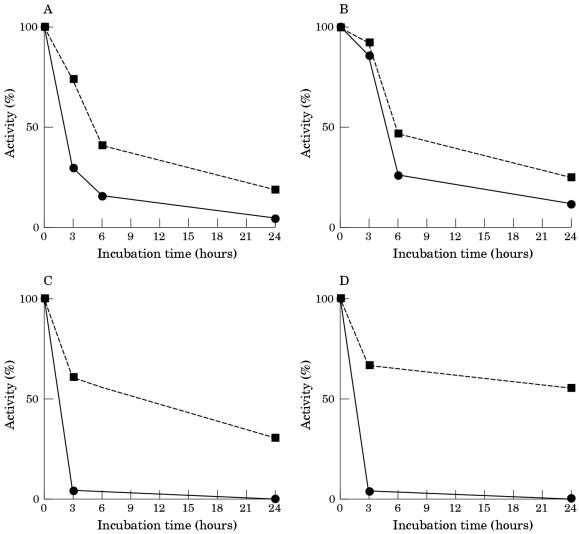
PGM activity from skeletal muscle was remarkably stable at 37°C in Tris buffer pH 7.5: after 24 hours of incubation there was a loss of less that 10% of activity (fig 2 ▶). In contrast, PGM rapidly lost its activity when incubated in human serum: the activity was reduced by 60% after six hours, and only 20% of the original activity remained after 24 hours of incubation. The addition of EDTA to the serum during the incubation reduced the decrease in PGM activity: over a 24 hour period there was a 50% loss of activity. The addition of DTT at the end of the various incubation periods greatly restored the lost enzymatic activity. No loss of PGM activity was seen when muscle extract was incubated for 24 hours in whole serum at 0°C (data not shown).
Figure 2.
Inactivation of phosphoglycerate mutase (PGM) in human muscle extracts incubated at 37°C in 20mM Tris/HCl buffer pH 7.5 (A,B), in whole serum (C,D), in dialysed serum (E,F), and in serum ultrafiltrate (G,H). Extracts were incubated in the absence (A,C,E,G) and in the presence (B,D,F,H) of 5mM EDTA. The activity of PGM was measured directly (continuous lines) and after 30 minutes of incubation at 30°C with 10mM dithiothreitol (discontinuous lines).
The incubation of muscle extract in dialysed serum in the absence of EDTA continued to inactivate PGM activity, although at a rate lower than that seen for incubation with whole serum. PGM activity could be restored to a large extent by the addition of DTT after incubation. The addition of EDTA to the dialysed serum greatly decreased the rate of inactivation: less that 10% of activity was lost in 24 hours. Heating the whole and dialysed serum at 60°C for 30 minutes did not affect its capacity to inactivate PGM (data not shown).
Incubation of muscle extract in serum ultrafiltrate inactivated PGM at a rate similar to that seen for dialysed serum. However, the addition of EDTA to the ultrafiltrate during incubation did not affect the rate of inactivation, and the activity was only partially restored by the addition of DTT after incubation.
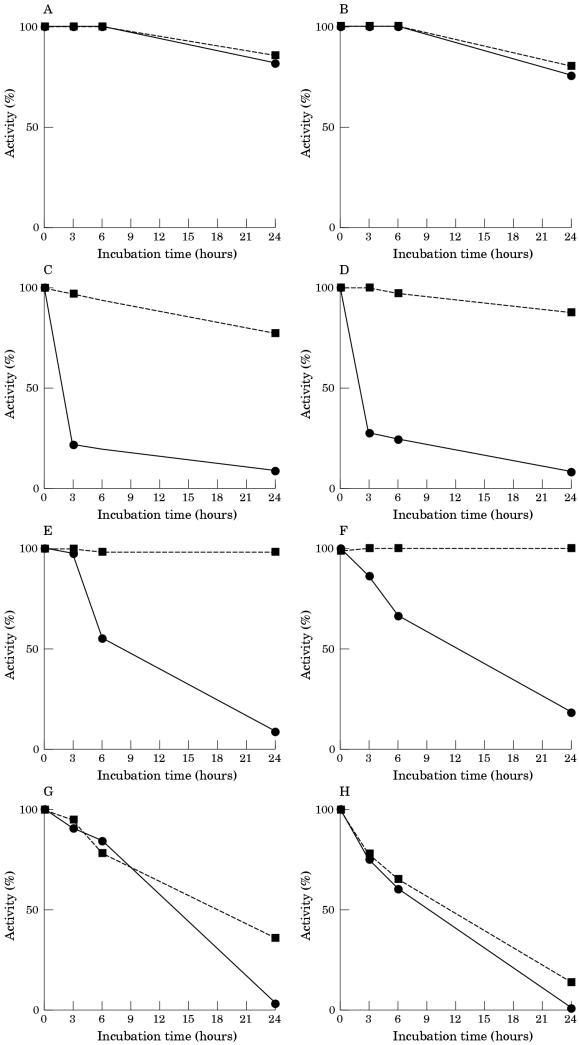
CK activity was fairly stable when muscle extract was incubated in Tris buffer, pH 7.5, at 37°C: after a 24 hour period of incubation 80% of the activity remained (fig 3 ▶). When incubated in human serum, CK was inactivated at a rate higher than PGM: after three hours of incubation the activity was reduced by 77%. CK was also inactivated when incubated in dialysed serum and in serum ultrafiltrate, although in both cases the rate of CK inactivation was lower than that produced by incubation in whole serum. The addition of EDTA to the incubation medium did not prevent the loss of CK activity in any case. The addition of DTT after incubation slightly restored the CK activity lost by incubation in serum ultrafiltrate, and fully restored the enzymatic activity lost by incubation in whole and in dialysed serum.
Figure 3.
Inactivation of creatine kinase (CK) activity of human muscle extracts incubated at 37°C in 20mM Tris/HCl buffer, pH 7.5 (A,B), in whole serum (C,D), in dialysed serum (E,F), and in serum ultrafiltrate (G,H). Extracts were incubated in the absence (A,C,E,G) and in the presence (B,D,F,H) of 5mM EDTA. The activity of CK was measured directly (continuous lines) and after 30 minutes of incubation at 30°C with 10mM dithiothreitol (discontinuous lines).
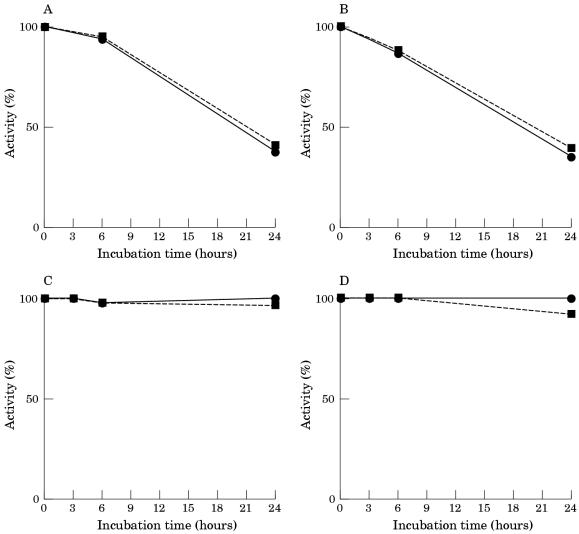
Figure 4 ▶ illustrates the rate of decay of PGM activity in brain extract during incubation in the different media. As shown, when brain extract was incubated in Tris buffer solution, pH 7.5, the PGM activity was 50% lower after 24 hours of incubation. The addition of EDTA during incubation did not prevent the loss of PGM activity, and the addition of DTT after different periods of incubation did not restore the enzymatic activity. In contrast, when the brain extract was incubated in whole serum, the PGM activity was highly stable over a 24 hour period of incubation.
Figure 4.
Inactivation of phosphoglycerate mutase (PGM) in human brain extracts incubated at 37°C in 20mM Tris HCl buffer pH 7.5 (A,B) and in whole serum (C,D). Extracts were incubated in the absence (A,C) and in the presence (B,D) of 5mM EDTA. The activity of PGM was measured directly (continuous lines) and after 30 minutes of incubation at 30°C with 10mM dithiothreitol (discontinuous lines).
CK was inactivated when brain extract was incubated in Tris buffer, pH 7.5 (fig 5 ▶). The addition of EDTA to the incubation solution did not significantly modify the rate of CK inactivation and the addition of DTT after incubation only partially restored the loss of activity. When brain extract was incubated in whole human serum, CK was almost fully inactivated after a three hour period of incubation. The addition of EDTA to the serum did not prevent the inactivation of CK. Treatment with DTT after inactivation partially restored the lost enzymatic activity. CK activity was also inactivated when the brain extract was incubated in dialysed serum and in serum ultrafiltrate. The addition of EDTA to the incubation medium did not prevent the inactivation of CK, and treatment with DTT after inactivation only partially restored the enzymatic activity (data not shown).
Figure 5.
Inactivation of creatine kinase (CK) in human brain extracts incubated at 37°C in 20mM Tris HCl buffer pH 7.5 (A,B) and in whole serum (C,D). Extracts were incubated in the absence (A,C) and in the presence (B,D) of 5mM EDTA. The activity of PGM was measured directly (continuous lines) and after 30 minutes of incubation at 30°C with 10mM dithiothreitol (discontinuous lines).
Effect of urate on the stability of PGM and CK isoenzymes
It has been shown that urate inactivates human and rabbit skeletal muscle CK, and that DTT partially restores the activity of the urate inactivated enzymes.10,11,15 Therefore, we compared the effects of urate on the activity of CK and PGM in extracts of human skeletal muscle, and on the activity of purified PGM and CK from rabbit muscle. When the extract of human skeletal muscle was incubated at 25°C with urate in Tris buffer solution, pH 7.4, after 24 hours of incubation the CK activity was reduced by 55% and the PGM activity was not affected; that is, it suffered the same slight inactivation (about 20%) in the absence and in the presence of urate (table 2 ▶). Treatment with DTT after inactivation partially restored the lost CK activity. Similar results were obtained when purified rabbit muscle CK and PGM were incubated with urate. After 24 hours of incubation, the activity of rabbit muscle CK was reduced by 60%, whereas the activity of rabbit muscle PGM was only slightly affected. Treatment with DTT after inactivation partially restored CK activity.
Table 2.
Inactivation of creatine kinase (CK) and phosphoglycerate mutase (PGM) activity by urate
| CK | PGM | |||
| No urate | Plus urate | No urate | Plus urate | |
| Human | 100 | 45 (71) | 77 | 80 |
| Rabbit | 88 (88) | 38 (59) | 90 (87) | 82 (82) |
Purified PGM and CK from rabbit muscle (3.8 mg/ml each) and human muscle extract (0.4 U/ml), previously reactivated by incubation with 10mM dithiothreitol (DTT) for 30 minutes at 30°C, were incubated at 25°C in buffer (50mM Tris/HCl, pH 7.4) in the presence and in the absence of 4mM urate. After 24 hours of incubation, the activities of PGM and CK were assayed directly and after a further 30 minutes of incubation at 30°C with 10mM DTT (numbers in parenthesis).
DISCUSSION
Our results confirm earlier findings that human serum inactivates PGM and show that the mechanism involved in PGM inactivation is complex and distinct from that implicated in CK inactivation.
Several studies have shown that CK is inactivated by human plasma and serum,8–24 and have shown that the three CK isoenzymes (MM, MB, and BB) are differently affected: MM CK is the most stable isoenzyme, MB CK is slightly less stable, and BB CK is the least stable isoenzyme.9,15–18,20,21 The rate of CK inactivation increased with temperature.16–20 Heating human serum at a temperature that should remove most enzymatic activity did not affect its capacity to inactivate CK isoenzymes.8,13,16,19 Ultrafiltration of plasma18 and serum8,10 through membrane filters that eliminate macromolecules did not prevent the inactivation of the MM and BB CK isoenzymes. Dialysis of serum produced contradictory results: human skeletal muscle CK was reported to be stable in dialysed serum,10 but rabbit muscle8 and human brain21 CK were found to be inactivated by dialysed serum. Albumin protected MM CK from inactivation when incubated in saline solution, but showed only a small protective effect when CK was incubated in whole serum, in dialysed serum, in heated serum, and in serum ultrafiltrate.8 Metal ion chelators did not prevent the inactivation of CK by whole serum21 or by heat inactivated serum.19 Thiol reagents produced only partial protection against inactivation of CK isoenzymes by plasma,12,18 by plasma ultrafiltrate,18 by whole serum,16,17,21 and by heat inactivated serum,13,16,19 and partially reactivated the CK isoenzymes inactivated by incubation in whole plasma,12,18 plasma ultrafiltrate,18 whole serum,10,11,16 heated serum,13 and serum ultrafiltrate.10
From all these data, which have been confirmed by our results, it was suggested that CK isoenzymes undergo two distinct types of inactivation when incubated in human plasma or serum: a rapid inactivation, presumably oxidative in nature, that is reversible with thiol agents, and a gradual inactivation, presumably thermal in nature, that is not reversible and is temperature dependent.8,15 The reversible CK inactivation seems to be mediated through a substance(s) in serum that is both protein bound and free.8 Two endogenous CK inhibitors have been isolated from human serum: urate10 and cystine.30
All three CK isoenzymes have been found to be inactivated by uric acid solutions.10,11,15 Serum proteins decreased the inactivation of CK produced by uric acid, and the inactivation was reversed by dilution.15 The SH groups of CK appeared to be involved in the inactivation produced by urate: it was shown that the thiol reagents prevent15 and reverse11,15 this inactivation, and that there is a reduction in the number of reactive SH groups from eight for each dimer to four in the inactivation of rabbit muscle CK.30,31 Paradoxically, urate retarded the irreversible thermal inactivation of CK isoenzymes and preserved their integrity.15 The mechanism of CK inhibition by cystine appeared to be non-competitive with respect to adenine nucleotide and guanidine substrates, and it has been postulated to involve a disulfide interchange reaction, reversible by reducing agents.29
“Our results show that there are several differences between the inactivation of phosphoglycerate mutase isoenzymes and the inactivation of creatine kinase isoenzymes by serum”
In addition to uric acid and cystine, catecholamines have been shown to inhibit CK. Kinetics indicated that the effect was non-competitive for ADP and creatine phosphate. Thiols gave partial protection against inactivation, and partially reversed catecholamine inactivation. Serum proteins decreased CK inactivation by catecholamines, and dilution but not dialysis reversed the inactivation. As for uric acid, catecholamines retarded the irreversible thermal inactivation of CK isoenzymes.15,32
Finally, it was suggested that one of the CK inhibitory substances present in serum could be calcium.8 Calcium ions act as a competitive inhibitor of magnesium in the CK reaction and at high concentrations they become a non-competitive inhibitor.33–35 However, the direct addition of calcium, at concentrations present in serum, to an albumin solution containing rabbit muscle CK did not inactivate the enzyme.8
Our results show that there are several differences between the inactivation of PGM isoenzymes and the inactivation of CK isoenzymes by serum, namely:
Type MM PGM, which is highly stable when incubated in saline solution is progressively inactivated when it is incubated in human whole serum. In contrast, the activity of type BB PGM is not affected when the enzyme is incubated in serum under similar conditions. Thus, human serum contains a PGM inactivation component(s), which is specific for the type M PGM subunit.
Type MM PGM is inactivated by dialysed serum, heated serum, and serum ultrafiltrate. This raises two possibilities: (a) the enzyme is inactivated by a serum component that is both protein bound and free, or (b) two types of PGM inactivators are present in serum, one of low molecular weight and one of high molecular weight.
The inactivation of MM PGM produced by the non-dialysable serum component(s) is greatly reduced by the presence of EDTA and is largely reversed by incubation with DTT. This suggests that this inactivation involves calcium ions and/or another chelatable metal ion, and implicates the SH groups of the enzyme.
The inactivation of MM PGM produced by the ultrafiltrable serum component(s) is not prevented by EDTA and is only partially reversed by DTT. This suggests that the mechanism of this inactivation is different to that of the inactivation produced by the non-ultrafiltrable serum component(s).
The incubation of MM PGM in urate solutions under conditions that inactivate MM CK does not affect the activity of PGM. Thus, urate is not the low molecular weight serum component that causes the inactivation of MM PGM.
From all these considerations it can be concluded that the inactivation of PGM by human serum is a complex process that involves serum components distinct from those implicated in the inactivation of CK. Human serum contains either a protein component or a protein bound component that inactivates MM PGM through a mechanism that seems to involve calcium and/or another chelatable metal ion, and probably the SH groups of the enzyme. This component does not affect MM CK, which is inactivated by a non-dialysable serum component, the mechanism of action of which does not involve chelatable metal ions. In addition, similar to MM CK, MM PGM is inactivated by a low molecular weight serum component with a metal ion independent mechanism of action, which could involve the SH groups of the enzyme. However, urate, which is one of the serum components that inactivates MM CK, does not affect MM PGM.
Take home messages.
These results confirm earlier findings that human serum inactivates phosphoglycerate mutase (PGM)
The mechanism of PGM inactivation is complex and distinct from that implicated in the inactivation of creatine kinase (CK)
The type M PGM subunit is inactivated by two different mechanisms, which appear to involve the thiol groups of the enzyme
One mechanism is caused by either a protein component or a protein bound serum component and involves calcium ions and/or another chelatable metal ion
The other mechanism is caused by a lower molecular weight serum component and is metal ion independent
Acknowledgments
This work was supported by Generalitat de Catalunya, grant GRQ 94–1036.
Abbreviations
CK, creatine kinase
DTT, dithiothreitol
PGM, phosphoglycerate mutase
REFERENCES
- 1.Fothergill-Gilmore LA, Watson HC. The phosphoglycerate mutases. Adv Enzymol 1989;62:227–313. [DOI] [PubMed] [Google Scholar]
- 2.Omenn GS, Cheung CY. Phosphoglycerate mutase isozyme marker for tissue differentiation in man. Am J Hum Genet 1974;26:393–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Mezquita J, Carreras J. Phylogeny and ontogeny of the phosphoglycerate mutases-I. Electrophoretic phenotypes of the glycerate-2,3-P2 dependent phosphoglycerate mutase in vertebrates. Comp Biochem Physiol B Biochem Mol Biol 1981;70B:237–45. [Google Scholar]
- 4.Bartrons R, Carreras J. Purification and characterization of phosphoglycerate mutase isozymes from pig heart. Biochim Biophys Acta 1982;708:167–77. [DOI] [PubMed] [Google Scholar]
- 5.Durany N, Carballo E, Joseph J, et al. Activity of phosphoglycerate mutase and its isoenzymes in serum after acute miocardial infarction. Mol Pathol 1996;49:M298–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chown PJ, Barnard EA, Barnard PJ, et al. Plasma phosphoglycerate mutase as a marker of muscular distrophy. J Neurol Sci 1984;65:201–10. [DOI] [PubMed] [Google Scholar]
- 7.Yates S, Jeffery S, Barnard P, et al. Plasma phosphoglycerate mutase muscle (M) isoenzyme is strikingly raised in Duchenne muscular distrophy. Biochemical Society Transactions 1986;14:1165–6. [Google Scholar]
- 8.Dalal R, Cilley J, Winsten S. A study of the problems of inactivation of creatine kinase in serum. Clin Chem 1972;18:330–4. [PubMed] [Google Scholar]
- 9.Frotscher U, Dominik B, Richter R, et al. Die Instabilität der Kreatin-Phosphokinase-Isoenzyme im Serum. Klin Wschr 1973;51:801–5. [DOI] [PubMed] [Google Scholar]
- 10.Warren W. Identification of a creatine kinase inhibitor in human serum. Clin Biochem 1976;8:247–53. [DOI] [PubMed] [Google Scholar]
- 11.Warren W, Madelian V. Creatine kinase activity in serum and uric acid solution. Clin Chim Acta 1976;70:285–8. [DOI] [PubMed] [Google Scholar]
- 12.Won Cho H, Meltzer HY, Joung JI, et al. Effect of incubation in human plasma on electrophoretic mobility of brain-type creatine phosphokinase. Clin Chim Acta 1976;73:257–65. [DOI] [PubMed] [Google Scholar]
- 13.Morin LG. Improved separation of creatine kinase cardiac isoenzyme in serum by batch fractionation. Clin Chem 1976;22:92–7. [PubMed] [Google Scholar]
- 14.Bayer PM, Gergely TH, Gabl F, et al. Serum-induced changes in electrophoretic mobility of creatine phosphokinase isoenzymes? Clin Chim Acta 1977;79:261–3. [DOI] [PubMed] [Google Scholar]
- 15.Morin LG. Creatine kinase: stability, inactivation, reactivation. Clin Chem 1977;23:646–52. [PubMed] [Google Scholar]
- 16.Nealon DA, Henderson AR. Stability of commonly used thiols and of human creatine kinase isoenzymes during storage at various temperatures in various media. Clin Chem 1977;23:816–29. [PubMed] [Google Scholar]
- 17.Szasz G, Gerhardt W, Gruber W. Creatine kinase in serum: 5. Effect of thiols on isoenzyme activity during storage at various temperatures. Clin Chem 1978;24:1557–63. [PubMed] [Google Scholar]
- 18.Won Cho H, Meltzer HY. Factors affecting stability of isozymes of creatine phosphokinase. Am J Clin Pathol 1979;71:75–82. [DOI] [PubMed] [Google Scholar]
- 19.Nealon DA, Pettit SM, Henderson AR. Effect of serum pH on storage stability and reaction lag phase of human creatine kinase isoenzymes. Clin Chem 1980;26:1165–9. [PubMed] [Google Scholar]
- 20.Heinbokel N, Hoo JJ, Benkmann HG, et al. Studies on the change of electrophoretic mobility and the decay of catalytic activity of brain-type creatine kinase isoenzyme (CK-BB) following incubation at 37°C. Clin Chim Acta 1981;24:47–53. [DOI] [PubMed] [Google Scholar]
- 21.Lott JA, Heinz JW. Transformation of creatine kinase-BB isoenzyme in vitro: effect of carboxylic acids, thiols, pH, cations, and chelators. Clin Chem 1982;28:2414–17. [PubMed] [Google Scholar]
- 22.Wevers R, Landeghem A, Soons J. Postsynthetic modification in the creatine kinase M-chain and the disappearance of the enzyme from the circulation. The role and function of a new serum protein. J Clin Chem Clin Biochem 1983;21:866–8. [Google Scholar]
- 23.Hashimoto H, Grace AM, Billadello JJ, et al. Nondenaturing quantification of subforms of canine MM creatine kinase isoenzymes (isoforms) and their interconversion. J Lab Clin Med 1984;103:470–84. [PubMed] [Google Scholar]
- 24.Chastain SLL, Ketchum CH, Grizzie WE. Stability and electrophoretic characteristics of creatine kinase BB extracted from human brain and intestine. Clin Chem 1988;34:489–92. [PubMed] [Google Scholar]
- 25.Beutler E, ed. Monophosphoglyceromutase (MPGM). In: Red cell metabolism. New York: Grune and Stratton, 1975:56–8.
- 26.Horder M, Elser RC, Gerhardt W, et al. Approved recommendation on IFCC methods for the measurement of catalytic concentrarion of enzymes, part 7. IFCC method for creatine kinase. Eur J Clin Chem Clin Biochem 1991;29:435–56. [PubMed] [Google Scholar]
- 27.Bradford M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54. [DOI] [PubMed] [Google Scholar]
- 28.Durany N, Carreras J. Distribution of phosphoglycerate mutase isozymes in rat, rabbit and human tissues. Comp Biochem Physiol 1996;113:217–23. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs HK, Philipp KH, Sundmark N, et al. Isolation and identification of a new potent inhibitor of creatine kinase from human serum. Clin Chim Acta 1978;85:299–309. [DOI] [PubMed] [Google Scholar]
- 30.Madelian V, Warren WA. Modification of sulfhydryl groups of creatine kinase by urate. Clin Biochem 1984;17:173–4. [DOI] [PubMed] [Google Scholar]
- 31.Madelian V. Modification of the sulfhydryl (SH) groups of creatine kinase (CK) by urate. Clin Chem 1977;23:1119. [Google Scholar]
- 32.Morin LG. Effects of serum constituents and drugs on cardiac isoenzyme (MB) of creatine kinase (CK). Clin Chem 1976;7:1163. [Google Scholar]
- 33.Szasz G, Waldenström J, Gruber W. Creatine kinase in serum: 6. Inhibition by endogenous polyvalent cations, and effect of chelators on the activity and stability of some assay components. Clin Chem 1979;25:446–52. [PubMed] [Google Scholar]
- 34.Urdal P, Stromme JH. Effects of Ca, Mg, and EDTA on creatine kinase activity in cerebrospinal fluid. Clin Chem 1979;25:147–50. [PubMed] [Google Scholar]
- 35.Nealon DA, Henderson AR. Effect of cations on the human creatine kinase isoenzymes. Clin Chem 1980;26:1137–9. [PubMed] [Google Scholar]