Abstract
CDK4/6 inhibitors (CDK4/6i) have significantly impacted on the treatment of HR + HER2 negative (HER2-) metastatic breast cancer (BC) when combined with endocrine therapy. Nonetheless, despite significant research efforts, the mechanisms of de novo and acquired resistance to CDK4/6i have not yet been fully elucidated, highlighting the need for a deeper understanding of these process. Additionally, the importance of dissecting CDK4/6i resistance from endocrine resistance for personalized treatment is increasingly recognized.
Liquid biopsy has emerged as a minimally invasive tool for identifying circulating biomarkers of resistance through the integration of multiparametric and dynamic assessments that encompass ctDNA, CTCs, exosomes, and epigenetic ctDNA alterations, representing a promising perspective for the clinical characterization of treatment resistance and guiding post-progression strategies to improve patient outcomes.
Aim of this review is summarize potential mechanisms of CDK4/6i resistance, along with the advantages of using liquid biopsy to identify resistance biomarkers in HR+/HER2- MBC patients treated with CDK 4/6 inhibitors.
Keywords: Metastatic breast cancer, CDK 4/6 inhibitors resistance, Liquid biopsy
Highlights
-
•
In MONALEESA-2, -3, -7 and PALOMA-3, copy number loss and/or loss of heterozygosity of RB1 detected in baseline ctDNA samples were associated with poorer PFS.
-
•
In PALOMA-3, CCNE1 gain detected through ctDNA was significantly associated with shorter PFS in the palbociclib plus fulvestrant arm but not in the placebo arm.
-
•
Role of ESR1 mutations is unclear in CDK4/6i resistance. Several trials showed benefit from CDK4/6i regardless the mutational status.
-
•
Preclinical data demonstrated that combining CDK4/6i with anti-PDL1 maymodulate immunity and overcome CDK4/6i resistance.
1. Introduction
An essential feature of cancer cells is their ability to proliferate in a sustained and uncontrolled way, a feature often achieved by altering the cell cycle control mechanisms. One of the critical checkpoints of this system is the passage from the G1 to the S phase, and in this context, the cyclin D-CDK4/6-INK4-RB axis is a key regulator [1]. Many mitogenic pathways converge on cyclin D up-regulation including steroid hormones, PI3K/AKT/mTOR, MAPKs, wnt/β-catenin, STATs, and NF-kB/IKK [2]. Activated by these pathways, D-type cyclins associate with CDK4 or CDK6 forming the active cyclin D-CDK4/6 complexes that phosphorylate the retinoblastoma protein (pRb), thus promoting the dissociation of the transcriptionally repressive pRb-E2F complex. Consequently, the E2F transcription factors, dissociated from pRb, are free to activate genes required for entry into the S phase and DNA replication [3]. This review aims to outline CDK4/6 inhibitor resistance mechanisms and how liquid biopsy may assist in detecting and bridging them to the clinical setting.
Literature research strategies and criteria are reported in the supplementary section.
1.1. CDK4/6i inhibitors: opportunities and current needs
The CDK4/6 pathway is often deregulated in hormone receptor positive (HR+) breast cancer (BC) and inhibitors of this pathway (CDK4/6i) appear as an ideal treatment in this setting [4]. CDK4/6i (i.e., palbociclib, ribociclib, and abemaciclib) have revolutionized the treatment of HR + HER2 negative (HER2-) metastatic BC (MBC). When combined with endocrine therapy (ET) in first-line or second-line regimens, CDK4/6i have demonstrated to significantly prolong progression free survival (PFS) and, in some instances, also overall survival (OS) [5].
Resistance still represents a major hindrance: up to 15 % of patients receiving CDK4/6i with aromatase inhibitor (AI) and about 30 % of those receiving CDK4/6i plus fulvestrant, experience disease progression shortly after treatment initiation (i.e., primary resistance). Moreover, primary or secondary resistance ultimately occurs in nearly all patients with MBC with a median PFS (mPFS) in first-line metastatic setting ranging from 23 to 28 months. A deeper understanding of the potential mechanisms of CDK4/6i resistance is therefore crucial for treatment personalization, treatment efficacy prediction and design of new post-progression strategies. This has taken on further relevance due to the recent registration of abemaciclib in the adjuvant setting for high-risk, node-positive early breast cancer [6]. With this regard, liquid biopsy is emerging as a useful tool for the detection of circulating biomarkers in a minimally invasive manner, overcoming the spatial and temporal limitations of tissue biopsies. In this review, we summarize the mechanisms of resistance to CDK4/6i taking advantages from landmark preclinical and clinical studies published up to October 2023, highlighting potential biomarkers that can be exploited to guide treatment in HR+/HER2- MBC patients, with a focus on the advantages of using liquid biopsy in identifying biomarker of resistance.
1.2. Liquid biopsy main technologies and clinical applications
The potential of liquid biopsy lies in the analysis of genetic material released by tumor cells, encompassing circulating tumor DNA (ctDNA, a fraction of cell-free DNA), microRNAs, non-coding RNA, circulating tumor cells (CTCs) and microvesicles such as exosomes. Particularly, ctDNA and CTCs currently stand as the most investigated biomarkers in both preclinical and clinical phases [7].
From a technical standpoint, targeted and non-targeted analysis are the two primary approaches regarding ctDNA. The former concerns PCR-based methods, including droplet digital PCR [8,9], real-time quantitative PCR (qPCR) and the BEAMing technique (Beads, Emulsion, Amplifying, and Magnetics) [10], ensuring high sensitivity but at the cost of monitoring only a few known mutations "a priori".
Conversely, the quest for unknown alterations can be pursued using Next Generation Sequencing (NGS) methods through genome-wide analysis, such as Whole Genome Sequencing (WGS) and Whole Exome Sequencing (WES) approaches. Nonetheless, these methods may exhibit limitations due to reduced sensitivity and the necessity for elevated concentrations of ctDNA [11].
Similarly, diverse are the techniques for analyzing Circulating Tumor Cells (CTCs) with prominent methods including CellSearch, a technology based on the epithelial marker EpCAM expression on the surface of CTCs [12,13], and Adnatest (QIAGEN), founded on an immunomagnetic cell selection system [13].
From a strategic point of view, the investigation of both ctDNA and CTCs at multiple timepoints serves varied purposes with significant implications. Individual analyses conducted at baseline or during progression seek to uncover prognostic or predictive biomarkers crucial for tailoring effective treatment strategies. Moreover, sampling throughout therapy at designated timepoints offers a unique window into the dynamic landscape of tumor biology, shedding light on the evolving clonal and subclonal tumor alterations and thereby potentially guiding treatment adaptations.
2. Intrinsic mechanisms and biomarkers of resistance
2.1. Cell cycle pathway
2.1.1. Cyclins E1-2/CDK2
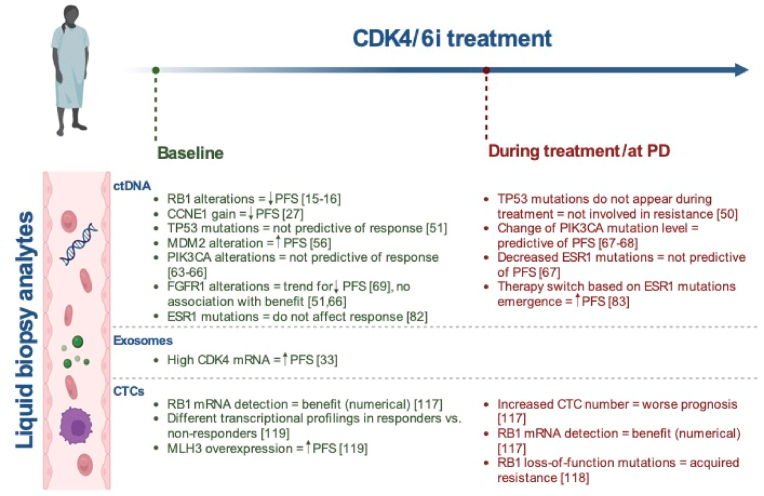
The cyclin E-CDK2 complex is another key player in the G1-S phase transition, and therefore a crucial resistance node, due to its role in pRb phosphorylation and in the regulation of E2F transcription factors [[14], [15], [16], [17]] (Fig. 1B). CCNE1 (encoding for cyclin E1) has been investigated as an intrinsic and acquired resistance candidate through amplification and/or overexpression [15,[17], [18], [19]]. Gene expression data from NeoPalAna showed an association between high expression of CCNE1 and resistance to neoadjuvant palbociclib plus anastrozole but not to anastrozole alone [20]. Consistently, gene expression of primary and metastatic tissue samples from 302 patients in the PALOMA-3 trial (Table 1) suggested that low CCNE1 mRNA expression was associated with better response to palbociclib [21]. In the palbociclib plus fulvestrant vs placebo plus fulvestrant arm, mPFS for patients with high CCNE1 levels was respectively 7.6 months vs 4.0 months (HR 0.85, 95 % CI 0.58 to 1.26), while it was 14.1 vs 4.8 months (HR, 0.32; 95 % CI, 0.20 to 0.50) in the subgroup with low CCNE1, with a significant interaction between treatment effect and CCNE1 mRNA expression (unadjusted P = 0.00238; FDR P = 0.0238).Notably, CCNE1 mRNA had a prognostic role when measured in metastatic samples (p < 0.001), but not in the primary site (p = 0.09), suggesting how temporal changes in tumor biology requires to examine samples as updated as possible.
Fig. 1.
Potential mechanisms of resistance to CDK4/6 inhibition and associated drugs.
Table 1.
Main clinical trials on CDK4/6i in HR-positive/HER2-negative breast cancer investigating the role of circulating biomarkers.
| Trial | Phase | Treatments | Setting | Circulating biomarker | Timepoint | Assay |
|---|---|---|---|---|---|---|
| PALOMA-3 (NCT01942135) | III | Palbociclib + Fulvestrant vs Placebo + Fulvestrant |
Advanced BC II line |
ctDNA | -Baseline -Cycle 1 day 15 -Progression |
Droplet Digital PCR |
| TREND (NCT02549430) | II | Palbociclib vs Palbociclib + ET earlier line |
Advanced BC ≥ II line |
CTCs | -Baseline -After cycle 1 -Progression |
Cellsearch |
| MONARCH-2(NCT02107703) | III | Abemaciclib + Fulvestrant vs Placebo + Fulvestrant |
Advanced BC II line |
ctDNA | -Baseline | Droplet Digital PCR |
| MONARCH-3 (NCT02246621) | III | Abemaciclib + NSAI vs Placebo + NSAI |
Advanced BC I line |
ctDNA | -Baseline -Progression |
Next generation Sequencing |
| nextMONARCH (NCT02747004) | II | Abemaciclib + tamoxifen Vs Abemaciclib Vs Abemaciclib + prophylactic loperamide |
Advanced BC ≥1 line |
ctDNA | - Baseline - Progression |
Next generation Sequencing |
| MONALEESA-2 (NCT01958021) | III | Ribociclib + Letrozole vs Placebo + Letrozole |
Advanced BC I line |
ctDNA | -Baseline | Next generation Sequencing |
| MONALEESA-3 (NCT02422615) | III | Ribociclib + Fulvestrant vs Placebo + Fulvestrant |
Advanced BC I or II line |
ctDNA | -Baseline | Next generation Sequencing |
| MONALEESA-7 (NCT02278120) | III | Ribociclib + Goserelin + NSAI or Tamoxifen vs Placebo + Goserelin + NSAI or Tamoxifen |
Advanced BC I line |
ctDNA | -Baseline | Next generation Sequencing |
| PACE (NCT03147287) | II | Fulvestrant Vs Fulvestrant + Palbociclib Vs Fulvestrant + Palbociclib + Avelumab |
Advanced BC II line |
ctDNA, CTCs | -Baseline -Cycle 3 -Progression |
ctDNA - > NGS CTCs - > CellSearch |
| PADA-1 (NCT03079011) | III | Palbociclib + AI Vs Palbociclib + Fulvestrant |
Advanced BC I/II line |
ctDNA | -Baseline -After one cycle, and then once every two cycles |
Droplet Digital PCR |
| MAINTAIN (NCT02632045) | II | Ribociclib plus switch of ET Vs Placebo plus switch of ET |
Advanced BC II line |
ctDNA | -Baseline | Next generation Sequencing |
Abbreviations: ET, endocrine therapy; NSAI, non-steroidal aromatase inhibitor; BC, breast cancer; ctDNA, circulating tumor DNA; CTCs, circulating tumor cells.
The role of high CCNE1 mRNA was also confirmed in an independent validation in the POP trial, in which high CCNE1 mRNA levels were associated with a lower antiproliferative response to palbociclib [22]. Similarly, in the MONALEESA-2 trial (Table 1), high tumor CCNE1 mRNA baseline levels were significantly associated with worse prognosis [23]. On the other hand, no associations between expression levels of CCNE1 and PFS were found in MONALEESA-3 and PALOMA-2 [24,25] (Table 1).
In PALOMA-3 (Table 1), CCNE1 gain detected through ctDNA was significantly associated with shorter PFS in the palbociclib plus fulvestrant arm (HR 5.71, 95 % CI 2.30 to 14.21, log-rank Q < 0.001) but not in the placebo arm [26]. On the other hand, in a genomic analysis of tissue biopsies from 59 patients treated with CDK4/6i no CCNE1 amplification was found, probably due to its relatively low prevalence, the proximity of CCNE1's locus to the centromere, and other technical challenges related to the assessment of amplifications via whole exome sequencing (WES) [27]. However, in this same study CCNE2 amplification was identified in 14.6 % of resistant tumors and only in 5.6 % of the sensitive casas, suggesting a potential role for CCNE2 amplification as a biomarker of resistance.
Since both up-regulation of CCNE1 and down-regulation of RB1 play a key role in CDK4/6i resistance, the CCNE1/RB1 ratio was investigated in the NeoPalAna trial showing a better prognostic value compared to the two genes taken separately [28].
2.1.2. CDK4, CDK6 and D-type cyclins
Although CDK4 overexpression/amplification may play a crucial role in CDK4/6i resistance since it is a major target of this drug class, evidence is still conflicting [29,30] (Fig. 1C).
The p27 protein, a member of the CIP/KIP family, exerts a pivotal role in stabilizing the dimer of cyclin D/CDK4. Its activity status depends upon the phosphorylation of tyrosin Y74, Y88, Y89 and elevated phosphorylation of Y88, leading to increased CDK4 activity, has been associated with the overexpression of Brk (breast tumor-related kinase, an intracellular tyrosine kinase) in BC cells, correlating with heightened resistance to palbociclib [31].
Tissue transcriptional analysis in PALOMA-2 showed that higher CDK4 mRNA levels were associated with endocrine therapy (ET) resistance, but an additional benefit in the palbociclib arm was still observed [32]., An analysis of exosome-derived mRNA in patients with advanced BC treated with fulvestrant and palbociclib showed that higher baseline levels of CDK4 were significantly associated with longer PFS [33].
Similarly, the expression of CDK6 may represent another potential node for resistance together with its kinase-independent functions [34]. CDK6 up-regulates the transcription of p16 in the presence of STAT3 and cyclin D and up-regulates VEGF-A in concert with c-Jun, promoting angiogenesis, another crucial mechanism of cancer progression [35,36]. In vitro models of palbociclib resistance have shown that CDK6 over-expression is dependent from TGF-beta pathway suppression via miR-432-5p, with resistance being transferable across contiguous cell populations via exosomal expression of miR-432-5p [37]. Therefore, not only ctDNA but also circulating exosomes may be a promising liquid biopsy resistance biomarker.
New compounds capable of degrading CDK4 and 6 are being investigated both in vitro and in vivo models [38]. All studies emphasize the importance of CDK6 in the development of CDK4/6i resistance; nevertheless, in both PALOMA-3 (Table 1) and PALOMA-2, CDK6 mRNA expression was not linked with a distinct benefit from palbociclib [39,40].To date there is no evidence that testing for CDK6 alone might help selecting patients for CDK4/6i treatment in clinical practice.
2.1.3. CDK7
CDK7 is a CDK-activating kinase (CAK) that exerts two main functions.
-
⁃
It is part of a complex (together with Cyclin H and MAT1) that phosphorylates CDK1, CDK2, CDK4, and CDK6, promoting their activation and, in turn, mitogen signaling during the G1 phase (Fig. 1E);
-
⁃
it is an element of the transcription factor TFIIH, which is involved in DNA transcription and repair [41].
Previous in vitro studies, respectively based on kinome and genome-wide CRISPR-Cas9 knockout screenings, found that CDK7 could be a potential target in both sensitive and palbociclib-resistant BC cell lines [42,43]. CDK7 inhibitors have been developed and are being tested for their antitumor activity [44]. Interestingly, an early report of the CDK7 inhibitor samuraciclib demonstrated acceptable tolerability and signs of clinical activity in 31 patients with HR+/HER2- BC progressing on prior CDK4/6i [45]. Two phase I/II studies are currently underway to assess the combination of samuraciclib with fulvestrant (SUMIT-BC) or elacestrant (SUMIT-ELA) in patients previously treated with CDK 4/6 inhibitors [46,47].
2.2. P53 pathway
2.2.1. MDM2
Mouse double minute 2 homolog (MDM2) is one of the major inhibitors of p53 activity [48] (Fig. 1L). Up to 30 % of all BC, especially the luminal B-like subtype, overexpress MDM2, with a negative prognostic impact [49].
Preclinical data suggest a senescence pathway disruption in CDK4/6i-resistant BC cells and patient-derived xenograft (PDX) models and a promising activity of CGM097, an MDM2 inhibitor [50]. CGM097 was effective alone or in combination with fulvestrant highlighting its capability to induce cell senescence, thus emphasizing the importance of MDM2 in overcoming CDK4/6i resistance [50].
MDM2 down-regulation was, moreover, required for a stable CDK4/6i-induced senescent phenotype in cell lines and MDM2 reduction in biopsies during palbociclib was associated with treatment time [51].
Interestingly, in a biomarker analysis of the MONALEESA trials (Table 1), patients with ctDNA-detected MDM2 alterations at baseline had a trend for increased PFS benefit of ribociclib vs placebo compared to those in the wild-type group [52] (Fig. 2).
Fig. 2.
Synthesis of mechanisms of resistance to CDK 4/6 inhibitors identified through different circulating tumor entities (ctDNA, Exosomes, CTCs) by liquid biopsy methods. Abbreviations: ctDNA, circulating tumor DNA; CTCs, circulating tumor cells; PFS, Progression Free Survival.
An intriguing pooled analysis of the MONALEESA trials by Andre et al., suggested that FRS2 and MDM2, both situated on the same amplicon (12q15), exhibited co-amplification in 34 out of 1045 patients (3 %) [53]. While this amplification within the genomic region might potentially act as a surrogate marker for other amplifications, patients displaying the co-amplification of FRS2/MDM2 showcased a notably enhanced PFS benefit from ribociclib compared to the placebo (HR 0.23, 95 % CI 0.11–0.51, P 0.0255) [53].
3. Dynamic mechanisms and biomarkers of resistance
3.1. Cell cycle pathway
3.1.1. Retinoblastoma protein (pRb)
pRb, encoded by the RB1 gene, is the main target of CDK4/6 and represents a fundamental mediator of their inhibition (Fig. 1A). Several preclinical and clinical studies have identified its loss as a driver of both intrinsic and acquired resistance to CDK4/6i [15,17,19,[54], [55], [56]].
By leveraging the potential of gene expression characterization, a signature associated with functional inactivation of pRb, called RBsig, was developed by analyzing 87 genes correlated with the expression of E2F1 and E2F2 [57]. Similarly to previous studies [57], quantification of RBsig in the neoMONARCH [58] and the NeoPalAna [15] showed that RBsig was significantly enriched in CDK4/6i intrinsically resistant tumors compared to sensitive. As a matter of fact, E2F overexpression can promote a complete bypass of CDK4/6 inhibition, suggesting how an expanded analysis of the Rb pathway could be useful in identifying CDK4/6i-resistant subpopulations (Fig. 1H).
In the PALOMA-3 trial (Table 1), when examining the frequency of copy number aberrations (CNAs) solely in patients with over 10 % circulating tumor fraction, due to technical challenges associated with samples having low circulating tumor fraction, copy number loss and/or loss of heterozygosity of RB1 were detected in 17.3 % of baseline circulating tumor DNA (ctDNA) samples through a ddPCR assay and were associated with poorer PFS in patients treated with palbociclib plus fulvestrant, suggesting a potential prognostic and/or predictive role for RB1 [59] (Fig. 2). Similar results were highlighted in a combined analysis of the MONALEESA-2, -3, and -7 trials (Table 1) where patients with RB1 alterations (29/1045; 3 %) had lower PFS benefit from ribociclib, with a median PFS of 3.8 vs 9.2 (HR 1.48, 95 % CI 0.65–3.38) compared with that of 18.9 vs 11.1 for those with WT RB1 (HR 0.56, 95 % CI 0.47–0.66) [53].
An analysis of genomic alterations acquired from ctDNA in the MONARCH 3 and nextMONARCH trials (Table 1) revealed a higher frequency of RB1 alterations in the abemaciclib arm, accounting for 6 % and 9 %, respectively [60]. Interestingly, in the young-PEARL study, RB1 loss was detected in 4 % of premenopausal women treated with palbociclib plus exemestane and GNRH agonist, and it was associated with a shorter PFS (log2 HR = 2.26, 95 % CI 0.51 to 4.01, P = 0.011) [61].
In addition, a recent study that examined tissue and liquid biopsy profiling as a part of routine clinical care, showed elevated levels of ESR1 and RB1 in MBC patients treated with a combination of endocrine therapy and CDK 4/6i [62]. Of note, when the association of acquired alterations with treatment patterns was assessed in a series of 196 patients with longitudinal genomic tests, acquired alterations in ESR1 and in RB1 were observed in 20.3 % and in 3.5 %, respectively [62].
3.2. p53 pathway
3.2.1. TP53
TP53 is a tumor suppressor gene mutated in nearly 30 % of CDK4/6i resistant MBC [63] (Fig. 1F). It is unclear, however, whether TP53 mutations alone are sufficient to confer resistance to these agents. Despite a significant enrichment in TP53 mutations in clinical specimens obtained from CDK4/6i-resistant patients, both wild-type and mutated cells demonstrated CDK4/6i sensitivity in vitro [27]. In a cohort of 447 patients with HR + MBC treated with first-line CDK 4/6i, loss of p53 was strongly correlated with a reduced long-term respons. Interestingly, p53 loss appeared to be mechanistically linked with a persistent phosphorylation of the p130 RB1-like protein, thereby promoting reentry into the cell cycle [64].
A ctDNA analysis in patients treated with palbociclib highlighted that TP53 mutations detected at progression were already present at baseline, suggesting that TP53 may not be involved in acquired CDK4/6i resistance [65]. Moreover, a ctDNA biomarker analysis of MONALEESA-2 and -3 (Table 1), showed that alterations of TP53 were not found to be predictive of response to ribociclib and benefit was observed regardless of TP53 status [66] (Fig. 2). In an analysis aimed at assessing the association between baseline ctDNA gene alterations and the response to ribociclib in the MONALEESA-7 trial, a comparable PFS benefit of ribociclib was observed in patients with wild-type TP53 (HR 0.48, 95 % CI 0.36 to 0.65) and those with altered TP53 (HR 0.47, 95 % CI, 0.27 to 0.82, P = 0.98) [67]. Although TP53 alterations were not predictive of the response to ribociclib, patients with altered TP53 exhibited a numerically shorter median PFS (9.2 vs. 7.2 months for ribociclib vs. placebo, respectively) compared to those with unaltered TP53 in both treatment arms (24.7 vs. 12.9 months, respectively) [67]. Similarly, in the PALOMA-3 trial, TP53 mutations were associated with worse PFS in both palbociclib plus fulvestrant and placebo plus fulvestrant arms (respectively HR = 2.00 and HR = 2.26) [68]. Overall, these studies suggest that TP53 alterations are associated with higher risk of early progression, representing a prognostic rather than a predictive biomarker for CDK4/6i.
3.3. Aurora Kinase A
Aurora Kinase A (AURKA) is a cell cycle serine/threonine kinase that is involved in centrosome duplication, maturation and separation, mitotic spindle formation and assembly, chromosomal alignment, and cytokinesis [69] (Fig. 1G). AURKA amplification has been reported in 27 % of CDK4/6i resistant MBC, implying that AURKA could be a potential molecular target for overcoming CDK4/6i resistance [27]. Consistently, it has been shown that RB1 loss-of-function mutations or palbociclib-resistance cell lines with reduced RB1 expression were sensitive to LY3295668, a highly specific AURKA inhibitor, similarly to what has been observed in AURKB hyper-reliant small cell lung cancer [70,71].
Pan selective Aurora kinase inhibitors as well as Aurora-A and Aurora-B selective inhibitors have been developed but, despite numerous ongoing trials, none of them has been yet approved in clinical practice [72].
3.4. PI3K/AKT/mTOR pathway
The PI3K/AKT/mTOR plays a major role in essential cellular activities. It is frequently hyperactivated in approximately 40 % of HR + BC, exerting a central role in resistance to endocrine therapy and, potentially, to CDK4/6i [73].
The up-regulation of the PI3K/mTOR/AKT pathway induces the up-regulation of cyclin D which, in turn, activates CDK2 thus driving cell progression [73]. Exposure to PI3K inhibitors (PI3Ki) could lead to reduced expression of cyclin D1, preventing early adaptions to CDK4/6i making the combination an interesting strategy [73].
An exploratory ctDNA analysis of the SOLAR-1 trial showed that in patients previously exposed to CDK4/6i, the impact on PFS of alpelisib (α-specific PI3Ki) was greater with respect to CDK4/6i naïve patients, suggesting a rationale for PI3Ki after progression to CDK4/6i [74].
In a recent exploratory analysis of the PALOMA-3 (Table 1), PIK3CA mutations were detected through ctDNA in 55 patients (17 %) at day 1 and in 52 patients (16 %) at the end of treatment. Regardless of PIK3CA status, the addition of palbociclib to fulvestrant provided a similar PFS and OS benefit compared to placebo, suggesting that PIK3CA alterations are not predictive of CDK4/6i benefit [75]. Similar results were highlighted in MONALEESA-3, MONALEESA-2, and MONARCH-2 (Table 1) [[76], [77], [78]]. Nevertheless, a longitudinal ctDNA analysis of PALOMA-3 (Table 1) showed that changes in PIK3CA ctDNA levels after 15 days of treatment were predictive of PFS in patients treated palbociclib and fulvestrant (HR 3.94, p = 0.0013), thus representing a potential biomarker of response, although the impact of overall tumor burden may not be excluded [79,80] (Fig. 2).
Alterations of other elements of the PI3K/AKT/mTOR pathway implicated in resistance to CDK4/6i may be detected through liquid biopsy, such as AKT1 and PTEN [26,81]. Beyond their role in resistance to CDK4/6, PTEN and AKT status may guide post-progression therapies (e.g., capivasertib) [82].
3.5. RTK/RAS pathway
Fibroblast growth factors (FGFs) transmit their signal through FGF receptors (FGFRs), thus regulating cellular proliferation, differentiation, and survival (Fig. 1N). Two FGFR2 mutations, N550K and M538I, have been shown to induce resistance to fulvestrant, palbociclib and the combinations of the two agents in ER + cells [83]. In endocrine-resistant BC cells, FGFR1 overexpression and amplification resulted in the increased activation of PI3K/AKT and RAS/MEK/ERK signaling pathways in response to FGF2. Moreover, the aberrant expression of FGFR1 exhibited constitutive ligand-independent signaling, as demonstrated in prior studies [84].
A ctDNA analysis performed in patients treated with palbociclib plus ET investigated the role of FGFR alterations on CDK4/6i resistance. FGFR alterations were found in 14 out of 34 baseline plasma specimens, primarily FGFR1 amplification (n = 9), FGFR2 amplification (n = 2), and FGFR1/2 activating mutations (n = 3) [85]. Moreover, FGFR1 alterations on ctDNA were reported in 20 of 427 patients (5 %) in the MONALEESA-2 trial (Table 1), with a trend toward a worse PFS in these patients compared to those with FGFR1 wild-type (10.6 vs. 24.8 months, p = 0.075) [85] (Fig. 2). However, in MONALEESA-3 and -2 (Table 1) the benefit from ribociclib was observed irrespectively of baseline ctDNA FGFR1 alterations [66,78] (Fig. 2).
In HR + BC harboring aberrant FGFR signaling, the combination of FGFRi with CDK4/6i and ET is being actively investigated. In patients with FGFR pathway amplification a phase II trial explored the combination of fulvestrant with either dovitinib, a potentFGFR inhibitor, or placebo showing an improved PFS in the experimental arm (mPFS 10.9 vs 5.5 months) [86].
Lucitanib, another RTK inhibitor active against FGFR1-3 but also VEGFR1-3, PDGFRα and CSF1R, is being investigated in several preclinical and clinical studies with discordant results [85,[87], [88], [89]].
The prognostic and predictive potential of KRAS status on ctDNA has been demonstrated in a cohort of 106 patients treated with palbociclib and fulvestrant. This analysis revealed that KRAS alterations were significantly associated with a worse PFS, increased recurrence within 6 months (p < 0.0001), and resistance to the combination of palbociclib and fulvestrant (p = 0.001) [90].
EGFR also belongs to the RTK/RAS pathway. A study in model systems has shown that inhibition of CDK4/6 is associated with increased expression of EGFR, resulting in a down-regulation of estrogen receptor (ER) signaling. Confirming this, the addition of neratinib, a pan-ERRB inhibitor, reduced EGFR phosphorylation, resulting in a re-expression of E-regulated genes and enhanced sensitivity to fulvestrant [42].
3.6. Hormone receptors
3.6.1. Estrogen and progesterone receptor (ER and PR) expression
ER has a crucial interplay with cyclin D-CDK4/6 in BC. Preclinical studies have confirmed a loss of ER after CDK4/6i resistance, but how this may drive CDK4/6 inhibition escape it is still unclear [42,91] (Fig. 1O). Through ChIP sequencing, Pancholi et al. demonstrated a reduced binding of ER to the promoter regions of classical ER-dependent genes (TFF1, PDZK1, and CCND1), likely as a result of chromatin remodeling, coupled with a reduction in ER signaling. Moreover, although TFF1, PGR, GREB1, and c-MYC levels were reduced after long-term palbociclib treatment, this reduction was not seen when cells were treated with palbociclib and the tyrosine kinase inhibitor neratinib [42].
In clinical samples, Wander et al. showed that loss of ER expression by immunohistochemistry (IHC) was observed only in CDK4/6i resistant tumors [92]. However, sub-analyses from the pivotal clinical trials with CDK4/6i in patients with HR+/HER2- MBC, including PALOMA-2, -3, MONALEESA-2, MONARCH-2 and -3 (Table 1) failed to demonstrate any predictive role for ER nor PR [[93], [94], [95], [96], [97]].
3.6.2. ESR1 mutations
ESR1, the gene encoding the alpha isoform of ER, can be fused, amplified, or mutated in BC, implying endocrine resistance primarily due to AI exposure in the metastatic setting [98] (Fig. 1O). The role of ESR1 mutations in CDK4/6i resistance is still unclear. However, a recent study has demonstrated an increase in the IC50 of Palbociclib in MCF-7 cells harboring the ESR1 Y537S and D538G mutations. Additionally, RNA sequencing highlighted a significant upregulation of cell cycle-related gene signatures such as E2F targets, G2/M checkpoints, and the mitotic spindle, suggesting a potential primary role of ESR1 in resistance to CDK 4/6i [99].
Although being detected in 25 % of baseline plasma samples from the PALOMA-3 trial (Table 1), the addition of palbociclib improved PFS regardless of ESR1 mutational status suggesting the combination of palbociclib and fulvestrant as an active therapeutic option in this setting [100]. Consistently, ctDNA dynamics showed that a decrease in ESR1 ctDNA after two weeks of treatment was not predictive of PFS nor of CDK4/6i sensitivity [79] (Fig. 2). Similar results were reported in the MONALEESA-3 and MONARCH-2 (Table 1) [76,78]. Interestingly, in a study aimed at assessing a prospective cohort of patients treated with palbociclib plus fulvestrant, the baseline mutation of ESR1 did not demonstrate a significant impact on PFS. However, patients experiencing early disease progression exhibited a significant increase in ctDNA mutants at day 30 (p < 0.0001) [101].
In PADA-1 (Table 1), patients receiving first-line palbociclib plus AIs were randomized to continue treatment or switch to fulvestrant plus palbociclib, resulting in a doubled mPFS for the switching strategy, suggesting a potential clinical utility for such characterization [102] (Fig. 2).
Overall, ESR1 mutations do not appear as specific biomarkers of CDK4/6i resistance but more generally of ET resistance.
3.7. Transcriptional activity of activator protein-1 (AP-1)
AP-1 is a collective term referring to homodimeric/heterodimeric transcription factors composed by the Jun, Fos, ATF, and MAF sub-families and it has been suggested that their over-expression could lead to increased biologic aggressiveness, resulting in a tumorigenic, invasive, and hormone-resistant phenotype as observed in palbociclib resistant in vitro models [103]. Similarly, abemaciclib increased the expression of some AP-1 factor genes (for example JUN, JUNB, FOSL2), in luminal breast cancer cell lines through a RB-dependent manner and regions gaining AP-1 binding proved to be responsible for the expression of genes regulating mammary differentiation, apoptotic evasion and interferon responses [104]. Although it is still unclear how AP-1 overexpression causes CDK4/6i resistance, it has been hypothesized that ER suppression by c-Jun leads to a loss of ER dependence, making CDK4/6 inhibition ineffective. On the other hand, this resistance mechanism could be explained by overexpression of cyclin D1, which is transcribed by c-Jun [105].
There have been numerous attempts to find natural products and small molecules inhibiting AP-1, however to date only T-5224, a c-Fos/AP-1 inhibitor, has shown promising results and it is currently being evaluated in a phase II clinical trial [106].
3.8. Serum thimidine kinase 1
Thymidine kinase 1 (TK1) is a cytosolic enzyme intricately involved in DNA replication and cellular proliferation, finely regulated by the RB1-E2F axis, thereby emerging as a potential biomarker for assessing CDK 4/6i activity. Numerous investigations have explored the association between serum thymidine kinase 1 activity (sTKa) and the effectiveness of CDKi.
In the TRend trial, which included patients treated with palbociclib alone or palbociclib plus ET, the baseline sTKa levels showed no significant prognostic value. However, patients exhibiting an early increase in sTKa at one month experienced notably worse PFS outcomes compared to their counterparts [107]. In contrast, the ALCINA trial (patients with ER+/HER2- MBC treated with a CDK 4/6i and ET) revealed a notable association between baseline sTKa levels and PFS, but subsequent assessments of sTKa kinetics at four weeks, however, showed no discernible impact [108]. Additionally, the PYTHIA trial, conducted on post-menopausal women with HR-positive/HER2-receiving palbociclib and fulvestrant, demonstrated a substantial pre-treatment, day 15, and day 28 sTKa impact, also suggesting the potential of early dynamic changes of sTKa as an identifying factor for primary resistance to CDK 4/6 inhibitors [109]. The multi-center phase IIIb study, BioItaLEE, provided further evidence of the prognostic value of sTKa levels pre-treatment (HR 2.21), at C1D15 (HR = 2.62), and C2D1 (HR = 3.05) [110]. Interestingly, the assessment of early dynamic changes in sTKa levels proved pivotal in differentiating patient groups, with sustained sTKa inhibition leading to the most favorable PFS status (mPFS not estimable), while inadequate inhibition at C1D15 was associated with the worst outcomes (mPFS 10.1 months), highlighting the relevance of dynamic sTKa evaluation as a novel and informative biomarker for monitoring the risk of disease progression during CDK 4/6i therapy [110].
Complementary to traditional sTKa assessment, another study investigated TK1 mRNA levels in plasma-derived exosomes from HR + HER2- MBC patients receiving palbociclib and endocrine therapy. The study highlighted that an increase in TK1 mRNA levels at T1 relative to T0 was linked to disease progression [111].
3.9. Immune regulation
CDK4/6i clinical activity has been mainly attributed to the effect on cancer cell growth inhibition, but evidence of a modulation on the immune system has been steadily accumulated during the last years.
CDK4/6i treatment increased antigen presentation and upregulated the expression of genes of the interferon (IFN) signaling [58,112,113]. On the other hand, tumoral IFN signaling was associated with intrinsic and acquired resistance to CDK4/6i in vitro and in vivo and may contribute to immune escape and overall poor prognosis in BC [114,115]. In addition, following CDK4/6i treatment, tumors upregulated PD-L1 expression, which is known to block T cell activation [116,117]. Interestingly, a recent comparative biomarker analysis from PALOMA-2 and -3 trials (Table 1) showed an enrichment in the interferon-gamma response, PD1 and its pathway among the resistance genes and an association between a gene signature of T cell inflamed tumor microenvironment and shorter PFS in the palbociclib plus letrozole arm of PALOMA-2 [118].
In multiple tumor cell models CDK4/6i treatment increased the infiltration of CD4+ and CD8+ cytotoxic T cells, reduced the numbers and proliferation of the regulatory immune-suppressive T cells (Treg) and induced a higher frequency of CD8+ memory-like T cells, which might have important implications for the long-term protective anti-tumor immunity [[119], [120], [121], [122], [123], [124]]. The downregulation of Treg and an association with treatment response was recently shown in patients with HR+/HER2- MBC receiving the combination of CDK4/6i and ET suggesting that failure to modulate the immune system might contribute to resistance [125,126].
Effects on cytokines have also been described, including an increase in the pro-inflammatory chemokines CXCL9, CXCL10 and CCL5 and a reduction in IL6, IL10, and IL23 levels and coinhibitory molecules, such as PD-1 and CTLA4 [120].
Immunotherapy (IT) has recently changed the clinical management of many solid tumors, but it is not yet approved for the treatment of HR+/HER2- BC. Preclinical evidence demonstrated that the combination of CDK4/6i and anti-PDL1 was more effective than CDK4/6i alone, suggesting that modulation of the immune system might help overcoming resistance to CDK4/6i [117,119,127,128]. A recent report of a phase I/II trial exploring the combination of palbociclib, the anti-PD1 antibody pembrolizumab and letrozole in patients with HR+/HER2- MBC reported a rate of complete response of 31 % [129]. Other clinical trials are testing the safety and efficacy of combinations of CDK4/6i and IT with early data showning good clinical activity but also high toxicity, suggesting that the combination of CDK4/6i and IT should be explored in selected patients with higher likelihood of response and careful evaluation of the side effects [130].
The phase II study PACE (Table 1) investigated the activity of continuing CDK4/6i beyond progression, with a change in ET to fulvestrant and the addition of PD-L1 inhibition with avelumab. Although combining palbociclib with fulvestrant beyond progression did not significantly improve PFS, the addition of avelumab to fulvestrant and palbociclib resulted in a longer PFS, an intriguing signal that warrants further investigation [131].
3.10. Circulating tumor cells
Beyond ctDNA, additional circulating biomarkers can be detected by liquid biopsy, such as CTCs. Despite difficulties in detecting CTCs due to their low concentration in the blood, the prognostic value of CTCs enumeration in patients with MBC has been widely demonstrated [132].
Overall CTCs appear as a useful tool to dissect resistance to CDK4/6i, representing a real-time snapshot of the complexity of MBC. Molecular characterization of CTCs, beyond the mere enumeration, holds promise for identifying predictive biomarkers and monitoring treatment response.
In a translational analysis of the TREND trial (Table 1), CTCs were analyzed as a biomarker of resistance to palbociclib [133]. The presence of at least one CTC/7.5 mL of blood and an increase of at least three CTCs after the first cycle of palbociclib were associated with worse prognosis, suggesting that the dynamics of CTCs during treatment, rather than their count at treatment initiation, may be useful as biological readout identifying patients with early treatment resistance (Fig. 2). Furthermore, in the same study, the dynamic of RB1 gene expression during palbociclib treatment was assessed through CTCs mRNA characterization. Although no significant differences were observed, patients with detectable RB1 expression at any time-point appeared to have a numerical benefit from palbociclib than those with undetectable RB1 levels (mPFS 7.6 vs. 4.8 months, p = 0.49) [133] (Fig. 2). Another study found that RB1 loss-of-function mutations were associated with palbociclib resistance in the context of a global transcriptional transition from palbociclib sensitivity to resistance. Interestingly, in this study, patient-specific in vitro expanded CTC cell lines were generated to further investigate mechanisms of drug resistance and, using drug screening assays, have been identified potential drugs that in combination with palbociclib could overcome the acquired resistance [134].
An additional ongoing study is evaluating the transcriptional profiling of CTCs at baseline and during palbociclib. Initial results suggested that CDK2, WWTR1, and YAP1 over-expression were more common in non-responder patients while MLH1 and NFKB1 were more common in responder than non-responders. Moreover, baseline MLH3 over-expression was significantly associated with prolonged PFS (p = 0.047) [135] (Fig. 2).
3.11. Clinical utility of liquid biopsy in CDK4/6 inhibitor resistance: current insights and future prospects
Despite CDK 4/6i combined with ET being considered the current first-line standard of care for HR + HER2- MBC patients, the majority of patients experience disease progression between 12 and 36 months [136]. Consequently, several trials have sought to leverage the potential of liquid biopsy to define the optimal treatment approach.
In the phase II MANTAIN study (Table 1), the combination of ribociclib plus a switch of ET was compared to placebo plus a switch of ET in patients who had progressed on CDK 4/6i plus ET [137]. The CDK 4/6i beyond progression (BP) strategy demonstrated a statistically significant impact on PFS (median 5.29 months vs. 2.76 months, HR 0.57). However, no benefit was observed in patients with ESR1 mutations in baseline ctDNA. Nonetheless, the limited number of patients with ESR1 mutations (n = 33) and the co-occurrence of CCND1 and FGFR1 alterations in this subgroup may have influenced the results.
Conversely, another analysis assessing the impact of CDK 4/6 BP strategy revealed significantly higher benefit from the presence of ESR1 mutations, suggesting that, in cases of prominent endocrine resistance, switching ET may prolong the efficacy of CDK 4/6i [138]. This was evident in the PADA-1 trial, where patients who switched from letrozole to fulvestrant upon detection of ESR1 mutations in plasma before clinical progression experienced significantly longer median PFS (HR = 0.004) [102]. These findings indicate that the effectiveness of a selective estrogen receptor degrader (SERD) can be maximized when an ESR1 mutation is identified, capitalizing on a reduced burden of resistant tumor cells [102].
A similar strategy is currently being evaluated in the SERENA-6 trial, analyzing the benefit of switching to camizestrant in patients with detectable ESR1 mutations during CDK4/6i plus ET treatment [139].
The PACE study assessed the activity of Palbociclib BP with ET change to fulvestrant and explored the addition of avelumab. In contrast to MANTAIN, no benefit was demonstrated in continuing palbociclib in terms of PFS or OS [140]. However, baseline ctDNA analyses suggested a trend of benefit from the combination of fulvestrant plus palbociclib or fulvestrant plus palbociclib plus avelumab in the ESR1-mutated or PIK3CA-mutated population [140]. Furthermore, in a recent exploratory analysis of ctDNA dynamics, the most common mutations identified at baseline were ESR1 (57 %), TP53 (38 %), PIK3CA (36 %), GATA3 (20 %), ATM (12 %), and RB1 (12 %). Correspondingly, at C3D1, the most prevalent mutations observed were ESR1 (47 %), TP53 (33 %), PIK3CA (26.1 %), GATA3 (18.1 %), ATM (13 %), and PTEN (8.6 %). Intriguingly, the prevalence of ESR1 and PIK3CA mutations decreased after two cycles of treatment (from 57 % to 44 % for ESR1 and from 36 % to 24 % for PIK3CA at C3D1 compared to baseline).Notably, interesting data emerged from the concurrent analysis of CTCs. Using the standard CTCs cutoff (≥5CTCs/7.5 mL) as a prognostic classifier (Stage IVindolent vs. StageIVaggressive), the Stage IVaggressive subgroup demonstrated a benefit from the fulvestrant plus palbociclib or fulvestrant plus palbociclib plus avelumab combinations compared to fulvestrant alone, suggesting that baseline CTCs analysis may predict the benefit of the CDK 4/6 BP strategy [141].
Concentrating exclusively on a single mutation entails the potential hazard of neglecting other resistance mechanisms that may emerge during the course of disease progression. Consequently, it becomes imperative to broaden the scope of the analytical panel to augment diagnostic sensitivity, as exemplified by the BioItaLEE study's utilization of a Next-Generation Sequencing (NGS) approach [142]. Alternatively, mutation-agnostic approaches like Low-pass Whole-Genome Sequencing (WGS) can be chosen, offering a more comprehensive and inclusive perspective despite potential sensitivity trade-offs [143].
3.12. Technical and strategic limitations: challenges ahead
In the context of CDK 4/6i resistance, the data summarized in this review underscores a crucial distinction: unlike tissue biopsies, which often belong to retrospective samples non-representative of the evolving tumor biology, liquid biopsy and its associated biomarkers emerge as an excellent tool for the real-time profiling of tumor burden and its dynamics, albeit burdened by numerous existing limitations yet to be overcome.
From a technical perspective, selectively choosing patients with a fraction of ctDNA above a specific cutoff (e.g., 1 % in the pooled analysis of the MONALEESA trials [53]) may identify a subgroup population with a poorer prognosis, potentially overlooking patients with lower levels of tumor shedding. Similarly, low concentrations of ctDNA might lead to missing gene amplifications, generally detectable only in tumors with a higher level of ctDNA. While it is increasingly evident that liquid biopsy will play a pivotal role in guiding CDK 4/6i treatment, it remains also challenging to determine which ctDNA-derived parameters will be most informative. On one hand, the PADA-1 [102] and SERENA6 [139] studies are evaluating the emergence of resistance mutations for treatment escalation by introducing a SERD, while on the other hand, the FAIM trial [144] is assessing the benefit of adding ipatasertib by analyzing early alterations in ctDNA dynamics. Concomitantly, in the adjuvant setting, the primary role of ctDNA will be to early identify minimal residual disease, as suggested by some data from the monarchE [145] and ongoing Phase II studies [144,146].
Methodologically, the findings presented in this review often relate to diverse therapeutic regimens involving various CDK 4/6 inhibitors, diverse endocrine therapy partners, and different lines of therapy. This multiplicity makes direct comparisons challenging and, alongside varying technical approaches (hypothesis-driven vs. 'unknown' analysis), partly explains the occasionally conflicting results.
Some limitations are additionally intrinsic to the structure of the evaluated studies. For instance, in the nextMonarch trial, focusing solely on the abemaciclib arms in monotherapy prevented contextualizing the achieved results [147]. Similarly, in the PADA-1 study, there existed a high risk of lead time bias and an underpowered design for OS analysis, a crucial endpoint in this context [102]. Consequently, the need for adequately powered and well-conceived biomarker-driven studies aimed at demonstrating the clinical utility of one or more resistance biomarkers becomes apparent.
Simultaneously, the outcomes of the monarchE [148] and NATALEE [149] trials in the early setting, along with various attempts in the neoadjuvant setting that have so far been less than satisfactory, such as in the NeoPAL [150], CORALLEEN [151], MONALEESA-1 [152] and neoMONARCH [152] studies, underscore the escalating importance of identifying biomarkers, not only for selecting patients who could benefit from CDK 4/6i but also for effectively guiding subsequent treatment strategies after progression in both adjuvant and advanced stages.
Furthermore, liquid biopsy stands out as a promising tool to aid clinicians in optimizing CDK 4/6i therapy in later lines, potentially pinpointing a select patients group most likely to respond to treatment approaches according to the nextMONARCH [153] and SONIA [154] studies.
In addition, although traditionally associated with luminal subtypes, CDK 4/6i are showing promising potential in triple-negative and HER2-positive breast cancers. This is evidenced by ongoing studies in various settings, including the early (e.g., PAveMenT [155] and NA-PHER [156]) and metastatic (PATINA [156], PATRICIA [157], and MONARCH-HER [157]) stages. Moreover, numerous active trials are aimed at evaluating the efficacy of combining CDK 4/6i not only with standard endocrine therapy but also with targeted therapies like inavolisib (INAVO-12) [158], gedatolisib (VIKTORIA-1) [158] and capivasertib (CAPITELLO-292) [159], underscoring the critical importance of identifying biomarkers that can accurately predict the clinical benefit of these drugs in an increasingly diverse and tailored therapeutic scenario.
4. Conclusions
Despite significant research efforts, the mechanisms of resistance to CDK4/6 inhibitors have not been yet fully elucidated. Clearly dissecting CDK4/6i resistance from endocrine resistance is one of the main unmet needs, together with tumor heterogeneity. Liquid biopsy may enable a real-time portrait of the patient's disease state and appears promising in different stages of CDK4/6i therapy such as prognostication, design of combinational regimens based on CDK4/6 inhibitors and treatment monitoring. Detected alterations may guide post-progression strategies to improve outcome of patients with endocrine-resistant HR-positive/HER2-negative BC. Given the multifactorial nature of CDK4/6i resistance, the integration of multiparametric and dynamic liquid biopsy assessments encompassing ctDNA, as well as CTCs, exosomes, and epigenetic ctDNA alterations may represent a promising perspective for the clinical characterization of treatment resistance.
CRediT authorship contribution statement
L. Foffano: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. L. Cucciniello: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. E. Nicolò: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. I. Migliaccio: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. C. Noto: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. C. Reduzzi: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. L. Malorni: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. M. Cristofanilli: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. L. Gerratana: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. F. Puglisi: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing.
Disclosures
M.C. reports advisory/consultancy fees from Merck and AstraZeneca, research grant/funding from Pfizer Inc, Menarini, Eli Lilly and G1 Therapeutics, consulting fees from Novartis, Menarini, Eli Lilly, Sermonix, G1 Therapeutics, AstraZeneca, Pfizer Inc and Foundation Medicine, and travel support from Foundation Medicine, L.G. reports advisory/consultancy fees from Novartis, Eli Lilly, AstraZeneca, Daiichi Sankyo, Incyte and GSK, F.P. reports advisory/consultancy fees from Amgen, AstraZeneca, Celgene, Daiichi Sankyo, Eisai, Eli Lilly, Gilead, Ipsen, MSD, Novartis, Pierre Fabre, Pfizer, Roche, Seagen, Takeda, and Viatris, research grant/funding from AstraZeneca, Eisai, and Roche; L.F. has no conflict of interests to declare; L.C. has no conflict of interests to declare; E.N. has no conflict of interests to declare; I.M. has no conflict of interests to declare; C.N. has no conflict of interests to declare; C.R. has no conflict of interests to declare; L.M. has no conflict of interests to declare.
Funding
The manuscript was supported the Ministry of Health Ricerca Finalizzata grant (Grant Number: RF-2016- 02362544) to FP; the CRO Aviano 5x1000 2014, redditi 2013 Cancer Specific Intramural Grant to LG; Eleonora Nicolò was supported by an American-Italian Cancer Foundation Post-Doctoral Research Fellowship.
Declaration of competing interests
L.F. has no conflict of interests to declare.
L.C. has no conflict of interests to declare.
E.N. has no conflict of interests to declare.
I.M. has no conflict of interests to declare.
C.N. has no conflict of interests to declare.
C.R. has no conflict of interests to declare.
L.M. reports advisory/consultancy fees from Novartis and Seagen, institutional research grant/funding from Pfizer;
M.C. reports advisory/consultancy fees from Merck and AstraZeneca, research grant/funding from Pfizer Inc, Menarini, Eli Lilly and G1 Therapeutics, consulting fees from Novartis, Menarini, Eli Lilly, Sermonix, G1 Therapeutics, AstraZeneca, Pfizer Inc and Foundation Medicine, and travel support from Foundation Medicine.
L.G. reports advisory/consultancy fees from Novartis, Eli Lilly, AstraZeneca, Daiichi Sankyo, Incyte and GSK.
F.P. reports advisory/consultancy fees from Amgen, AstraZeneca, Celgene, Daiichi Sankyo, Eisai, Eli Lilly, Gilead, Ipsen, MSD, Novartis, Pierre Fabre, Pfizer, Roche, Seagen, Takeda, and Viatris, research grant/funding from AstraZeneca, Eisai, and Roche.
Biographies
Lorenzo Foffano, M.D. Graduated in Medicine and Surgery at the University of Udine in 2022, currently Medical Oncology resident at the University of Udine. He works in the Unit of medical Oncology and Cancer prevention at the National cancer Institute, IRCCS Centro di Riferimento Oncologico, Aviano (PN).
Linda Cucciniello, M.D. Graduated in Medicine and Surgery at the Cattolica University (Rome) in 2020, currently Medical Oncology resident at the University of Udine. She works in the Unit of medical Oncology and Cancer prevention at the National cancer Institute, IRCCS Centro di Riferimento Oncologico, Aviano (PN).
Eleonora Nicolò, M.D. Graduated in Medicine and Surgery at the Sapienza University (Rome) in 2017. After finishing his fellowship in Medical Oncology at the European Institute of Oncology, she is currently Research Fellow at Weill Cornell Medicine.
Ilenia Migliaccio, MD PhD, is a Senior Pathologist at the Translational Research Unit of the Hospital of Prato. Her research is mainly focused on endocrine resistance mechanisms that she previously conducted at University Federico II of Naples and Baylor College of Medicine.
Claudia Noto M.D. Graduated in Medicine and Surgery at the University of Madrid in 2018, currently Medical Oncology resident at the University of Udine. She works in the Unit of medical Oncology and Cancer prevention at the National cancer Institute, IRCCS Centro di Riferimento Oncologico, Aviano (PN), Italy
Carolina Reduzzi PhD. She is a Research Associate at Weill Cornell Medicine, where she holds the position of Director of the Cristofanilli Circulating Tumor Cells Laboratory. After obtaining her PhD, she pursued her postdoctoral scholar position at Northwestern University. Her research mainly focuses on liquid biopsy in breast carcinoma and cholangiocarcinoma.
Luca Malorni MD, PhD. He is a senior medical oncologist at Hospital of Prato, Italy, where he is the Director of the Translational Research Unit. After obtaining his degree in Medicine and Surgery at the University Federico II of Naples and obtaining his Ph.D. at Baylor College of Medicine, he primarily focused his research on the mechanisms underlying endocrine resistance.
Massimo Cristofanilli, MD. He is the Director of Breast Medical Oncology at Weill Cornell Medicine and NewYork-Presbyterian, the Associate Director of Precision Oncology at the Meyer Cancer Center (MCC), and the co-leader of the MCC Breast Cancer Disease Management Team, as well as the Scientific Director of the Englander Institute of Precision Medicine (EIPM). Dr. Cristofanilli’s research has focused on biomarkers of endocrine resistance in breast cancer, liquid biopsies and novel drug development.
Lorenzo Gerratana, MD. He is a Breast Cancer Physician scientist at the IRCCS CRO Aviano National cancer Institute and a Research fellow at the Department of Medicine at the University of Udine (Uniud). After graduating as a Medical Doctor at Uniud, he focused on DNA repair in vitro models in Triple Negative Breast Cancer, clinical methodology and endpoint analysis. His mixed background resulted in a pragmatic imprinting that still guides his current translational research towards clinical utility and transferability. After finishing his fellowship in Medical Oncology at Uniud he acts as Attending Physician at IRCCS CRO Aviano with a commitment in translational and big data-driven research, with the aim of transferring promising tissue and liquid biopsy-based biomarkers and treatment strategies from the bench to the bedside.
Fabio Puglisi, M.D., Ph.D. He is Full Professor of Medical Oncology and Head of the School of Medical Oncology at the University of Udine, Italy. He is the Chief of Department of medical Oncology and the Director of the Unit of Medical Oncology and Cancer Prevention at the National Cancer Institute, IRCCS Centro di Riferimento Oncologico, Aviano (Pn), Italy. He is author of several pubblications in scientific peer-reviewed journals, focused on clinical and translational studies on breast cancer.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2024.103863.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Diehl J.A. Cycling to cancer with cyclin D1. Cancer Biol Ther. 2002;1 doi: 10.4161/cbt.72. [DOI] [PubMed] [Google Scholar]
- 2.Witzel I.I., Koh L.F., Perkins N.D. Regulation of cyclin D1 gene expression. Biochem Soc Trans. 2010;38 doi: 10.1042/BST0380217. [DOI] [PubMed] [Google Scholar]
- 3.Malumbres M., Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9 doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 4.Koboldt D.C., Fulton R.S., McLellan M.D., Schmidt H., Kalicki-Veizer J., McMichael J.F., et al. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/NATURE11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piezzo M., Chiodini P., Riemma M., Cocco S., Caputo R., Cianniello D., et al. Progression-free survival and overall survival of CDK 4/6 inhibitors plus endocrine therapy in metastatic breast cancer: a systematic review and meta-analysis. Int J Mol Sci. 2020;21:6400. doi: 10.3390/ijms21176400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston S.R.D., Harbeck N., Hegg R., Toi M., Martin M., Shao Z.M., et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE) J Clin Oncol. 2020;38:3987–3998. doi: 10.1200/JCO.20.02514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buono G., Gerratana L., Bulfoni M., Provinciali N., Basile D., Giuliano M., et al. Circulating tumor DNA analysis in breast cancer: is it ready for prime-time? Cancer Treat Rev. 2019;73:73–83. doi: 10.1016/j.ctrv.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Wang D., Cai X., Ven Te C. Detecting human interferences to low flows through base flow recession analysis. Water Resour Res. 2009;45 doi: 10.1029/2009WR007819. [DOI] [Google Scholar]
- 9.Gevensleben H., Garcia-Murillas I., Graeser M.K., Schiavon G., Osin P., Parton M., et al. Noninvasive detection of HER2 amplification with plasma DNA digital PCR. Clin Cancer Res. 2013;19:3276–3284. doi: 10.1158/1078-0432.CCR-12-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Board R.E., Williams V.S., Knight L., Shaw J., Greystoke A., Ranson M., et al. Isolation and extraction of circulating tumor DNA from patients with small cell lung cancer. Ann N Y Acad Sci. 2008;1137:98–107. doi: 10.1196/ANNALS.1448.020. [DOI] [PubMed] [Google Scholar]
- 11.Corcoran R.B., Chabner B.A. Application of cell-free DNA analysis to cancer treatment. N Engl J Med. 2018;379:1754–1765. doi: 10.1056/NEJMRA1706174. [DOI] [PubMed] [Google Scholar]
- 12.Liang D.H., Hall C., Lucci A. Circulating tumor cells in breast cancer. Recent results. Cancer Res. 2020;215:127–145. doi: 10.1007/978-3-030-26439-0_7. [DOI] [PubMed] [Google Scholar]
- 13.Müller V., Riethdorf S., Rack B., Janni W., Fasching P.A., Solomayer E., et al. Prognostic impact of circulating tumor cells assessed with the CellSearch SystemTM and AdnaTest BreastTM in metastatic breast cancer patients: the DETECT study. Breast Cancer Res. 2012;14 doi: 10.1186/BCR3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dean J.L., Thangavel C., McClendon A.K., Reed C.A., Knudsen E.S. Therapeutic CDK4/6 inhibition in breast cancer: key mechanisms of response and failure. Oncogene. 2010;29:4018–4032. doi: 10.1038/ONC.2010.154. [DOI] [PubMed] [Google Scholar]
- 15.Guarducci C., Bonechi M., Benelli M., Biagioni C., Boccalini G., Romagnoli D., et al. Cyclin E1 and Rb modulation as common events at time of resistance to palbociclib in hormone receptor-positive breast cancer. NPJ Breast Cancer. 2018;4 doi: 10.1038/S41523-018-0092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor-Harding B., Aspuria P.-J., Agadjanian H., Cheon D.-J., Mizuno T., Greenberg D., et al. Cyclin E1 and RTK/RAS signaling drive CDK inhibitor resistance via activation of E2F and ETS. Oncotarget. 2014;6:696–714. doi: 10.18632/ONCOTARGET.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrera-Abreu M.T., Palafox M., Asghar U., Rivas M.A., Cutts R.J., Garcia-Murillas I., et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. 2016;76 doi: 10.1158/0008-5472.CAN-15-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knudsen E.S., Witkiewicz A.K. The strange case of CDK4/6 inhibitors: mechanisms, resistance, and combination strategies. Trends Cancer. 2017;3 doi: 10.1016/j.trecan.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor-Harding B., Aspuria P.-J., Agadjanian H., Cheon D.-J., Mizuno T., Greenberg D., et al. Cyclin E1 and RTK/RAS signaling drive CDK inhibitor resistance via activation of E2F and ETS. Oncotarget. 2014;6:696–714. doi: 10.18632/ONCOTARGET.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma C.X., Gao F., Luo J., Northfelt D.W., Goetz M., Forero A., et al. NeoPalAna: neoadjuvant palbociclib, a cyclin-dependent kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor–positive breast cancer. Clin Cancer Res. 2017;23 doi: 10.1158/1078-0432.CCR-16-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner N.C., Liu Y., Zhu Z., Loi S., Colleoni M., Loibl S., et al. Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2019;37 doi: 10.1200/JCO.18.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnedos M., Bayar M.A., Cheaib B., Scott V., Bouakka I., Valent A., et al. Modulation of Rb phosphorylation and antiproliferative response to palbociclib: the preoperative-palbociclib (POP) randomized clinical trial. Ann Oncol. 2018;29:1755–1762. doi: 10.1093/ANNONC/MDY202. [DOI] [PubMed] [Google Scholar]
- 23.Hortobagyi G.N., Paluch-Shimon S., Petrakova K., Villanueva C., Chan A., Nusch A., et al. First-line ribociclib (RIB) + letrozole (LET) in hormone receptor-positive (HR+), HER2-negative (HER2–) advanced breast cancer (ABC): MONALEESA-2 biomarker analyses. J Clin Oncol. 2018;36 doi: 10.1200/jco.2018.36.15_suppl.1022. [DOI] [Google Scholar]
- 24.Finn R., Liu Y., Martin M., Rugo H., Dieras V., Im S.-A., et al. Abstract P2-09-10: comprehensive gene expression biomarker analysis of CDK 4/6 and endocrine pathways from the PALOMA-2 study. 2018. [DOI]
- 25.Chia S., Su F., Neven P., Im S.-A., Petrakova K., Bianchi G.V., et al. Abstract PD2-08: gene expression analysis and association with treatment response in postmenopausal patients with hormone receptor-positive, HER2-negative advanced breast cancer in the MONALEESA-3 study. Cancer Res. 2020;80:PD2–8. doi: 10.1158/1538-7445.SABCS19-PD2-08. [DOI] [Google Scholar]
- 26.O’leary B., Cutts R.J., Huang X., Hrebien S., Liu Y., André F., et al. Circulating tumor DNA markers for early progression on fulvestrant with or without palbociclib in ER+ advanced breast cancer. J Natl Cancer Inst. 2021;113:309–317. doi: 10.1093/JNCI/DJAA087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wander S.A., Cohen O., Gong X., Johnson G.N., Buendia-Buendia J.E., Lloyd M.R., et al. The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors in patients with hormone receptor–positive metastatic breast cancer. Cancer Discov. 2020 doi: 10.1158/2159-8290.cd-19-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guarducci C., Bonechi M., Benelli M., Biagioni C., Boccalini G., Romagnoli D., et al. Cyclin E1 and Rb modulation as common events at time of resistance to palbociclib in hormone receptor-positive breast cancer. NPJ Breast Cancer. 2018;4 doi: 10.1038/s41523-018-0092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y.X., Sicinska E., Czaplinski J.T., Remillard S.P., Moss S., Wang Y., et al. Antiproliferative effects of CDK4/6 inhibition in CDK4-amplified human liposarcoma in vitro and in vivo. Mol Cancer Ther. 2014;13:2184–2193. doi: 10.1158/1535-7163.MCT-14-0387/175477/AM/ANTIPROLIFERATIVE-EFFECTS-OF-CDK4-6-INHIBITION-IN. [DOI] [PubMed] [Google Scholar]
- 30.Olanich M.E., Sun W., Hewitt S.M., Abdullaev Z., Pack S.D., Barr F.G. CDK4 amplification reduces sensitivity to CDK4/6 inhibition in fusion-positive rhabdomyosarcoma. Clin Cancer Res. 2015;21 doi: 10.1158/1078-0432.CCR-14-2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel P., Asbach B., Shteyn E., Gomez C., Coltoff A., Bhuyan S., et al. Brk/protein tyrosine kinase 6 phosphorylates p27 KIP1 , regulating the activity of cyclin D–cyclin-dependent kinase 4. Mol Cell Biol. 2015;35 doi: 10.1128/mcb.01206-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finn R.S., Liu Y., Zhu Z., Martin M., Rugo H.S., Dieras V., et al. Biomarker analyses of response to cyclin-dependent kinase 4/6 inhibition and endocrine therapy in women with treatment-naïve metastatic breast cancer. Clin Cancer Res. 2020;26:110–121. doi: 10.1158/1078-0432.CCR-19-0751. [DOI] [PubMed] [Google Scholar]
- 33.del Re M., Bertolini I., Crucitta S., Fontanelli L., Rofi E., de Angelis C., et al. Overexpression of TK1 and CDK9 in plasma-derived exosomes is associated with clinical resistance to CDK4/6 inhibitors in metastatic breast cancer patients. Breast Cancer Res Treat. 2019;178 doi: 10.1007/s10549-019-05365-y. [DOI] [PubMed] [Google Scholar]
- 34.Li Z., Razavi P., Li Q., Toy W., Liu B., Ping C., et al. Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the hippo pathway. Cancer Cell. 2018;34 doi: 10.1016/j.ccell.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kollmann K., Heller G., Schneckenleithner C., Warsch W., Scheicher R., Ott R.G., et al. A kinase-independent function of CDK6 links the cell cycle to tumor angiogenesis. Cancer Cell. 2013;24 doi: 10.1016/j.ccr.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tigan A.S., Bellutti F., Kollmann K., Tebb G., Sexl V. CDK6-a review of the past and a glimpse into the future: from cell-cycle control to transcriptional regulation. Oncogene. 2016;35 doi: 10.1038/onc.2015.407. [DOI] [PubMed] [Google Scholar]
- 37.Cornell L., Wander S.A., Visal T., Wagle N., Shapiro G.I. MicroRNA-Mediated suppression of the TGF-β pathway confers transmissible and reversible CDK4/6 inhibitor resistance. Cell Rep. 2019;26 doi: 10.1016/j.celrep.2019.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Q., Jiang B., Guo J., Shao H., del Priore I.S., Chang Q., et al. INK4 tumor suppressor proteins mediate resistance to CDK4/6 kinase inhibitors. Cancer Discov. 2022;12:356–371. doi: 10.1158/2159-8290.CD-20-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finn R.S., Liu Y., Zhu Z., Martin M., Rugo H.S., Dieras V., et al. Biomarker analyses of response to cyclin-dependent kinase 4/6 inhibition and endocrine therapy in women with treatment-naïve metastatic breast cancer. Clin Cancer Res. 2020;26:110–121. doi: 10.1158/1078-0432.CCR-19-0751. [DOI] [PubMed] [Google Scholar]
- 40.Turner N.C., Liu Y., Zhu Z., Loi S., Colleoni M., Loibl S., et al. Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2019;37:1169–1178. doi: 10.1200/JCO.18.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fisher R.P. Secrets of a double agent: CDK7 in cell-cycle control and transcription. J Cell Sci. 2005;118 doi: 10.1242/jcs.02718. [DOI] [PubMed] [Google Scholar]
- 42.Pancholi S., Ribas R., Simigdala N., Schuster E., Nikitorowicz-Buniak J., Ressa A., et al. Tumour kinome re-wiring governs resistance to palbociclib in oestrogen receptor positive breast cancers, highlighting new therapeutic modalities. Oncogene. 2020;39:4781–4797. doi: 10.1038/s41388-020-1284-6. 25 2020;39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guarducci C., Nardone A., Feiglin A., Migliaccio I., Malorni L., Bonechi M., et al. Abstract PD7-12: inhibition of CDK7 overcomes resistance to CDK4/6 inhibitors in hormone receptor positive breast cancer cells. Cancer Res. 2019;79:PD7–12. doi: 10.1158/1538-7445.SABCS18-PD7-12. [DOI] [Google Scholar]
- 44.Sava G.P., Fan H., Coombes R.C., Buluwela L., Ali S. CDK7 inhibitors as anticancer drugs. Cancer Metastasis Rev. 2020;39:805–823. doi: 10.1007/S10555-020-09885-8. 3 2020;39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coombes C., Howell S.J., Krebs M.G., Lord S., Kenny L.M., Bahl A., et al. Abstract GS3-10: study of samuraciclib (CT7001), a first-in-class, oral, selective inhibitor of CDK7, in combination with fulvestrant in patients with advanced hormone receptor positive HER2 negative breast cancer (HR+BC) Cancer Res. 2022;82:GS3–10. doi: 10.1158/1538-7445.SABCS21-GS3-10. [DOI] [Google Scholar]
- 46.Glen Clack et al. (PO3-04-12) SUMIT-BC: Phase 2 randomized study of fulvestrant with or without the cyclin-dependent kinase 7 inhibitor (CDK7i) samuraciclib in advanced hormone receptor positive (HR+) breast cancer after CDK4/6i n.d. https://sabcs2023.eventscribe.net/fsPopup.asp?Mode=posterinfo&PosterID=624827(accessed January 3, 2024)..
- 47.Amita Patnaik et al. (PO3-04-13) SUMIT-ELA: Phase 1b/2 combination of cyclin-dependent kinase 7 inhibitor (CDK7i) samuraciclib and selective estrogen receptor degrader (SERD) elacestrant in advanced hormone receptor positive (HR+) breast cancer after CDK4/6i n.d. https://sabcs2023.eventscribe.net/fsPopup.asp?Mode=posterinfo&PosterID=624826(accessed January 3, 2024)..
- 48.Efeyan A., Ortega-Molina A., Velasco-Miguel S., Herranz D., Vassilev L.T., Serrano M. Induction of p53-dependent senescence by the MDM2 antagonist nutlin-3a in mouse cells of fibroblast origin. Cancer Res. 2007;67 doi: 10.1158/0008-5472.CAN-07-0200. [DOI] [PubMed] [Google Scholar]
- 49.Curtis C., et al. Comprehensive molecular portraits of human breast tumors the Cancer Genome Atlas Network. Nature. 2012;490 doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim E., Portman N., Alexandrou S., Haupt S., Haupt Y., Caldon E. Abstract P4-04-12: therapeutic targeting of CDK4/6 inhibitor resistant breast cancer. 2018. [DOI]
- 51.Kovatcheva M., Liu D.D., Dickson M.A., Klein M.E., O'Connor R., Wilder F.O., et al. MDM2 turnover and expression of ATRX determine the choice between quiescence and senescence in response to CDK4 inhibition. Oncotarget. 2015;6 doi: 10.18632/oncotarget.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andre F., Su F., Solovieff N., Arteaga C.L., Hortobagyi G.N., Chia S.K.L., et al. Pooled ctDNA analysis of the MONALEESA (ML) phase III advanced breast cancer (ABC) trials. J Clin Oncol. 2020;38 doi: 10.1200/JCO.2020.38.15_suppl.1009. 1009–1009. [DOI] [PubMed] [Google Scholar]
- 53.André F., Su F., Solovieff N., Hortobagyi G., Chia S., Neven P., et al. Journal Pre-proof Pooled ctDNA analysis of MONALEESA phase III advanced breast cancer trials Pooled ctDNA analysis of MONALEESA phase III advanced breast cancer trials. 2023. [DOI] [PubMed]
- 54.Witkiewicz A.K., Knudsen E.S. Retinoblastoma tumor suppressor pathway in breast cancer: prognosis, precision medicine, and therapeutic interventions. Breast Cancer Res. 2014;16 doi: 10.1186/bcr3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Finn R.S., Dering J., Conklin D., Kalous O., Cohen D.J., Desai A.J., et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11 doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Condorelli R., Spring L., O'Shaughnessy J., Lacroix L., Bailleux C., Scott V., et al. Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann Oncol. 2018;29:640–645. doi: 10.1093/ANNONC/MDX784. [DOI] [PubMed] [Google Scholar]
- 57.Malorni L., Piazza S., Ciani Y., Guarducci C., Bonechi M., Biagioni C., et al. A gene expression signature of retinoblastoma loss-of-function is a predictive biomarker of resistance to palbociclib in breast cancer cell lines and is prognostic in patients with ER positive early breast cancer. Oncotarget. 2016;7 doi: 10.18632/ONCOTARGET.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hurvitz S.A., Martin M., Press M.F., Chan D., Fernandez-Abad M., Petru E., et al. Potent cell-cycle inhibition and upregulation of immune response with abemaciclib and anastrozole in Neomonarch, phase II neoadjuvant study in HR+/HER2- Breast cancer. Clin Cancer Res. 2020;26:566–580. doi: 10.1158/1078-0432.CCR-19-1425/75358/AM/POTENT-CELL-CYCLE-INHIBITION-AND-UPREGULATION-OF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’leary B., Cutts R.J., Huang X., Hrebien S., Liu Y., André F., et al. Circulating tumor DNA markers for early progression on fulvestrant with or without palbociclib in ER+ advanced breast cancer. J Natl Cancer Inst. 2021;113:309–317. doi: 10.1093/JNCI/DJAA087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goetz M.P., Hamilton E.P., Campone M., Hurvitz S.A., Cortes J., Johnston S.R.D., et al. Acquired genomic alterations in circulating tumor DNA from patients receiving abemaciclib alone or in combination with endocrine therapy. 2020. Https://DoiOrg/101200/JCO20203815_suppl3519. [DOI] [PMC free article] [PubMed]
- 61.Park K., Kim G.M., Jung K.H., Kang S.Y., Park I.H., Kim J.H., et al. Abstract PS5-19: exploratory biomarker analysis of Young-PEARL [palbociclib plus exemestane with GnRH agonist versus capecitabine in premenopausal women with HR (hormone receptor)-positive, HER2-negative metastatic breast cancer (MBC)] study. Cancer Res. 2021;81:PS5–19. doi: 10.1158/1538-7445.SABCS20-PS5-19. [DOI] [Google Scholar]
- 62.Sivakumar S., Jin D.X., Tukachinsky H., Murugesan K., McGregor K., Danziger N., et al. Tissue and liquid biopsy profiling reveal convergent tumor evolution and therapy evasion in breast cancer. Nat Commun. 2022;13(1) doi: 10.1038/s41467-022-35245-x. 2022;13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shahbandi A., Nguyen H.D., Jackson J.G. TP53 mutations and outcomes in breast cancer: reading beyond the headlines. Trends Cancer. 2020;6:98–110. doi: 10.1016/J.TRECAN.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kudo Rei, et al. (PS12-03) p53 loss enables HR+ breast cancer escape from CDK4/6 inhibitor-induced quiescence via CDK2 2023. https://sabcs2023.eventscribe.net/fsPopup.asp?Mode=posterinfo&PosterID=625149
- 65.O'Leary B., Cutts R.J., Liu Y., Hrebien S., Huang X., Fenwick K., et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov. 2018;8:1390–1403. doi: 10.1158/2159-8290.CD-18-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hortobagyi G., Stemmer S., Campone M., Sonke G., Arteaga C., Paluch-Shimon S., et al. Abstract PD4-06: first-line ribociclib + letrozole in hormone receptor-positive. HER2-negative advanced breast cancer: Efficacy by baseline circulating tumor DNA alterations in MONALEESA- 2018;2 doi: 10.1158/1538-7445.sabcs17-pd4-06. [DOI] [Google Scholar]
- 67.Bardia A., Su F., Solovieff N., Im S.-A., Sohn J., Lee K.S., et al. Genomic profiling of premenopausal HR+ and HER2– metastatic breast cancer by circulating tumor DNA and association of genetic alterations with therapeutic response to endocrine therapy and ribociclib. 2021. Https://DoiOrg/101200/PO2000445. [DOI] [PMC free article] [PubMed]
- 68.O’leary B, Cutts RJ, Huang X, Hrebien S, Liu Y, Andr F, et al. Circulating Tumor DNA Markers for Early Progression on Fulvestrant With or Without Palbociclib in ER1 Advanced Breast Cancer n.d. 10.1093/jnci/djaa087.. [DOI] [PMC free article] [PubMed]
- 69.Yan M., Wang C., He B., Yang M., Tong M., Long Z., et al. Aurora-A kinase: a potent oncogene and target for cancer therapy. Med Res Rev. 2016;36 doi: 10.1002/med.21399. [DOI] [PubMed] [Google Scholar]
- 70.Gong X., Du J., Parsons S.H., Merzoug F.F., Webster Y., Iversen P.W., et al. Aurora a kinase inhibition is synthetic lethal with loss of the RB1 tumor suppressor gene. Cancer Discov. 2019;9 doi: 10.1158/2159-8290.CD-18-0469. [DOI] [PubMed] [Google Scholar]
- 71.Oser M.G., Fonseca R., Chakraborty A.A., Brough R., Spektor A., Jennings R.B., et al. Cells lacking the RB1 tumor suppressor gene are hyperdependent on aurora B kinase for survival. Cancer Discov. 2019;9 doi: 10.1158/2159-8290.CD-18-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jing X.L., Chen S.W. Aurora kinase inhibitors: a patent review (2014-2020) Expert Opin Ther Pat. 2021;31 doi: 10.1080/13543776.2021.1890027. [DOI] [PubMed] [Google Scholar]
- 73.Herrera-Abreu M.T., Palafox M., Asghar U., Rivas M.A., Cutts R.J., Garcia-Murillas I., et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. 2016;76 doi: 10.1158/0008-5472.CAN-15-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Juric D., Ciruelos E., Rubovszky G., Campone M., Loibl S., Rugo H., et al. Abstract GS3-08: alpelisib + fulvestrant for advanced breast cancer: subgroup analyses from the phase III SOLAR-1 trial. 2019. [DOI]
- 75.Cristofanilli Massimo, Rugo H.S., Im S.-A., Slamon D.J., Harbeck N., Igor, et al. Overall survival with palbociclib and fulvestrant in women with HR+/HER2- ABC: updated exploratory analyses of PALOMA-3, a double-blind, phase 3 randomized study. Clin Cancer Res. 2022 doi: 10.1158/1078-0432.CCR-22-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tolaney S.M., Toi M., Neven P., Sohn J., Grischke E.-M., Llombart-Cussac A., et al. Abstract 4458: clinical significance of PIK3CA and ESR1 mutations in ctDNA and FFPE samples from the MONARCH 2 study of abemaciclib plus fulvestrant. Cancer Res. 2019;79 doi: 10.1158/1538-7445.AM2019-4458. 4458–4458. [DOI] [Google Scholar]
- 77.Hortobagyi G., Stemmer S., Campone M., Sonke G., Arteaga C., Paluch-Shimon S., et al. Abstract PD4-06: first-line ribociclib + letrozole in hormone receptor-positive, HER2-negative advanced breast cancer: efficacy by baseline circulating tumor DNA alterations in MONALEESA-2. Cancer Res. 2018;78:PD4–6. doi: 10.1158/1538-7445.SABCS17-PD4-06. [DOI] [Google Scholar]
- 78.Neven P., Petrakova K., Val Bianchi G., de la Cruz-Merino L., Jerusalem G., Sonke G., et al. Abstract PD2-05: biomarker analysis by baseline circulating tumor DNA alterations in the MONALEESA-3 study. Cancer Res. 2019;79:PD2–5. doi: 10.1158/1538-7445.SABCS18-PD2-05. [DOI] [Google Scholar]
- 79.O'Leary B., Hrebien S., Morden J.P., Beaney M., Fribbens C., Huang X., et al. Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat Commun. 2018;9 doi: 10.1038/s41467-018-03215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jacob S., Davis A.A., Gerratana L., Velimirovic M., Shah A.N., Wehbe F., et al. The use of serial circulating tumor DNA to detect resistance alterations in progressive metastatic breast cancer. Clin Cancer Res. 2021;27:1361–1370. doi: 10.1158/1078-0432.CCR-20-1566. [DOI] [PubMed] [Google Scholar]
- 81.Andre F., Su F., Solovieff N., Arteaga C.L., Hortobagyi G.N., Chia S.K.L., et al. Pooled ctDNA analysis of the MONALEESA (ML) phase III advanced breast cancer (ABC) trials. 2020. 38:1009–1009. [DOI] [PubMed]
- 82.Turner N.C., Oliveira M., Howell S.J., Dalenc F., Cortes J., Gomez Moreno H.L., et al. Capivasertib in hormone receptor–positive advanced breast cancer. N Engl J Med. 2023;388:2058–2070. doi: 10.1056/NEJMOA2214131/SUPPL_FILE/NEJMOA2214131_DATA-SHARING.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mao P., Kusiel J., Cohen O., Wagle N. Abstract PD4-01: the role of FGF/FGFR axis in resistance to SERDs and CDK4/6 inhibitors in ER+ breast cancer. 2018. [DOI]
- 84.Turner N., Pearson A., Sharpe R., Lambros M., Geyer F., Lopez-Garcia M.A., et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res. 2010;70 doi: 10.1158/0008-5472.CAN-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Formisano L., Lu Y., Servetto A., Hanker A.B., Jansen V.M., Bauer J.A., et al. Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer. Nat Commun. 2019;10 doi: 10.1038/s41467-019-09068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Musolino A., Campone M., Neven P., Denduluri N., Barrios C.H., Cortes J., et al. Phase II, randomized, placebo-controlled study of dovitinib in combination with fulvestrant in postmenopausal patients with HR+, HER2- breast cancer that had progressed during or after prior endocrine therapy. Breast Cancer Res. 2017;19 doi: 10.1186/S13058-017-0807-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bello E., Colella G., Scarlato V., Oliva P., Berndt A., Valbusa G., et al. E-3810 is a potent dual inhibitor of VEGFR and FGFR that exerts antitumor activity in multiple preclinical models. Cancer Res. 2011;71 doi: 10.1158/0008-5472.CAN-10-2700. [DOI] [PubMed] [Google Scholar]
- 88.Soria J.C., DeBraud F., Bahleda R., Adamo B., Andre F., Dientsmann R., et al. Phase I/IIa study evaluating the safety, efficacy, pharmacokinetics, and pharmacodynamics of lucitanib in advanced solid tumors. Ann Oncol. 2014;25 doi: 10.1093/annonc/mdu390. [DOI] [PubMed] [Google Scholar]
- 89.Hui R., Pearson A., Cortes J., Campbell C., Poirot C., Azim H.A., et al. Lucitanib for the treatment of HRþ/HER2- Metastatic breast cancer: results from the multicohort phase II FINESSE study. Clin Cancer Res. 2020;26 doi: 10.1158/1078-0432.CCR-19-1164. [DOI] [PubMed] [Google Scholar]
- 90.Raimondi L., Raimondi F.M., Pietranera M., Di Rocco A., Di Benedetto L., Miele E., et al. Assessment of resistance mechanisms and clinical implications in patients with KRAS mutated-metastatic breast cancer and resistance to CDK4/6 inhibitors. Cancers. 2021;13 doi: 10.3390/CANCERS13081928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kharenko O.A., Patel R.G., Calosing C., van der Horst E.H. Combination of ZEN-3694 with CDK4/6 inhibitors reverses acquired resistance to CDK4/6 inhibitors in ER-positive breast cancer. Cancer Gene Ther. 2022;29:859–869. doi: 10.1038/S41417-021-00375-9. [DOI] [PubMed] [Google Scholar]
- 92.Wander S.A., Cohen O., Gong X., Johnson G.N., Buendia-Buendia J.E., Lloyd M.R., et al. The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors in patients with hormone receptor–positive metastatic breast cancer. Cancer Discov. 2020 doi: 10.1158/2159-8290.cd-19-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cristofanilli M., Turner N.C., Bondarenko I., Ro J., Im S.A., Masuda N., et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 94.Turner N.C., Liu Y., Zhu Z., Loi S., Colleoni M., Loibl S., et al. Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2019;37:1169–1178. doi: 10.1200/JCO.18.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Finn R.S., Liu Y., Zhu Z., Martin M., Rugo H.S., Dieras V., et al. Biomarker analyses of response to cyclin-dependent kinase 4/6 inhibition and endocrine therapy in women with treatment-naïve metastatic breast cancer. Clin Cancer Res. 2020;26:110–121. doi: 10.1158/1078-0432.CCR-19-0751. [DOI] [PubMed] [Google Scholar]
- 96.Hortobagyi G.N., Stemmer S.M., Burris H.A., Yap Y.S., Sonke G.S., Paluch-Shimon S., et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29:1541–1547. doi: 10.1093/ANNONC/MDY155. [DOI] [PubMed] [Google Scholar]
- 97.di Leo A., O'Shaughnessy J., Sledge G.W., Martin M., Lin Y., Frenzel M., et al. Prognostic characteristics in hormone receptor-positive advanced breast cancer and characterization of abemaciclib efficacy. Npj Breast Cancer. 2018;4 doi: 10.1038/s41523-018-0094-2. 1 2018;4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brett J.O., Spring L.M., Bardia A., Wander S.A. ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res. 2021;23 doi: 10.1186/s13058-021-01462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rosario Chica-Parrado M., et al. 2023. (PO3-23-09) ESR1 mutations drive resistance to CDK4/6 inhibitors in ER+ Breast Cancer. [Google Scholar]
- 100.Fribbens C., O'Leary B., Kilburn L., Hrebien S., Garcia-Murillas I., Beaney M., et al. Plasma ESR1 Mutations and the treatment of estrogen receptor-Positive advanced breast cancer. J Clin Oncol. 2016;34 doi: 10.1200/JCO.2016.67.3061. [DOI] [PubMed] [Google Scholar]
- 101.Jeannot E., Darrigues L., Michel M., Stern M.H., Pierga J.Y., Rampanou A., et al. A single droplet digital PCR for ESR1 activating mutations detection in plasma. Oncogene. 2020;39:2987–2995. doi: 10.1038/S41388-020-1174-Y. [DOI] [PubMed] [Google Scholar]
- 102.Bidard F.C., Hardy-Bessard A.C., Dalenc F., Bachelot T., Pierga J.Y., DE L.A., de la Motte Rouge T., et al. Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2022;23:1367–1377. doi: 10.1016/S1470-2045(22)00555-1. [DOI] [PubMed] [Google Scholar]
- 103.De Angelis C., Nardone A., Cataldo M., Veeraraghavan J., Fu X., Giuliano M., et al. Abstract P4-03-05: AP-1 as a potential mediator of resistance to the cyclin-dependent kinase (CDK) 4/6-inhibitor palbociclib in ER-positive endocrine-resistant breast cancer. 2018. [DOI]
- 104.Watt A.C., Cejas P., DeCristo M.J., Metzger-Filho O., Lam E.Y.N., Qiu X., et al. CDK4/6 inhibition reprograms the breast cancer enhancer landscape by stimulating AP-1 transcriptional activity. Nature Cancer. 2020;2 doi: 10.1038/s43018-020-00135-y. 1 2020;2:34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shaulian E., Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20 doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 106.Tewari D., Nabavi S.F., Nabavi S.M., Sureda A., Farooqi A.A., Atanasov A.G., et al. Targeting activator protein 1 signaling pathway by bioactive natural agents: possible therapeutic strategy for cancer prevention and intervention. Pharmacol Res. 2018;128 doi: 10.1016/j.phrs.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 107.McCartney A., Bonechi M., De Luca F., Biagioni C., Curigliano G., Moretti E., et al. Plasma thymidine kinase activity as a biomarker in patients with luminal metastatic breast cancer treated with palbociclib within the TREnd trial. Clin Cancer Res. 2020;26:2131–2139. doi: 10.1158/1078-0432.CCR-19-3271. [DOI] [PubMed] [Google Scholar]
- 108.Cabel L., Rosenblum D., Lerebours F., Brain E., Loirat D., Bergqvist M., et al. Plasma thymidine kinase 1 activity and outcome of ER+ HER2- metastatic breast cancer patients treated with palbociclib and endocrine therapy. Breast Cancer Res. 2020;22 doi: 10.1186/S13058-020-01334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Malorni L., Tyekucheva S., Hilbers F.S., Ignatiadis M., Neven P., Colleoni M., et al. Serum thymidine kinase activity in patients with hormone receptor-positive and HER2-negative metastatic breast cancer treated with palbociclib and fulvestrant. Eur J Cancer. 2022;164:39–51. doi: 10.1016/J.EJCA.2021.12.030. [DOI] [PubMed] [Google Scholar]
- 110.Malorni L., Bianchini G., Caputo R., Zambelli A., Puglisi F., Bianchi G.V., et al. Serum thymidine kinase activity in patients with HR-positive/HER2-negative advanced breast cancer treated with ribociclib plus letrozole: results from the prospective BioItaLEE trial. Eur J Cancer. 2023;186:1–11. doi: 10.1016/J.EJCA.2023.03.001. [DOI] [PubMed] [Google Scholar]
- 111.Del Re M., Bertolini I., Crucitta S., Fontanelli L., Rofi E., De Angelis C., et al. Overexpression of TK1 and CDK9 in plasma-derived exosomes is associated with clinical resistance to CDK4/6 inhibitors in metastatic breast cancer patients. Breast Cancer Res Treat. 2019;178:57–62. doi: 10.1007/S10549-019-05365-Y/METRICS. [DOI] [PubMed] [Google Scholar]
- 112.Goel S., Decristo M.J., Watt A.C., Brinjones H., Sceneay J., Li B.B., et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548:471–475. doi: 10.1038/NATURE23465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schaer D.A., Beckmann R.P., Dempsey J.A., Huber L., Forest A., Amaladas N., et al. The CDK4/6 inhibitor abemaciclib induces a T cell inflamed tumor microenvironment and enhances the efficacy of PD-L1 checkpoint blockade. Cell Rep. 2018;22:2978–2994. doi: 10.1016/J.CELREP.2018.02.053. [DOI] [PubMed] [Google Scholar]
- 114.de Angelis C., Fu X., Cataldo M.L., Nardone A., Pereira R., Veeraraghavan J., et al. Activation of the IFN signaling pathway is associated with resistance to CDK4/6 inhibitors and immune checkpoint activation in ER-positive breast cancer. Clin Cancer Res. 2021;27:4870–4882. doi: 10.1158/1078-0432.CCR-19-4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pandey K., Lee E., Park N., Hur J., Cho Y bin, Katuwal N.B., et al. Deregulated immune pathway associated with palbociclib resistance in preclinical breast cancer models: integrative genomics and transcriptomics. Genes. 2021;12:1–14. doi: 10.3390/GENES12020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Uzhachenko R.v., Bharti V., Ouyang Z., Blevins A., Mont S., Saleh N., et al. Metabolic modulation by CDK4/6 inhibitor promotes chemokine-mediated recruitment of T cells into mammary tumors. Cell Rep. 2021;35 doi: 10.1016/J.CELREP.2021.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang J., Bu X., Wang H., Zhu Y., Geng Y., Nihira N.T., et al. Cyclin D–CDK4 kinase destabilizes PD-L1 via cullin 3–SPOP to control cancer immune surveillance. Nature. 2018;553:91–95. doi: 10.1038/nature25015. 7686 2017;553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu Z., Turner N.C., Loi S., André F., Martin M., Diéras V., et al. Comparative biomarker analysis of PALOMA-2/3 trials for palbociclib. npj Precis Oncol. 2022;6 doi: 10.1038/s41698-022-00297-1. 1 2022;6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Goel S., Decristo M.J., Watt A.C., Brinjones H., Sceneay J., Li B.B., et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548:471–475. doi: 10.1038/NATURE23465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Deng J., Wang E.S., Jenkins R.W., Li S., Dries R., Yates K., et al. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov. 2018;8:216–233. doi: 10.1158/2159-8290.CD-17-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schaer D.A., Beckmann R.P., Dempsey J.A., Huber L., Forest A., Amaladas N., et al. The CDK4/6 inhibitor abemaciclib induces a T cell inflamed tumor microenvironment and enhances the efficacy of PD-L1 checkpoint blockade. Cell Rep. 2018;22:2978–2994. doi: 10.1016/J.CELREP.2018.02.053. [DOI] [PubMed] [Google Scholar]
- 122.Uzhachenko R.v., Bharti V., Ouyang Z., Blevins A., Mont S., Saleh N., et al. Metabolic modulation by CDK4/6 inhibitor promotes chemokine-mediated recruitment of T cells into mammary tumors. Cell Rep. 2021;35 doi: 10.1016/J.CELREP.2021.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lelliott E.J., Kong I.Y., Zethoven M., Ramsbottom K.M., Martelotto L.G., Meyran D., et al. CDK4/6 inhibition promotes antitumor immunity through the induction of T-cell memory. Cancer Discov. 2021;11:2582–2601. doi: 10.1158/2159-8290.CD-20-1554. [DOI] [PubMed] [Google Scholar]
- 124.Heckler M., Ali L.R., Clancy-Thompson E., Qiang L., Ventre K.S., Lenehan P., et al. Inhibition of CDK4/6 promotes CD8 T-cell memory formation. Cancer Discov. 2021;11:2564–2581. doi: 10.1158/2159-8290.CD-20-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Peuker C.A., Yaghobramzi S., Grunert C., Keilholz L., Gjerga E., Hennig S., et al. Treatment with ribociclib shows favourable immunomodulatory effects in patients with hormone receptor-positive breast cancer—findings from the RIBECCA trial. Eur J Cancer. 2022;162:45–55. doi: 10.1016/J.EJCA.2021.11.025. [DOI] [PubMed] [Google Scholar]
- 126.Scirocchi F., Scagnoli S., Botticelli A., di Filippo A., Napoletano C., Zizzari I.G., et al. Immune effects of CDK4/6 inhibitors in patients with HR+/HER2− metastatic breast cancer: relief from immunosuppression is associated with clinical response. EBioMedicine. 2022;79 doi: 10.1016/J.EBIOM.2022.104010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schaer D.A., Beckmann R.P., Dempsey J.A., Huber L., Forest A., Amaladas N., et al. The CDK4/6 inhibitor abemaciclib induces a T cell inflamed tumor microenvironment and enhances the efficacy of PD-L1 checkpoint blockade. Cell Rep. 2018;22:2978–2994. doi: 10.1016/J.CELREP.2018.02.053. [DOI] [PubMed] [Google Scholar]
- 128.Tong J., Tan X., Song X., Gao M., Risnik D., Hao S., et al. CDK4/6 inhibition suppresses p73 phosphorylation and activates DR5 to potentiate chemotherapy and immune checkpoint blockade. Cancer Res. 2022;82:1340–1352. doi: 10.1158/0008-5472.CAN-21-3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yuan Y., Lee J.S., Yost S.E., Frankel P.H., Ruel C., Egelston C.A., et al. Phase I/II trial of palbociclib, pembrolizumab and letrozole in patients with hormone receptor-positive metastatic breast cancer. Eur J Cancer. 2021;154:11–20. doi: 10.1016/J.EJCA.2021.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guo X., Chen H., Zhou Y., Shen L., Wu S., Chen Y. Cyclin-dependent kinase inhibition and its intersection with immunotherapy in breast cancer: more than CDK4/6 inhibition. Expert Opin Investig Drugs. 2022;31:933–944. doi: 10.1080/13543784.2022.2097067. [DOI] [PubMed] [Google Scholar]
- 131.Mayer E.L., Ren Y., Wagle N., Mahtani R., Ma C., DeMichele A., et al. PACE: palbociclib after CDK and endocrine therapy A randomized phase II study of fulvestrant +/- palbociclib after progression on CDK4/6 inhibitor for hr+/HER2- metastatic breast cancer. San Antonio Breast Cancer Symposium. 2022:GS3–6. [Google Scholar]
- 132.Cristofanilli M., Budd G.T., Ellis M.J., Stopeck A., Matera J., Miller M.C., et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMOA040766. [DOI] [PubMed] [Google Scholar]
- 133.Galardi F., de Luca F., Biagioni C., Migliaccio I., Curigliano G., Minisini A.M., et al. Circulating tumor cells and palbociclib treatment in patients with ER-positive, HER2-negative advanced breast cancer: results from a translational sub-study of the TREnd trial. Breast Cancer Res. 2021;23 doi: 10.1186/s13058-021-01415-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Trumpp A., Becker L., Fletcher M., Würth R., Thippeswamy A., Haas S., et al. Abstract PD2-01: exploring CDK4/6 inhibitor therapy response and drug resistance development at the single cell level in metastatic breast cancer CTCs. Cancer Res. 2020;80 doi: 10.1158/1538-7445.SABCS19-PD2-01. PD2-01. [DOI] [Google Scholar]
- 135.Kasimir-Bauer S., Gruber C., Hoffmann O., Kimmig R., Keup C. Abstract P5-13-33: longitudinal transcriptional profiling of CTCs in metastatic breast cancer patients receiving the CDK4/6 inhibitor Palbociclib to predict therapy response. Cancer Res. 2022;82 doi: 10.1158/1538-7445.SABCS21-P5-13-33. P5-13–33. [DOI] [Google Scholar]
- 136.Piezzo M., Chiodini P., Riemma M., Cocco S., Caputo R., Cianniello D., et al. Progression-free survival and overall survival of CDK 4/6 inhibitors plus endocrine therapy in metastatic breast cancer: a systematic review and meta-analysis. Int J Mol Sci. 2020;21:1–17. doi: 10.3390/IJMS21176400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kalinsky K., Accordino M.K., Chiuzan C., Mundi P.S., Sakach E., Sathe C., et al. Randomized phase II trial of endocrine therapy with or without ribociclib after progression on cyclin-dependent kinase 4/6 inhibition in hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: MAINTAIN trial. J Clin Oncol. 2023 doi: 10.1200/JCO.22.02392. JCO2202392. [DOI] [PubMed] [Google Scholar]
- 138.Gerratana L., Davis A.A., Velimirovic M., Reduzzi C., Clifton K., Bucheit L., et al. Cyclin-dependent kinase 4/6 inhibitors beyond progression in metastatic breast cancer: a retrospective real-world biomarker analysis. JCO Precis Oncol. 2023;7 doi: 10.1200/PO.22.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Phase III study to assess AZD9833+ CDK4/6 inhibitor in HR+/HER2-MBC with detectable ESR1m before progression (SERENA-6) - Full text view - ClinicalTrials.gov n.d. https://classic.clinicaltrials.gov/ct2/show/NCT04964934
- 140.SABCS Palbociclib after CDK4/6i and endocrine therapy (PACE): a randomized phase II study of fulvestrant, palbociclib, and avelumab for endocrine pre-treated er+/HER2- metastatic breast cancer n.d. 2022. https://clin.larvol.com/abstract-detail/SABCS%202022/60535046
- 141.Gerratana L., Ren Y., Reduzzi C., Regan M.M., Mahtani R.L., Ma C.X., et al. Circulating tumor cells (CTCs) dynamics after CDK4/6i for hormone-receptor positive (HR+) metastatic breast cancer (MBC): a biomarker analysis of the PACE randomized phase II study. 2023. 41:1059–1059. [DOI]
- 142.Arpino G., Bianchini G., Malorni L., Zambelli A., Puglisi F., Mastro L Del, et al. Circulating tumor DNA (ctDNA) and serum thymidine kinase 1 activity (TKa) matched dynamics in patients (pts) with hormone receptor–positive (HR+), human epidermal growth factor 2–negative (HER2-) advanced breast cancer (ABC) treated in first-line (1L) with ribociclib (RIB) and letrozole (LET) in the BioItaLEE trial. 2022. 40:1012–1012. [DOI]
- 143.Kim M.H., Kim G.M., Ahn J.M., Ryu W.J., Kim S.G., Kim J.H., et al. Copy number aberrations in circulating tumor DNA enables prognosis prediction and molecular characterization of breast cancer. J Natl Cancer Inst. 2023;115:1036–1049. doi: 10.1093/JNCI/DJAD080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Turner N., Phillips E.R., Bunce C., Robert M., Bailleux C., Garcia-Murillas I., et al. Abstract OT1-01-01: a randomised phase II trial of palbociclib and fulvestrant vs standard endocrine therapy in patients with ER positive HER2 negative breast cancer and ctDNA detected molecular relapse during adjuvant endocrine therapy (TRAK-ER) Cancer Res. 2023;83 doi: 10.1158/1538-7445.SABCS22-OT1-01-01. OT1-01–01. [DOI] [Google Scholar]
- 145.Sherene Loi et al. (PS06-01) Results from a pilot study exploring ctDNA detection using a tumor-informed assay in the monarchE trial of adjuvant abemaciclib with endocrine therapy in HR+, HER2-, node-positive, high-risk early breast cancer n.d. https://sabcs2023.eventscribe.net/fsPopup.asp?PresentationID=1328872&returl=YWpheGNhbGxzL3Nlc3Npb25pbmZvLmFzcD9QcmVzZW50YXRpb25JRD0xMzM2NDYw&mode=presInfo(accessed January 3, 2024)..
- 146.Study Details | DNA-Guided Second Line Adjuvant Therapy For High Residual Risk, Stage II-III, Hormone Receptor Positive, HER2 Negative Breast Cancer | ClinicalTrials.govn.d. https://clinicaltrials.gov/study/NCT04567420 (accessed January 3, 2024)..
- 147.Goetz M.P., Toi M., Campone M., Trédan O., Bourayou N., Sohn J., et al. Monarch 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35 doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 148.Slamon D.J., Stroyakovskiy D., Yardley D.A., Huang C.-S., Fasching P.A., Crown J., et al. Ribociclib and endocrine therapy as adjuvant treatment in patients with HR+/HER2- early breast cancer: primary results from the phase III NATALEE trial. 2023. 41:LBA500–LBA500. [DOI]
- 149.Johnston S.R.D., Harbeck N., Hegg R., Toi M., Martin M., Shao Z.M., et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE) J Clin Oncol. 2020;38:3987–3998. doi: 10.1200/JCO.20.02514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Prat A., Saura C., Pascual T., Hernando C., Muñoz M., Paré L., et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2020;21:33–43. doi: 10.1016/S1470-2045(19)30786-7. [DOI] [PubMed] [Google Scholar]
- 151.Curigliano G., Gómez Pardo P., Meric-Bernstam F., Conte P., Lolkema M.P., Beck J.T., et al. Ribociclib plus letrozole in early breast cancer: a presurgical, window-of-opportunity study. Breast. 2016;28:191–198. doi: 10.1016/J.BREAST.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 152.Hurvitz S.A., Martin M., Press M.F., Chan D., Fernandez-Abad M., Petru E., et al. Potent cell-cycle inhibition and upregulation of immune response with abemaciclib and anastrozole in neoMONARCH, phase II neoadjuvant study in HR+/HER2- breast cancer. Clin Cancer Res. 2020;26:566–580. doi: 10.1158/1078-0432.CCR-19-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hamilton E., Cortes J., Ozyilkan O., Chen S.C., Petrakova K., Manikhas A., et al. nextMONARCH: abemaciclib monotherapy or combined with tamoxifen for metastatic breast cancer. Clin Breast Cancer. 2021;21:181–190.e2. doi: 10.1016/J.CLBC.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 154.Sonke G.S., Van Ommen - Nijhof A., Wortelboer N., van der Noort V., Swinkels A.C.P., Blommestein H.M., et al. Primary outcome analysis of the phase 3 SONIA trial (BOOG 2017-03) on selecting the optimal position of cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors for patients with hormone receptor-positive (HR+) HER2-negative (HER2-) advanced breast cancer (ABC) 2023;41 doi: 10.1200/JCO.2023.41.17_SUPPL.LBA1000. LBA1000–LBA1000. [DOI] [Google Scholar]
- 155.Gianni L., Bisagni G., Colleoni M., Del Mastro L., Zamagni C., Mansutti M., et al. Neoadjuvant treatment with trastuzumab and pertuzumab plus palbociclib and fulvestrant in HER2-positive, ER-positive breast cancer (NA-PHER2): an exploratory, open-label, phase 2 study. Lancet Oncol. 2018;19:249–256. doi: 10.1016/S1470-2045(18)30001-9. [DOI] [PubMed] [Google Scholar]
- 156.Study Details | Randomized, Open Label, Clinical Study of the Targeted Therapy, Palbociclib, to Treat Metastatic Breast Cancer | ClinicalTrials.gov n.d. https://www.clinicaltrials.gov/study/NCT02947685 (accessed January 3, 2024).
- 157.Tolaney S.M., Wardley A.M., Zambelli S., Hilton J.F., Troso-Sandoval T.A., Ricci F., et al. Abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemotherapy in women with hormone receptor-positive, HER2-positive advanced breast cancer (monarcHER): a randomised, open-label, phase 2 trial. Lancet Oncol. 2020;21:763–775. doi: 10.1016/S1470-2045(20)30112-1. [DOI] [PubMed] [Google Scholar]
- 158.Hurvitz S. et al. (PO2-20-02) A Phase 3 study of gedatolisib plus fulvestrant with and without palbociclib in patients with HR+/HER2- advanced breast cancer previously treated with a CDK4/6 inhibitor plus a non-steroidal aromatase inhibitor (VIKTORIA-1) n.d. https://sabcs2023.eventscribe.net/fsPopup.asp?Mode=posterinfo&PosterID=624775 (accessed January 3, 2024).
- 159.Rugo H. et al. (PO2-19-10) CAPItello-292 Phase 3: An open-label, randomized study of capivasertib, fulvestrant, and investigator's choice of CDK4/6 inhibitor (palbociclib or ribociclib) in HR+/HER2– advanced breast cancer n.d. https://sabcs2023.eventscribe.net/fsPopup.asp?Mode=posterinfo&PosterID=624646 (accessed January 3, 2024).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.