Abstract
FliL is a bacterial flagellar protein demonstrated to associate with, and regulate ion flow through, the stator complex in a diverse array of bacterial species. FliL is also implicated in additional functions such as stabilizing the flagellar rod, modulating rotor bias, sensing the surface, and regulating gene expression. How can one protein do so many things? Its location is paramount to understanding its numerous functions. This review will look at the evidence, attempt to resolve some conflicting findings, and offer new thoughts on FliL.
Keywords: flagella, FliL, motor torque, rotor bias, stators, surface sensing, swarming, swimming
1 |. INTRODUCTION
FliL is a well-conserved flagellar protein in bacteria, found thus far in at least 10 phyla, most frequently in Proteobacteria (Figure 1; Potter et al., 2018). Phenotypes associated with FliL mutants have thus far been reported in Gammaproteobacteria (Escherichia coli, Salmonella enterica, Proteus mirabilis, Pseudomonas aeruginosa, and Vibrio alginolyticus), Alphaproteobacteria (Bradyrhizobium diazoefficiens, Caulobacter crescentus, Rhodobacter sphaeroides, Silicibacter sp., and Sinorhizobium meliloti), Betaproteobacteria (Herminiimonas arsenicoxydans), Campylobacterota (Helicobacter pylori), Firmicutes (Bacillus subtilis), and Spirochetes (Borrelia burgdorferi). FliL binds to protein complexes called stators, a physical association first reported in Salmonella (Partridge et al., 2015). Stators conduct protons (or sodium ions in marine bacteria) to power the motor (Berg, 2003). Two recent reviews largely covered the role of FliL in modulating stator function, and readers are referred to them for more extensive citations (Guo & Liu, 2022; Subramanian & Kearns, 2019).
FIGURE 1.
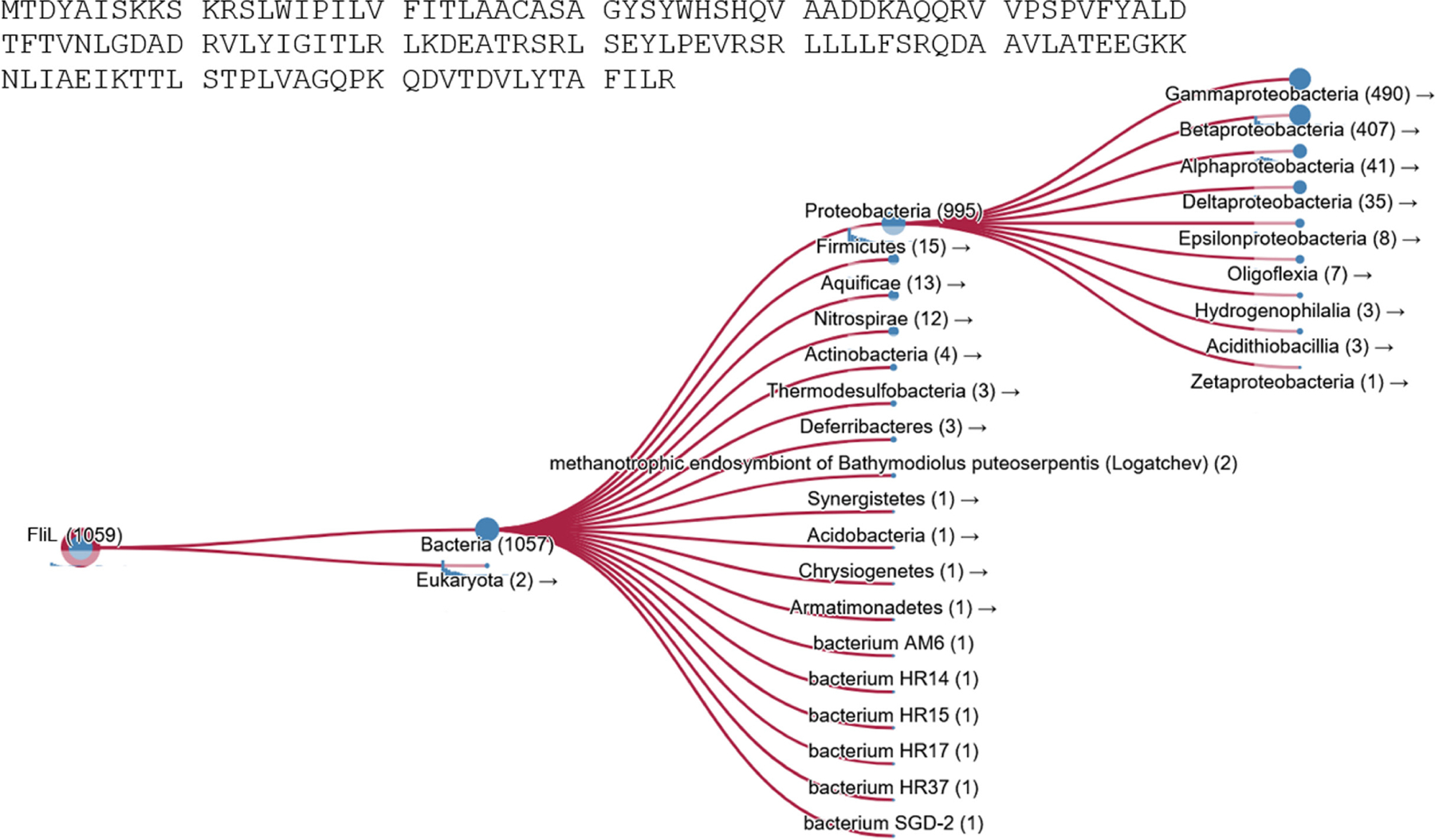
Taxonomic prevalence of the FliL protein. Results of searching the HMMER database (Potter et al., 2018) using the FliL protein sequence from Escherichia coli (sourced from Ecocyc).
2 |. FliL LOCATION AND CONFIGURATION
Flagellar rotation is powered by the stator complex MotAB located at the flagellar base in the inner membrane (Figure 2, left). The disposition of FliL with respect to MotAB is clearly evident in the cryo-ET images from B. burgdorferi (referred to henceforth as Borrelia), where FliL subunits encircle this complex (Guo et al., 2022). Because an equivalent FliL structure is not available in Gammaproteobacteria where the majority of the varied FliL phenotypes have been reported, we have taken the liberty of docking FliL from Borrelia onto MotAB and apposing this complex to the flagellar base of Salmonella where structures of a large number of flagella components are available (Mondino et al., 2022) (Figure 2, left). Most components shown in the figure are present in both bacterial species where they function in an equivalent manner, although the Borrelia basal body structure is much larger. MotA interacts with FliG at the top of the cytoplasmic C-ring whose size changes depending on the direction of motor rotation, as first observed in Borrelia (Chang et al., 2020), and now reported in Salmonella as well (Johnson et al., 2024; Singh et al., 2024; Tan et al., 2024). FliG interdigitates with the transmembrane MS-ring (FliF) above, which in turn is attached to a periplasmic rod made of four different proteins (FlgB/FlgC/FlgF/FlgG; Figure 2, right). The C-ring/MS-ring/rod/PL-ring unit is generally called the flagellar basal body. The rod attaches to the long flagellar filament (FliC) via a short flexible hook (FlgE). In most bacteria, the hook and filament are external to the cell; in Borrelia, they are periplasmic.
FIGURE 2.
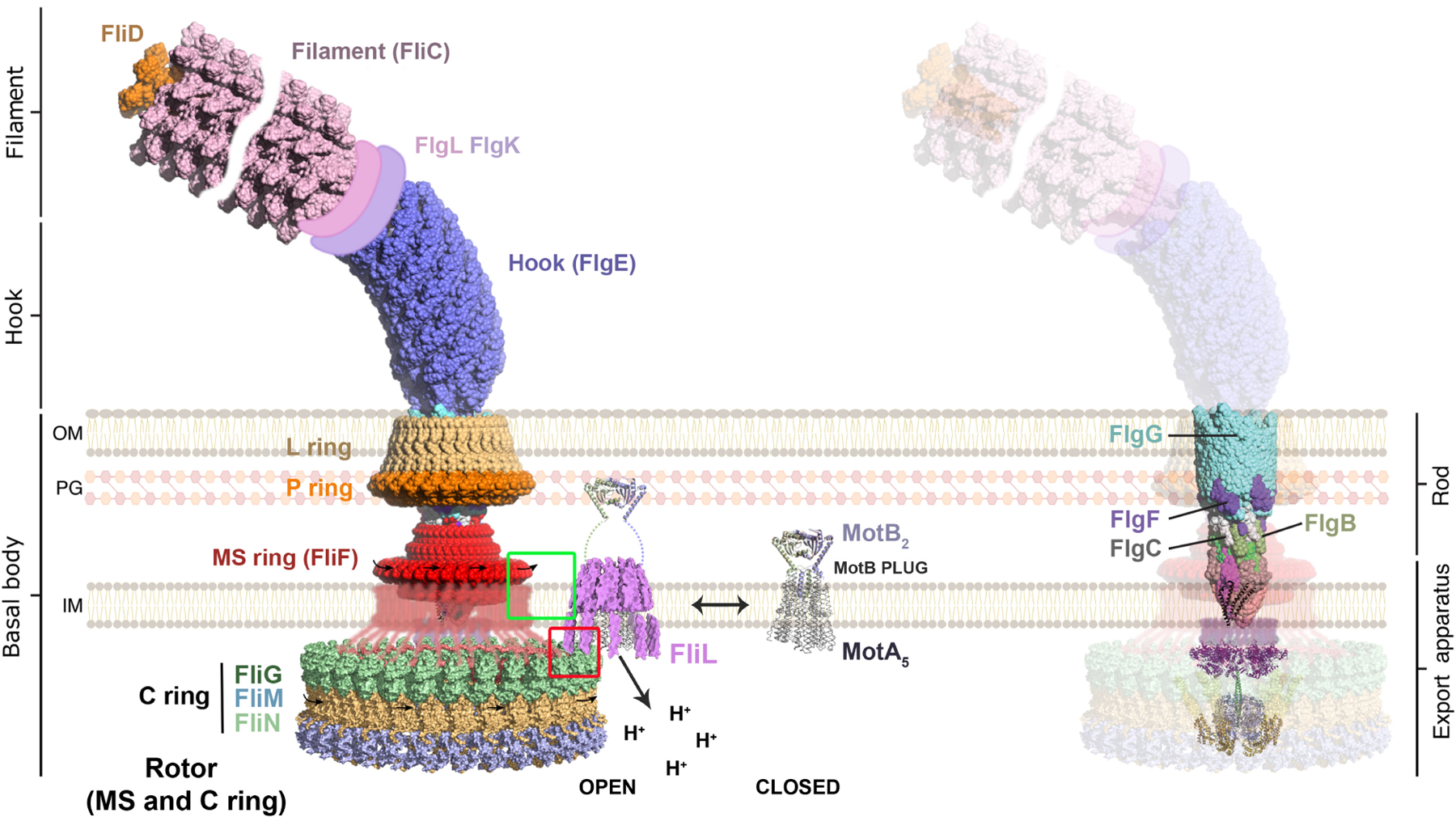
A model for FliL positioning at the flagella motor. Left: The bulk of the figure is derived from Mondino et al., 2022 (reproduced under the open access terms of the Creative Commons CC-BY license), where a complete representation of a bacterial flagellum was constructed using available high-resolution structures of subassemblies from several bacterial species, all drawn to scale (for full details, see Figure 1 in Mondino et al., 2022). In light of the recently published complete C-ring structures (see text), Mondino et al. (2022) kindly updated the C-ring in their figure using PDB 8T8O (Alejandro Buschiazzo, personal communication), shown here in its CCW orientation indicated by the black rightward-pointing arrows placed on the periplasmic RBM-3 domain of FliF. Also added was a schematic representation of FliF RBM-3 loops (red) that descend into the cytoplasm and interdigitate with the FliG (green) at the top of the C-ring. In Salmonella/Escherichia coli, FliL interacts with RBM-3 in both locations as highlighted by the green and red squares (see text). The “closed” configuration of the MotAB complex was rendered by repurposing the previously generated “open” complex (Mondino et al., 2022). The FliL supramolecular complex (magenta) is taken from (Guo & Liu, 2022), and positioned to surround the “open” stator complex that associates with the basal body; Borrelia FliL (178 residues) is similar in length to Salmonella FliL (154 residues) and folds similarly in Alphfold. Right: Rod subunits not visible in the image on the left are highlighted. IM, inner membrane; OM, outer membrane; PG, peptidoglycan.
Stators are composed of pentameric MotA and dimeric MotB proteins (Deme et al., 2020; Santiveri et al., 2020). They are independent units that drift in the inner membrane in a “closed” ion-nonconducting form until they encounter the flagellar basal body, upon which they “open” for business (Figure 2, left). In Borrelia, FliL assembles as a decameric ring above the MotA pentamer, encircling and contacting a “plug” or valve segment of the MotB dimer, which opens upon basal body contact to allow ion flow. The now-extended MotB attaches securely to the peptidoglycan (PG). This attachment is not permanent; stators can engage and disengage from the basal body by sensing the external load exerted on the filament, higher loads retaining more stators (Baker & O’Toole, 2017). Protons transiting through the MotAB complex cause conformational changes in MotA that power rotation of the pentameric MotA ring around the stationary MotB (Guo & Liu, 2022). In the cytoplasm, the altered conformation of the rotary MotA pushes on FliG, generating torque that results not only in the rotation of the C-ring, but also of all the flagellar parts linked to the C-ring (MS-ring, rod, hook, and filament; PL-rings serve as bushings and do not rotate), producing a rotating jamboree! Not shown in the Figure 2 composite is a large periplasmic collar in Borrelia that surrounds the rod/MS-ring/FliL-MotAB assembly, and provides many functions that assure structural stability and maximal power (Chang et al., 2021); there is no collar in E. coli/Salmonella. In Borrelia, the FliL ring rests against the collar. FliL is a single-pass transmembrane (TM) protein, whose short N-terminal segment in the cytoplasm was not resolved in the Borrelia cryo-ET images (Guo et al., 2022). However, since the TM segment of FliL lies between the MS-ring and MotA, the cytoplasmic N-terminal segment is also expected to reside between these two proteins (Figure 2, left). In Salmonella, biochemical experiments are consistent with existence of a FliL ring (Partridge et al., 2015). Pull-down and two-hybrid assays showed a strong interaction between FliL and the upper portion of the MS-ring (RBM-3 domain of FliF; see Figure 2 legend) (Partridge et al., 2023). This domain of FliF interdigitates with the periplasmic rod above and loops down into the cytoplasm to also interdigitate with FliG at the top of the C-ring (see Partridge et al., 2023). Biochemical experiments in E. coli and Salmonella are consistent with the short cytoplasmic segment of FliL interacting with cytoplasmic RBM-3 of FliF as well as with FliG (Partridge et al., 2015, 2023). Thus, as deduced for Borrelia, FliL lies at the junction of MS-ring-FliG-MotA proteins in E. coli/Salmonella as well, a prime location for manifesting its many functions.
3 |. FliL AS A POWER BROKER
The location of the FliL ring encircling the MotB plug, as well as the multiple contacts between the two seen in the cryo-ET images from Borrelia, implicate FliL in modulating proton flow via the plug. The clearest experimental support for this scenario comes from the observation that mutations in FliL impair flagella rotation or abolish swimming motility in the Alphaproteobacteria C. crescentus, R. sphaeroides, S. meliloti, and Silicibacter sp. (Belas et al., 2009; Jenal et al., 1994; Sobe et al., 2022; Suaste-Olmos et al., 2010); suppressor mutations that restored motility in R. sphaeroides mapped to a region of MotB equivalent to that identified as the plug in E. coli (Hosking et al., 2006). The Alphaproteobacterium B. diazoefficiens has two distinct kinds of flagella – subpolar (S) and lateral (L), each with a dedicated FliL (FliLS and FliLL): a ΔfliLS strain expressing only S flagella showed reduced swimming speed, while a ΔfliLL strain expressing only L flagella was non-motile, similar to the motility-arrest seen in the absence of FliL in other Alphaproteobacteria (Mengucci et al., 2020). Absence of FliL also abolished swimming in H. pylori (Tachiyama et al., 2022). Thus, FliL is essential for motility in all these bacteria. Such an essential function for FliL in swimming motility has not been reported in other bacterial species. However, mutations in the MotB plug region that FliL contacts (likely to regulate proton flow), improved motility of a ΔfliL Salmonella strain (Partridge et al., 2015). Also, partial defects in swimming motility assayed by various methodologies (migration in soft agar, tracking swimming speeds, and single motor bead assays), have been observed for FliL mutants in Borrelia, E. coli, Salmonella, and Vibrio (Attmannspacher et al., 2008; Motaleb et al., 2011; Partridge et al., 2015; Zhu et al., 2015).
Bacteria can migrate collectively over the surface of semi-solid medium in the process called swarming. Viscous forces on the flagellar filament are expected to be high in this environment as the bacteria encounter numerous physical forces such as surface tension, adhesion, friction, and capillary action (Partridge & Harshey, 2013). It takes increased motor torque to navigate on a surface as witnessed by the requirement for increased flagella numbers (Ghelardi et al., 2012; McCarter et al., 1988; Mukherjee et al., 2015), special stators (Toutain et al., 2005), and stator-associated proteins such as FliL or SwrD during swarming in different bacterial species (Attmannspacher et al., 2008; Hall et al., 2018). The need for FliL is especially critical in E. coli and Salmonella while swarming, where FliL contributes not only to increased speed but also to an altered rotor bias (see below). A FliL requirement for movement through viscous media was reported for B. diazoefficiens (Mengucci et al., 2020) and for V. alginolyticus (Zhu et al., 2015). At high viscosities, more stators are expected to be recruited, supplying more power to the motor. In P. aeruginosa, which has two sets of stators—MotAB and MotCD (the latter essential for swarming; Toutain et al., 2005), FliL was shown to increase torque of MotCD motors, an effect more pronounced at higher viscosities (Zhang et al., 2022).
A more indirect role for FliL as a power broker is in stabilizing stator retention at the rotor. In Borrelia, stator occupancy was reduced by 60% in the absence of FliL (Chang et al., 2019), whereas in V. alginolyticus, polar localization of stators was affected (Zhu et al., 2015). In Salmonella, the presence of FliL strengthened MotA–MotB interactions and slightly improved stator dwell time at the rotor (Partridge et al., 2015). In P. aeruginosa, FliL enhanced the dwell time of MotCD stators at the rotor (Zhang et al., 2022). Thus, in addition to regulating ion flow, FliL appears to optimize stator function, perhaps by promoting secure attachment of stators to the cell wall and motor.
4 |. FliL AS A BULWARK
FliL not only assists stator retention at the basal body, but also stabilizes the rotating assembly in the periplasm. This was deduced by the finding that in the absence of FliL the periplasmic rod fractures in E. coli and Salmonella as they attempt to swarm (Attmannspacher et al., 2008; Partridge et al., 2015). In these bacterial species, subunits of the four rod proteins (FlgB, FlgC, FlgF, and FlgG) interdigitate and pack against the inside of the MS-ring β-collar (a topmost portion of FliF) (Johnson et al., 2021; Tan et al., 2021) (Figure 2, right). This arrangement presumably exists to allow some “give” and provide structural stability to the rotating unit, which is also supported by the non-rotating PL-rings positioned around the FlgF/FlgG junction. This junction must be vulnerable, because during swarming, when viscous forces on the filament are expected to be high, the Salmonella rod breaks between FlgF and FlgG in the absence of FliL (Attmannspacher et al., 2008) (Figure 3a). A similar-looking break in the rod was observed in a spontaneous mutant of the MS-ring (FliF) in Salmonella, but only when the bacteria were propagated in a viscous liquid (Figure 3b) (Okino et al., 1989). Rod vulnerability at a similar location has been exploited by C. crescentus to eject its flagellum, facilitating its transition from a motile to a sessile state (Figure 3c) (Kanbe et al., 2005). Interestingly, the ejection process is linked to FliL-dependent developmental degradation of FliF (Aldridge & Jenal, 1999). By positioning alongside the MS-ring in E. coli/Salmonella, the FliL ring likely acts as a bulwark to absorb the rotational stress transmitted to the rod in high-viscosity regimes (but see next section). In non-swarming Borrelia, absence of FliL not only impacts stator assembly, but the periplasmic flagella located at both ends of the bacterium orient abnormally (Motaleb et al., 2011), suggesting some manner of a fortification function for FliL in this bacterium as well.
FIGURE 3.
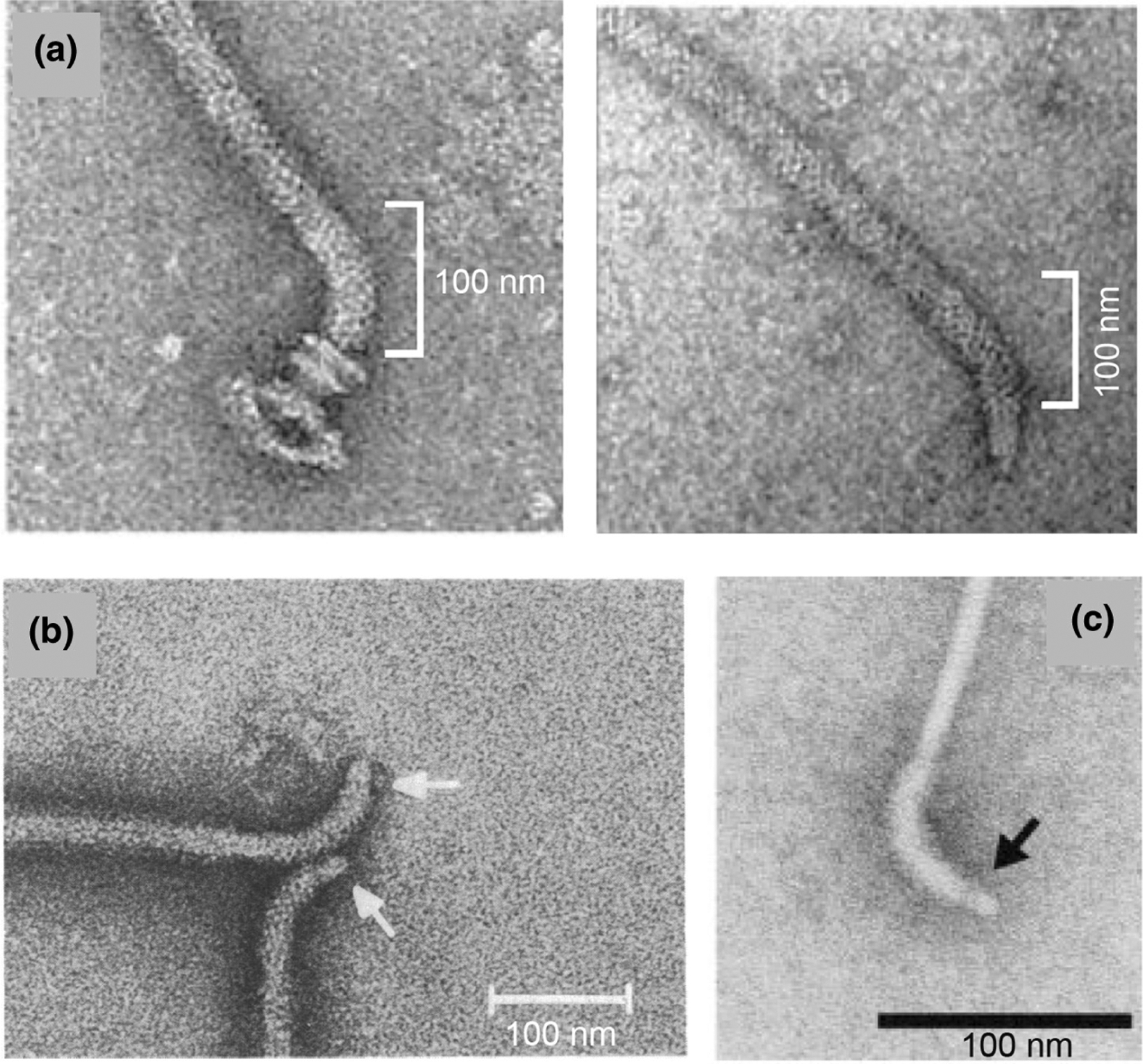
Flagellar filaments from Salmonella enterica and Caulobacter crescentus severed within the rod. EM images of negatively stained filament-hook-rod structures from: (a) Wild-type (left) and ΔfliL (right) strains of Salmonella propagated on swarm medium (white bracket highlights curved hook). This panel is reproduced from (Attmannspacher et al., 2008) under the rights of re-use afforded the author. (b) A spontaneous Salmonella fliF (MS-ring) mutant propagated in viscous liquid medium. This panel is reproduced from (Okino et al., 1989) under ASM free use Creative Commons Attribution license. (c) Ejected flagella during developmental life cycle of C. crescentus. This panel is reproduced from (Kanbe et al., 2005), with republication permission obtained from the Copyright Clearance Center (license ID 1493052–1).
5 |. FliL AS A MODULATOR OF ROTORBIAS
The flagellar motor rotates bidirectionally in the bacterial species being discussed in this section. In E. coli and Salmonella, the default state of rotation is counterclockwise (CCW). Bidirectionality is imposed on the rotor by a chemosensory system, which in response to environmental stimuli, phosphorylates the signaling protein CheY (Hazelbauer et al., 2008). CheY~P binds at the bottom of the C-ring to bring about a large change in FliG conformation at the top of the ring (Johnson et al., 2024; Singh et al., 2024; Tan et al., 2024), reversing the motor direction to clockwise (CW). FliG and MotA function as engaged gears as evidenced by both cryo-EM and genetic data (Guo & Liu, 2022 and citations in Partridge et al., 2023). Therefore, anything that affects their interaction is expected to affect rotor bias as well. Thus, when CheY~P binding alters FliG conformation, MotA positioning with respect to FliG also changes, leading the stators to move closer to the MS-ring in the CW state compared to the CCW state as shown in Borrelia (Chang et al., 2020), and as modeled for Salmonella [figure 6d in (Tan et al., 2024)]. We note in this regard that in the CCW orientation of the C-ring, FliL is close to the RBM-3 domain of FliF in the cytoplasm, but not so in the periplasm (Figure 2, left). In the CW orientation, however, when the stators move closer to the MS ring, FliL would be expected to interact closely with FliF. At an organismal level, the CCW/CW rotor bias impacts bacterial run-tumble behavior. CW episodes generate tumbles and CCW episodes produce runs in E. coli. The chemosensory system enables an increase in CCW durations, allowing the bacteria to extend runs and climb attractant gradients.
As shown in Figure 2 and discussed in section 2 above, FliL not only associates intimately with the stators and contacts the MS-ring in the periplasm in E. coli/Salmonella, but its cytoplasmic N-terminus is likely also sandwiched between the MS-ring and MotA and in contact with FliG (Partridge et al., 2015). Given that FliG-MotA interaction dictates motor bias, it is not surprising that absence of FliL alters the normal rotor bias in both bacterial species (Partridge et al., 2015), as it does in P. aeruginosa as well (Zhang et al., 2022). This property of FliL is particularly evident during swarming (Partridge et al., 2023), where bacteria increase speed and running episodes (higher CCW bias), and suppress tumbling (lower CW bias), a phenomenon seen in several bacterial species (Partridge et al., 2019). Tumble suppression likely helps maintain/promote the side-by-side alignment of cells moving within a swarm (Partridge et al., 2020) (see Video S1). Contribution of FliL to a decreased CW bias during swarming is also seen in P. mirabilis and B. subtilis, where FliL is not essential for swarming (Partridge et al., 2023). We have called this dual property of FliL in increasing cell speed and lowering CW bias during swarming “motor remodeling.” Both swarming and motor remodeling could be restored either fully or partially to an E. coli fliL mutant by complementation with fliL genes from P. mirabilis and B. subtilis, showing conservation of a swarming-associated FliL function across phyla (Partridge et al., 2023).
The CCW conformation of the C-ring during swarming suggests that FliL positioning at the edge of the C-ring (Figure 2, left) might counteract CheY~P signals acting on FliG to adopt a CW conformation. Why is this FliL-dependent CCW conformation adopted only during swarming? We offer two possible explanations. In bacterial species where RNA and protein profiles have been compared, cell physiologies are distinctly different between swarmer and planktonic states (Bhattacharyya et al., 2020; Gode-Potratz et al., 2011; Morgenstein et al., 2010; Overhage et al., 2008; Pearson et al., 2010; Wang et al., 2006). A common observation in swarmers is up-regulation of genes involved in energy metabolism, and changes in membrane lipids and proteins. Given that flagella are embedded in membranes, changes in membrane composition or in periplasmic space would be expected to impact how flagella orient/anchor in swarm cells, changing FliL positioning at the rotor. Alternatively, additional proteins expressed only during swarming might bridge the distance between the MS-ring and FliL, stabilizing the CCW state. We note that in both Borrelia and H. pylori, as yet unidentified proteins occupy the space between these two rings (Guo et al., 2022; Tachiyama et al., 2022).
6 |. FliL AS A SURFACE SENSOR FOR REGULATING GENE EXPRESSION
The periplasmic region of FliL shows remarkable structural similarity to the mammalian stomatin (SPFH) domain, which is known to modulate ion transport/channels in eukaryotes (Tachiyama et al., 2022; Takekawa et al., 2020). These type of proteins are linked to the formation of mechanosensors in Caenorhabditis elegans, and mechanosensory transduction in mammalian neurons (Goodman et al., 2002; Wetzel et al., 2007), but their specific mechanism is still unknown (Cheng et al., 2018). Could a protein located in the periplasm be a mechanosensor?
A role for the flagellar filament as a surface sensor was first proposed in V. parahaemolyticus, which swims using a polar flagellum but uses hundreds of lateral flagella (Laf) to swarm (McCarter et al., 1988). Induction of Laf is seen when the bacteria are inoculated on the surface of agar, and also when viscosity of the liquid medium is increased (Belas et al., 1986). However, deletion of MotA or MotB associated with the polar flagellum did not affect Laf induction (Boles & McCarter, 2000), suggesting that this surface response is independent of stators (and hence FliL) [see (Harshey, 2016)]. In B. diazoefficiens, which similarly upregulates Laf, FliLS associated with the subpolar flagellum was implicated in Laf induction (Mengucci et al., 2020). In P. mirabilis, a FliL mutant with altered 14 amino acid residues at its C-terminus was not as proficient in upregulating the flagellar regulon as WT (Cusick et al., 2012), and a ΔfliL mutant of S. meliloti was mostly aflagellate, and had reduced transcription of the flagellar gene flaA (Sobe et al., 2022). Also, a FliL null strain produced “precocious” swarming of P. mirabilis over surfaces with low viscosity that normally impede WT swarming, leading the authors to suggest a role for FliL in detecting “appropriate” surface conditions (Lee & Belas, 2015).
The examples cited above imply a role for FliL in gene regulation. A reasonable suggestion of how FliL might work as a surface sensor might be gleaned from a study monitoring swimmer to stalk cell transition in C. crescentus, where surface contact increases c-di-GMP levels that stimulate synthesis of a sticky holdfast, conditions which also promote FliL-dependent degradation of the flagellum (Aldridge & Jenal, 1999; Christen et al., 2007). Curiously, holdfast production is independent of the rod, hook and external filament, but dependent on MotAB/FliL (Hug et al., 2017). The authors have suggested that the stator complexes may act as mechanosensitive channels, detecting mechanical forces affecting the cell envelope upon surface contact and communicating them to the cytoplasm via a change in ion flux through the stators. A resulting transient pH change could conceivably be sensed by gene expression regulators positioned near the flagellum.
FliL is located at an ideal position to link signals coming down from the external filament to the rod and MS-ring, relaying them to both the stators and the cytoplasmic C-ring (Figure 2). Whether these signals activate “mechanosenstive” FliL channels remains to be explored. FliL is apparently not involved in sensing external load on the filament as demonstrated in single motor experiments conducted with planktonic cells of E. coli, where stator recruitment at the basal body in response to external load applied on the filament, did so irrespective of FliL (Chawla et al., 2017). However, given that swarm cells are physiologically different from their planktonic counterparts, these experiments deserve to be repeated in swarm cells before ruling out a load-detection function for FliL.
7 |. CONFLICTING FliL PHENOTYPES
It is amply evident from the sections above that FliL is an essential component of the flagella machinery in some bacterial species. In others, the FliL requirement is only manifested when moving on surfaces or through viscous media. Our lab first reported the critical role of FliL during swarming in E. coli and Salmonella (Attmannspacher et al., 2008). In our hands, FliL null mutants of both bacterial species show similar phenotypes– impaired swimming in 0.3% soft agar (“swim” or “chemotaxis”) medium, and no swarming on medium with 0.5–0.7% agar (“swarm”) (Attmannspacher et al., 2008; Partridge et al., 2015, 2023). Since FliL affects rotor bias in these bacteria, the reduced motility of fliL mutants in soft agar could at least in part be due to sub-optimal chemotaxis. However, chemotaxis proficiency is not required for swarming in these bacteria (Burkart et al., 1998), and is actually suppressed (Partridge et al., 2019). The non-swarming phenotype of FliL mutants can perhaps be explained fully by the observed fracture of flagella rods (see Figure 3a), and by a requirement for more motor power on the surface. The conflicting data we consider here is that of Chawla et al. (2017) and Lee and Belas (2015) who reported that fliL null mutants of E. coli could swarm.
Flagella must push against a fluid to perform work, and there is not much fluid on a surface terrain. Swarmers secrete polysaccharides and surfactants, thought to draw water from beneath the surface of the swarm medium (Partridge & Harshey, 2013). E. coli struggles to do so, based on the observed sensitivity of its swarming to the following multiple factors: (1) commercial source of the agar [see (Harshey, 2010) for the discovery of Eiken agar and other quirks], (2) batch number of agar obtained from the same commercial source, (3) concentration of agar used to solidify the media, (4) addition of 0.5% sugars to the media, (5) quality of the water used in media preparation, (6) drying time between pouring swarm media and inoculating bacteria, (7) humidity of the room/incubator. All these conditions likely affect how “wet” the surface is. A “wetter” surface would be expected to lower the resistance to flagella rotation and hence lower the difficulty level of moving. If the plates are not dried sufficiently after pouring (Chawla et al., 2017) or if the humidity in the incubation chamber is high (Lee & Belas, 2015), fliL mutants might swarm. To test this possibility, we tried to reproduce the conditions described in the two publications, and indeed succeeded in getting partial swarming in ΔfliL E. coli when we hydrated the surface by spritzing water (figure S2b in Partridge et al., 2023). Salmonella is less fastidious than E. coli and will swarm on Bacto agar with added sugar (Harshey & Matsuyama, 1994). However, Salmonella swarms more efficiently on Eiken agar and ΔfliL Salmonella can migrate farther on this medium (figure S2a in Partridge et al., 2023). If this explanation for the difference in the fliL swarming phenotype for E. coli reported by different labs is correct, there is perhaps no conflict. However, Chawla et al. (2017) reported in addition that lack of FliL did not affect motor torque or rotor switching in planktonic cells, clearly contradicting data from single motor assays as well as cell tracking assays of planktonic cells of E. coli/Salmonella. As noted above (see section 5), there is ample evidence for contribution of FliL to both motor torque and rotor bias not only in E. coli, but also in several bacterial species. Assuming the strain genotype used by Chawla et al. (2017) was correct, we have no explanation for the difference in their motor data at this time.
The discussion above also highlights our lack of understanding of physicochemical properties of a surface terrain that the bacteria clearly do. Given all the variables that affect swarming, we advise caution when deducing FliL function by simply surveying the literature as was done in a recent report, which concluded that FliL truncations that leave the N-terminal and TM regions intact, block motility, whereas a complete FliL deletion allows normal motility (Liu et al., 2023). This report inferred from the survey that this N-terminal segment of FliL interacts with MotB to prevent plug opening. We specifically note here that both E. coli and Salmonella show similar swim and swarm phenotypes irrespective of whether the FliL truncation retains the TM segment (Attmannspacher et al., 2008), or not (Partridge et al., 2023). Also, the N-terminal segment and TM regions of FliL are unlikely to be in contact with MotB in the inner membrane as discussed above.
8 |. FINAL THOUGHTS
Understanding FliL function has been a challenge. In biology, function follows form. The structure and positioning of FliL as revealed by cryoET and other studies is giving credence to the real estate mantra that “location is everything” in helping us understand its many functions. The leitmotif of FliL is to regulate ion flow through stators as revealed by swimming phenotypes in diverse bacteria. The ability of FliL to influence rotor bias as well as to stabilize the rotating periplasmic assembly (or chassis if you will), is clear from studies on swarming. FliL location is ideal for serving as a center point from which to influence the many flagellar properties. A useful FliL analogy that comes to mind is that of a timing belt in a motor car, which syncs rotation of different units in a car engine in order for all valves and pistons to open and close correctly. Whether and how FliL functions as a mechanosensor to regulate gene expression is still uncharted territory.
The idea that the flagellar motor might rotate came to Howard Berg as a distinct possibility when he saw the several rings in Julius Adler’s electron micrographs of the E. coli basal body (Berg & Anderson, 1973). Does the FliL ring evoke a similar possibility? Mounted on top of the rotary MotA ring and juxtaposed against the rotary FliF ring, must the FliL ring also rotate? Time will tell.
Supplementary Material
ACKNOWLEDGMENTS
We thank graduate student Brady Wilkins for generating the Graphical Abstract image in Microsoft Designer. Swarming work in our lab is supported by Public Health Service Grant GM118085 to R.M.H.
Footnotes
ETHICS STATEMENT
The authors declare that no human or animal subjects were used in this study.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Aldridge P & Jenal U (1999) Cell cycle-dependent degradation of a flagellar motor component requires a novel-type response regulator. Molecular Microbiology, 32, 379–91. [DOI] [PubMed] [Google Scholar]
- Attmannspacher U, Scharf BE & Harshey RM (2008) FliL is essential for swarming: motor rotation in absence of FliL fractures the flagellar rod in swarmer cells of Salmonella enterica. Molecular Microbiology, 68, 328–41. [DOI] [PubMed] [Google Scholar]
- Baker AE & O’Toole GA (2017) Bacteria, rev your engines: stator dynamics regulate Flagellar motility. Journal of Bacteriology, 199, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belas R, Horikawa E, Aizawa S & Suvanasuthi R (2009) Genetic determinants of Silicibacter sp. TM1040 motility. Journal of Bacteriology, 191, 4502–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belas R, Simon M & Silverman M (1986) Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. Journal of Bacteriology, 167, 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg HC (2003) The rotary motor of bacterial flagella. Annual Review of Biochemistry, 72, 19–54. [DOI] [PubMed] [Google Scholar]
- Berg HC & Anderson RA (1973) Bacteria swim by rotating their flagellar filaments. Nature, 245, 380–2. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Walker DM & Harshey RM (2020) Dead cells release a ‘necrosignal’ that activates antibiotic survival pathways in bacterial swarms. Nature Communications, 11, 4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles BR & McCarter LL (2000) Insertional inactivation of genes encoding components of the sodium-type flagellar motor and switch of Vibrio parahaemolyticus. Journal of Bacteriology, 182, 1035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkart M, Toguchi A & Harshey RM (1998) The chemotaxis system, but not chemotaxis, is essential for swarming motility in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America, 95, 2568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Moon KH, Zhao X, Norris SJ, Motaleb MA & Liu J (2019) Structural insights into flagellar stator-rotor interactions. eLife, 8, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Xu H, Motaleb MA & Liu J (2021) Characterization of the Flagellar collar reveals structural plasticity essential for spirochete motility. MBio, 12, e0249421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Zhang K, Carroll BL, Zhao X, Charon NW, Norris SJ et al. (2020) Molecular mechanism for rotational switching of the bacterial flagellar motor. Nature Structural & Molecular Biology, 27, 1041–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla R, Ford KM & Lele PP (2017) Torque, but not FliL, regulates mechanosensitive flagellar motor-function. Scientific Reports, 7, 5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YR, Jiang BY & Chen CC (2018) Acid-sensing ion channels: dual function proteins for chemo-sensing and mechano-sensing. Journal of Biomedical Science, 25, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen M, Christen B, Allan MG, Folcher M, Jeno P, Grzesiek S et al. (2007) DgrA is a member of a new family of cyclic diguanosine monophosphate receptors and controls flagellar motor function in Caulobacter crescentus. Proceedings of the National Academy of Sciences of the United States of America, 104, 4112–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusick K, Lee YY, Youchak B & Belas R (2012) Perturbation of FliL interferes with Proteus mirabilis swarmer cell gene expression and differentiation. Journal of Bacteriology, 194, 437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deme JC, Johnson S, Vickery O, Aron A, Monkhouse H, Griffiths T et al. (2020) Structures of the stator complex that drives rotation of the bacterial flagellum. Nature Microbiology, 5, 1553–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghelardi E, Salvetti S, Ceragioli M, Gueye SA, Celandroni F & Senesi S (2012) Contribution of surfactin and SwrA to flagellin expression, swimming, and surface motility in Bacillus subtilis. Applied and Environmental Microbiology, 78, 6540–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gode-Potratz CJ, Kustusch RJ, Breheny PJ, Weiss DS & McCarter LL (2011) Surface sensing in Vibrio parahaemolyticus triggers a programme of gene expression that promotes colonization and virulence. Molecular Microbiology, 79, 240–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman MB, Ernstrom GG, Chelur DS, O’Hagan R, Yao CA & Chalfie M (2002) MEC-2 regulates C. elegans DEG/ENaC channels needed for mechanosensation. Nature, 415, 1039–42. [DOI] [PubMed] [Google Scholar]
- Guo S & Liu J (2022) The bacterial Flagellar motor: insights into torque generation, rotational switching, and Mechanosensing. Frontiers in Microbiology, 13, 911114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Xu H, Chang Y, Motaleb MA & Liu J (2022) FliL ring enhances the function of periplasmic flagella. Proceedings of the National Academy of Sciences of the United States of America, 119, e2117245119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AN, Subramanian S, Oshiro RT, Canzoneri AK & Kearns DB (2018) SwrD (YlzI) promotes swarming in Bacillus subtilis by increasing power to Flagellar motors. Journal of Bacteriology, 200, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshey RM (2010) Swarming Adventures. In: Maloy SM, Casadesus J & Hughes KT (eds). The lure of bacterial genetics: a tribute to John Roth. Washington, D.C.: American Society for Microbiology, pp. 163–72. [Google Scholar]
- Harshey RM (2016) The flagellum as a sensor. In: de Bruijn FJ (Ed.) Stress and environmental control of gene expression in bacteria. Hoboken, USA: Wiley Blackwell. [Google Scholar]
- Harshey RM & Matsuyama T (1994) Dimorphic transition in Escherichia coli and salmonella typhimurium: surface-induced differentiation into hyperflagellate swarmer cells. Proceedings of the National Academy of Sciences of the United States of America, 91, 8631–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelbauer GL, Falke JJ & Parkinson JS (2008) Bacterial chemoreceptors: high-performance signaling in networked arrays. Trends in Biochemical Sciences, 33, 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking ER, Vogt C, Bakker EP & Manson MD (2006) The Escherichia coli MotAB proton channel unplugged. Journal of Molecular Biology, 364, 921–37. [DOI] [PubMed] [Google Scholar]
- Hug I, Deshpande S, Sprecher KS, Pfohl T & Jenal U (2017) Second messenger-mediated tactile response by a bacterial rotary motor. Science, 358, 531–4. [DOI] [PubMed] [Google Scholar]
- Jenal U, White J & Shapiro L (1994) Caulobacter flagellar function, but not assembly, requires FliL, a non-polarly localized membrane protein present in all cell types. Journal of Molecular Biology, 243, 227–44. [DOI] [PubMed] [Google Scholar]
- Johnson S, Deme JC, Furlong EJ, Caesar JJE, Chevance FFV, Hughes KT et al. (2024) Structural basis of directional switching by the bacterial flagellum. Nature Microbiology, 9, 1282–92. [DOI] [PubMed] [Google Scholar]
- Johnson S, Furlong EJ, Deme JC, Nord AL, Caesar JJE, Chevance FFV et al. (2021) Molecular structure of the intact bacterial flagellar basal body. Nature Microbiology, 6, 712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanbe M, Shibata S, Umino Y, Jenal U & Aizawa SI (2005) Protease susceptibility of the Caulobacter crescentus flagellar hook-basal body: a possible mechanism of flagellar ejection during cell differentiation. Microbiology, 151, 433–8. [DOI] [PubMed] [Google Scholar]
- Lee YY & Belas R (2015) Loss of FliL alters Proteus mirabilis surface sensing and temperature-dependent swarming. Journal of Bacteriology, 197, 159–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Roujeinikova A & Ottemann KM (2023) FliL functions in diverse microbes to negatively modulate motor output via its N-terminal region. MBio, 14, e0028323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter L, Hilmen M & Silverman M (1988) Flagellar dynamometer controls swarmer cell differentiation of V. parahaemolyticus. Cell, 54, 345–51. [DOI] [PubMed] [Google Scholar]
- Mengucci F, Dardis C, Mongiardini EJ, Althabegoiti MJ, Partridge JD, Kojima S et al. (2020) Characterization of FliL proteins in Bradyrhizobium diazoefficiens: lateral FliL supports swimming motility, and subpolar FliL modulates the lateral Flagellar system. Journal of Bacteriology, 202, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondino S, San Martin F & Buschiazzo A (2022) 3D cryo-EM imaging of bacterial flagella: novel structural and mechanistic insights into cell motility. The Journal of Biological Chemistry, 298, 102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstein RM, Szostek B & Rather PN (2010) Regulation of gene expression during swarmer cell differentiation in Proteus mirabilis. FEMS Microbiology Reviews, 34, 753–63. [DOI] [PubMed] [Google Scholar]
- Motaleb MA, Pitzer JE, Sultan SZ & Liu J (2011) A novel gene in-activation system reveals altered periplasmic flagellar orientation in a Borrelia burgdorferi fliL mutant. Journal of Bacteriology, 193, 3324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Bree AC, Liu J, Patrick JE, Chien P & Kearns DB (2015) Adaptor-mediated Lon proteolysis restricts Bacillus subtilis hyperflagellation. Proceedings of the National Academy of Sciences of the United States of America, 112, 250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okino H, Isomura M, Yamaguchi S, Magariyama Y, Kudo S & Aizawa SI (1989) Release of flagellar filament-hook-rod complex by a salmonella typhimurium mutant defective in the M ring of the basal body. Journal of Bacteriology, 171, 2075–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overhage J, Bains M, Brazas MD & Hancock RE (2008) Swarming of Pseudomonas aeruginosa is a complex adaptation leading to increased production of virulence factors and antibiotic resistance. Journal of Bacteriology, 190, 2671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge JD, Dufour Y, Hwang Y & Harshey RM (2023) Flagellar motor remodeling during swarming requires FliL. Molecular Microbiology, 120, 670–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge JD & Harshey RM (2013) Swarming: flexible roaming plans. Journal of Bacteriology, 195, 909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge JD, Nhu NTQ, Dufour YS & Harshey RM (2019) Escherichia coli remodels the chemotaxis pathway for swarming. MBio, 10, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge JD, Nhu NTQ, Dufour YS & Harshey RM (2020) Tumble suppression is a conserved feature of swarming motility. MBio, 11, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge JD, Nieto V & Harshey RM (2015) A new player at the flagellar motor: FliL controls both motor output and bias. MBio, 6, e02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson MM, Rasko DA, Smith SN & Mobley HL (2010) Transcriptome of swarming Proteus mirabilis. Infection and Immunity, 78, 2834–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter SC, Luciani A, Eddy SR, Park Y, Lopez R & Finn RD (2018) HMMER web server: 2018 update. Nucleic Acids Research, 46, W200–W204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiveri M, Roa-Eguiara A, Kuhne C, Wadhwa N, Hu H, Berg HC et al. (2020) Structure and function of stator units of the bacterial Flagellar motor. Cell, 183(244–257), e216. [DOI] [PubMed] [Google Scholar]
- Singh PK, Sharma P, Afanzar O, Goldfarb MH, Maklashina E, Eisenbach M et al. (2024) CryoEM structures reveal how the bacterial flagellum rotates and switches direction. Nature Microbiology, 1271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobe RC, Gilbert C, Vo L, Alexandre G & Scharf BE (2022) FliL and its paralog MotF have distinct roles in the stator activity of the Sinorhizobium meliloti flagellar motor. Molecular Microbiology, 118, 223–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suaste-Olmos F, Domenzain C, Mireles-Rodriguez JC, Poggio S, Osorio A, Dreyfus G et al. (2010) The flagellar protein FliL is essential for swimming in Rhodobacter sphaeroides. Journal of Bacteriology, 192, 6230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S & Kearns DB (2019) Functional regulators of bacterial flagella. Annual Review of Microbiology, 73, 225–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachiyama S, Chan KL, Liu X, Hathroubi S, Peterson B, Khan MF et al. (2022) The flagellar motor protein FliL forms a scaffold of circumferentially positioned rings required for stator activation. Proceedings of the National Academy of Sciences of the United States of America, 119, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekawa N, Imada K & Homma M (2020) Structure and energy-conversion mechanism of the bacterial Na(+)-driven Flagellar motor. Trends in Microbiology, 28, 719–31. [DOI] [PubMed] [Google Scholar]
- Tan J, Zhang L, Zhou X, Han S, Zhou Y & Zhu Y (2024) Structural basis of the bacterial flagellar motor rotational switching. bioRxiv: 2024.2004.2030.591856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Zhang X, Wang X, Xu C, Chang S, Wu H et al. (2021) Structural basis of assembly and torque transmission of the bacterial flagellar motor. Cell, 184, e2619. [DOI] [PubMed] [Google Scholar]
- Toutain CM, Zegans ME & O’Toole GA (2005) Evidence for two flagellar stators and their role in the motility of Pseudomonas aeruginosa. Journal of Bacteriology, 187, 771–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Mariconda S, Suzuki A, McClelland M & Harshey RM (2006) Uncovering a large set of genes that affect surface motility in Salmonella enterica serovar Typhimurium. Journal of Bacteriology, 188, 7981–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel C, Hu J, Riethmacher D, Benckendorff A, Harder L, Eilers A et al. (2007) A stomatin-domain protein essential for touch sensation in the mouse. Nature, 445, 206–9. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wu Z, Zhang R & Yuan J (2022) FliL differentially interacts with two stator systems to regulate Flagellar motor output in Pseudomonas aeruginosa. Applied and Environmental Microbiology, 88, e0153922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Kumar A, Kojima S & Homma M (2015) FliL associates with the stator to support torque generation of the sodium-driven polar flagellar motor of vibrio. Molecular Microbiology, 98, 101–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


