Abstract
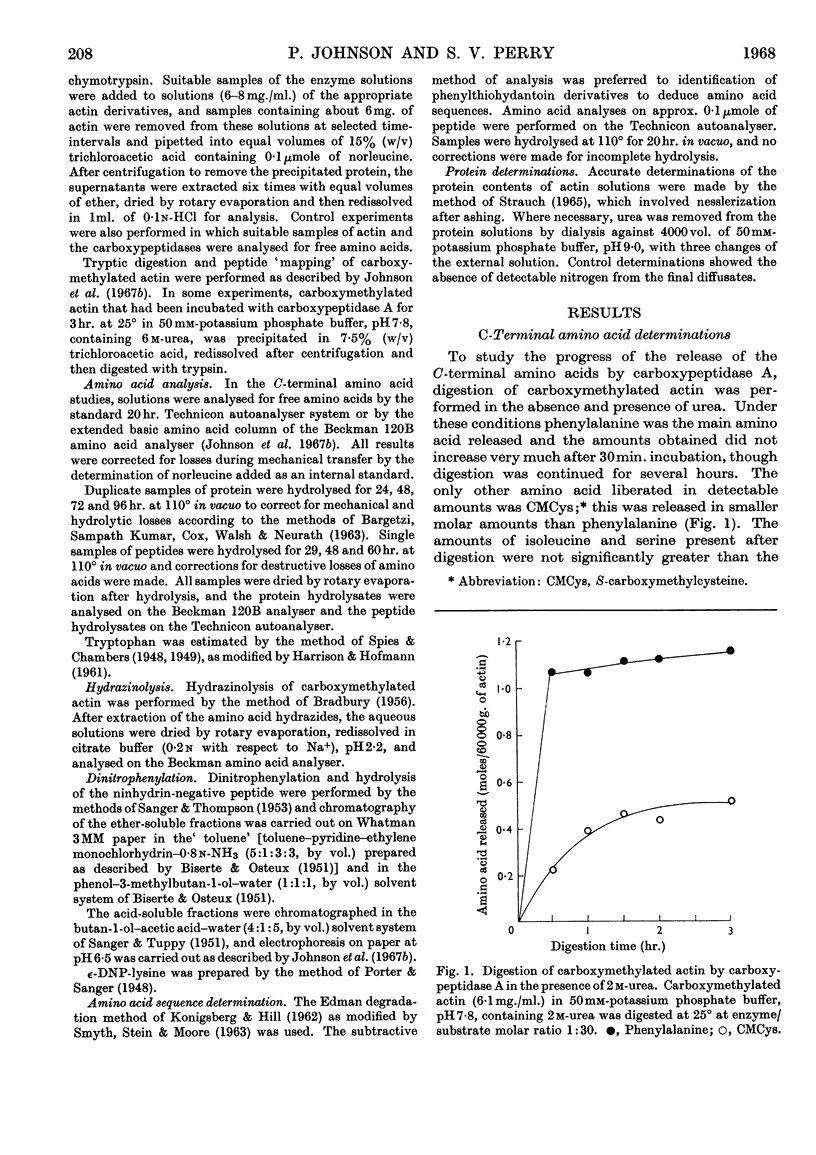
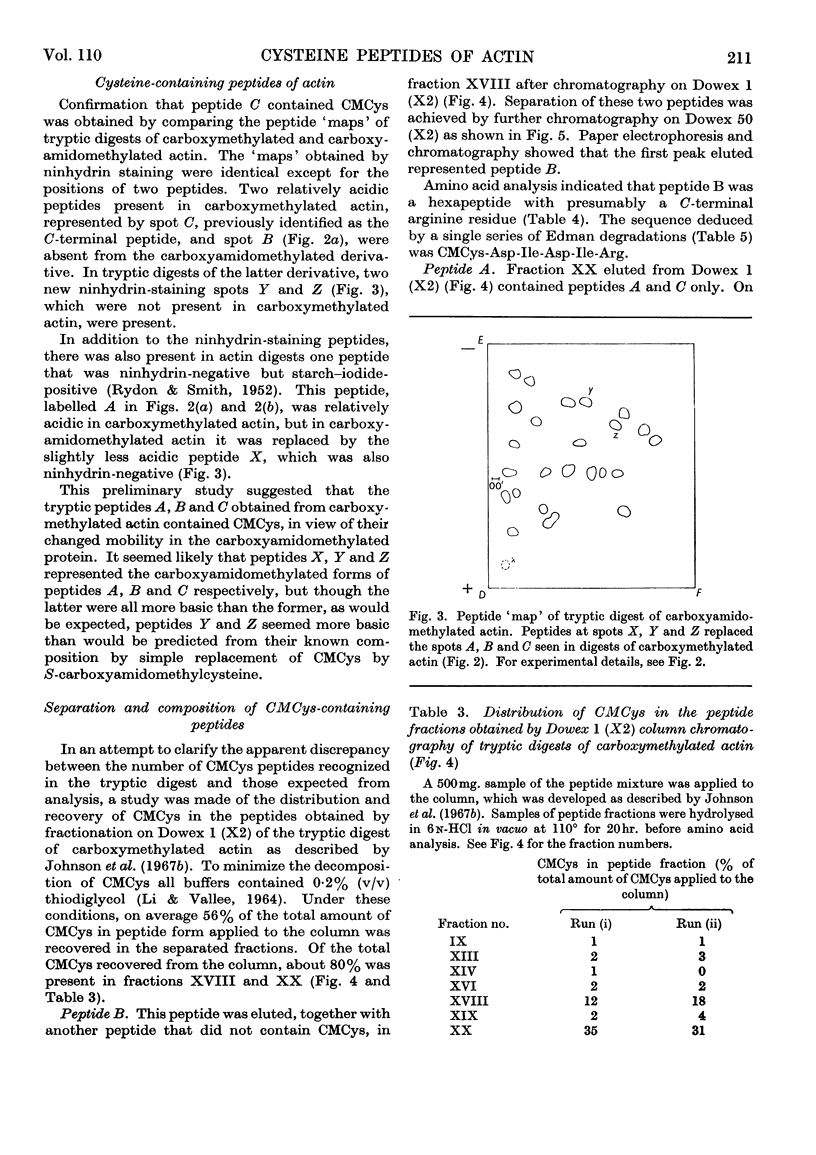
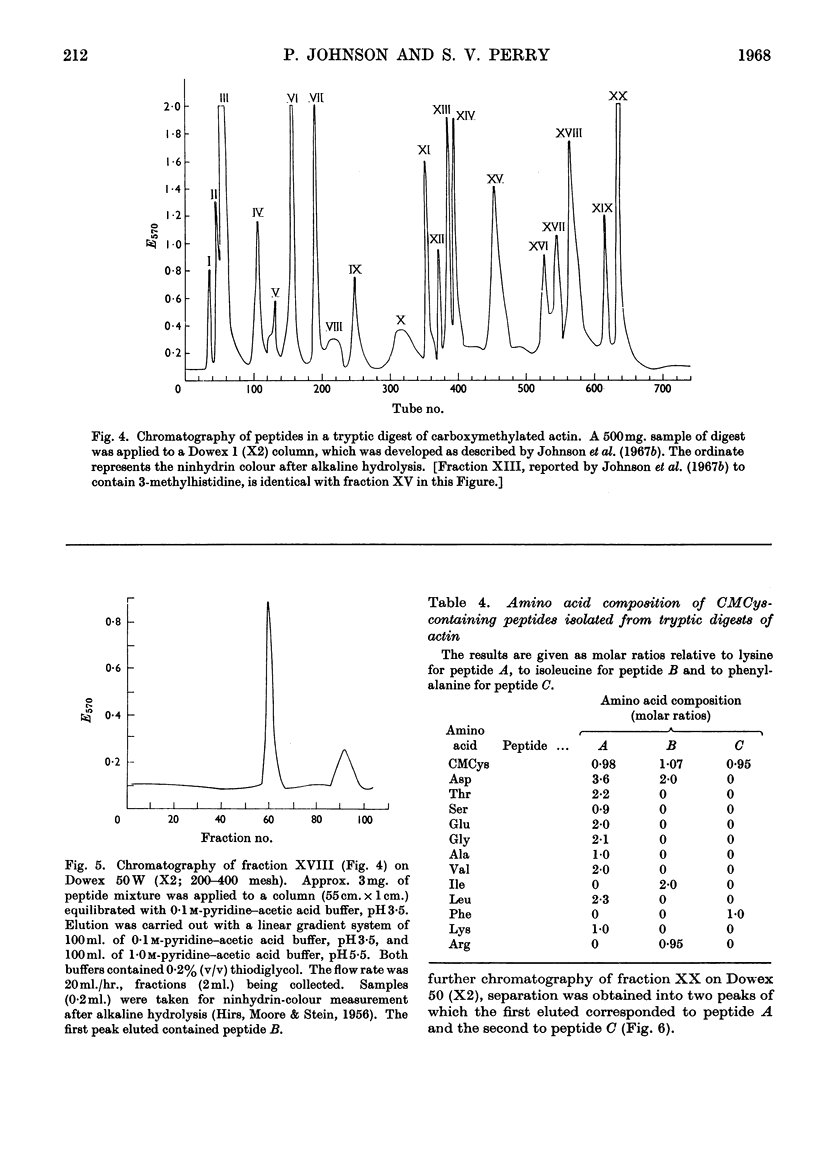
1. On exhaustive digestion of carboxymethylated actin in 6m-urea solutions with carboxypeptidase A, 1 mole of phenylalanine was liberated/43000g. of protein. At a lower urea concentration and in the absence of urea, carboxymethyl-cysteine (CMCys) was also liberated. 2. Three cysteine-containing peptides were identified by the study of peptide `maps' of tryptic digests of actin treated with thiol reagents. 3. The three peptides, each containing one residue of CMCys, were isolated from tryptic digests of carboxymethylated actin by ion-exchange chromatography. 4. One of these peptides was possibly the N-terminal peptide and contained about 17–18 residues; another was CMCys-Asp-Ile-Asp-Ile-Arg; the other, CMCys-Phe, was the C-terminal tryptic peptide. 5. The chemical evidence suggests that the actin molecule consists of a single polypeptide chain of molecular weight about 44000.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asatoor A. M., Armstrong M. D. 3-methylhistidine, a component of actin. Biochem Biophys Res Commun. 1967 Jan 23;26(2):168–174. doi: 10.1016/0006-291x(67)90229-x. [DOI] [PubMed] [Google Scholar]
- BARANY M. Studies on the actin-actin bonding. Biochim Biophys Acta. 1956 Mar;19(3):560–562. doi: 10.1016/0006-3002(56)90488-7. [DOI] [PubMed] [Google Scholar]
- BARGETZI J. P. KUMAR KS, COX DJ, WALSH KA, NEURATH H: THE AMINO ACID COMPOSITION OF BOVINE PANCREATIC CARBOXYPEPTIDASE A. Biochemistry. 1963 Nov-Dec;2:1468–1474. doi: 10.1021/bi00906a046. [DOI] [PubMed] [Google Scholar]
- Bailin G., Bárány M. Studies on actin-actin and actin-myosin interaction. Biochim Biophys Acta. 1967 Jun 27;140(2):208–221. doi: 10.1016/0005-2795(67)90461-8. [DOI] [PubMed] [Google Scholar]
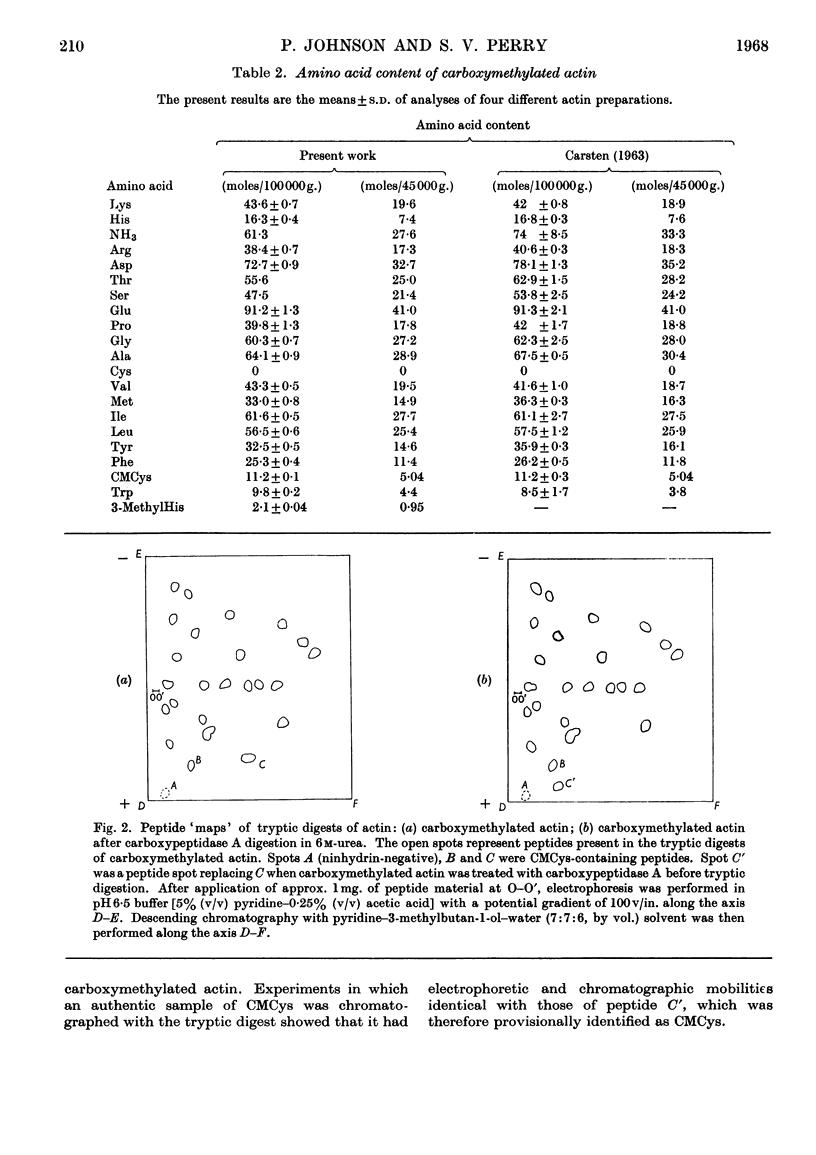
- CARSTEN M. E. Actin, its amino acid composition and its reaction with iodoacetate. Biochemistry. 1963 Jan-Feb;2:32–34. doi: 10.1021/bi00901a007. [DOI] [PubMed] [Google Scholar]
- CARSTEN M. E., KATZ A. M. ACTIN: A COMPARATIVE STUDY. Biochim Biophys Acta. 1964 Sep 4;90:534–541. doi: 10.1016/0304-4165(64)90232-6. [DOI] [PubMed] [Google Scholar]
- Carsten M. E. Actin. Its thiol groups. Biochemistry. 1966 Jan;5(1):297–300. doi: 10.1021/bi00865a038. [DOI] [PubMed] [Google Scholar]
- GUIDOTTI G. The action of carboxypeptidases A and B on the separated alpha and beta chains of normal adult human hemoglobin. Biochim Biophys Acta. 1960 Jul 29;42:177–179. doi: 10.1016/0006-3002(60)90774-5. [DOI] [PubMed] [Google Scholar]
- Gaetjens E., Bárány M. N-acetylaspartic acid in G-actin. Biochim Biophys Acta. 1966 Mar 28;117(1):176–183. doi: 10.1016/0304-4165(66)90164-4. [DOI] [PubMed] [Google Scholar]
- HALSEY Y. D., NEURATH H. The terminal carboxyl groups of denatured yeast triosephosphate dehydrogenase. J Biol Chem. 1955 Nov;217(1):247–252. [PubMed] [Google Scholar]
- HIRS C. H., MOORE S., STEIN W. H. Peptides obtained by tryptic hydrolysis of performic acid-oxidized ribonuclease. J Biol Chem. 1956 Apr;219(2):623–642. [PubMed] [Google Scholar]
- Johnson P., Harris C. I., Perry S. V. 3-methylhistidine in actin and other muscle proteins. Biochem J. 1967 Oct;105(1):361–370. doi: 10.1042/bj1050361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ A. M., MOMMAERTS W. F. The sulfhydryl groups of actin. Biochim Biophys Acta. 1962 Nov 19;65:82–92. doi: 10.1016/0006-3002(62)90151-8. [DOI] [PubMed] [Google Scholar]
- KATZ A. M. The influence of cations on the reactivity of the sulfhydryl groups of actin. Biochim Biophys Acta. 1963 May 14;71:397–407. doi: 10.1016/0006-3002(63)91094-1. [DOI] [PubMed] [Google Scholar]
- KUSCHINSKY G., TURBA F. Uber die Rolle der SH-Gruppen bei Vorgängen am Aktomyosin, Myosin und Aktin. Biochim Biophys Acta. 1951 Jan;6(3):426–433. doi: 10.1016/0006-3002(50)90114-4. [DOI] [PubMed] [Google Scholar]
- LAKI K., STANDAERT J. The minimal molecular weight of actin estimated with the use of carboxypeptidase A. Arch Biochem Biophys. 1960 Jan;86:16–18. doi: 10.1016/0003-9861(60)90360-x. [DOI] [PubMed] [Google Scholar]
- LI T. K., VALLEE B. L. ACTIVE-CENTER PEPTIDES OF LIVER-ALCOHOL DEHYDROGENASE. I. THE SEQUENCE SURROUNDING THE ACTIVE CYSTEINYL RESIDUES. Biochemistry. 1964 Jun;3:869–873. doi: 10.1021/bi00894a025. [DOI] [PubMed] [Google Scholar]
- LOCKER R. H. C-Terminal groups in myosin, tropomyosin and actin. Biochim Biophys Acta. 1954 Aug;14(4):533–542. doi: 10.1016/0006-3002(54)90233-4. [DOI] [PubMed] [Google Scholar]
- MARTONOSI A., GOUVEA M. A. Studies on actin. VI. The interaction of nucleoside triphosphates with actin. J Biol Chem. 1961 May;236:1345–1352. [PubMed] [Google Scholar]
- MIHASHI K. MOLECULAR CHARACTERISTICS OF G-ADP ACTIN. Arch Biochem Biophys. 1964 Sep;107:441–448. doi: 10.1016/0003-9861(64)90300-5. [DOI] [PubMed] [Google Scholar]
- MIHASHI K., OOI T. LOCATION OF ABNORMAL TYROSINES IN ACTIN. Biochemistry. 1965 May;4:805–813. doi: 10.1021/bi00881a003. [DOI] [PubMed] [Google Scholar]
- Martonosi A. The sulfhydryl groups of actin. Arch Biochem Biophys. 1968 Jan;123(1):29–40. doi: 10.1016/0003-9861(68)90100-8. [DOI] [PubMed] [Google Scholar]
- POGLAZOV B. F., BAEV A. A. Role of sulfhydryl groups in polymerization of actin. Biokhimiia. 1961 Nov-Dec;26:475–479. [PubMed] [Google Scholar]
- Perry S. V., Cotterill J. The action of thiol inhibitors on the interaction of F-actin and heavy meromyosin. Biochem J. 1964 Sep;92(3):603–608. doi: 10.1042/bj0920603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. R., Sanger F. The free amino groups of haemoglobins. Biochem J. 1948;42(2):287–294. doi: 10.1042/bj0420287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGER F., THOMPSON E. O. P. The amino-acid sequence in the glycyl chain of insulin. I. The identification of lower peptides from partial hydrolysates. Biochem J. 1953 Feb;53(3):353–366. doi: 10.1042/bj0530353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGER F., TUPPY H. The amino-acid sequence in the phenylalanyl chain of insulin. I. The identification of lower peptides from partial hydrolysates. Biochem J. 1951 Sep;49(4):463–481. doi: 10.1042/bj0490463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STROHMAN R. C., SAMORODIN A. J. The requirements for adenosine triphosphate binding to globular actin. J Biol Chem. 1962 Feb;237:363–370. [PubMed] [Google Scholar]
- TIETZE F., GLADNER J. A., FOLK J. E. Release of C-terminal S-(beta-aminoethyl)-cysteine residues by carboxypeptidase-B. Biochim Biophys Acta. 1957 Dec;26(3):659–659. doi: 10.1016/0006-3002(57)90125-7. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Hartley B. S. Selective purification of the thiol peptides of myosin. Biochem J. 1968 Apr;107(4):531–548. doi: 10.1042/bj1070531. [DOI] [PMC free article] [PubMed] [Google Scholar]


